Space use and daily movement patterns in an arid zone agamid lizard†
Adam Bernich A * , Kimberly Maute A , Isabella C. Contador-Kelsall A , Paul G. Story B , Grant C. Hose C and Kristine French A
A * , Kimberly Maute A , Isabella C. Contador-Kelsall A , Paul G. Story B , Grant C. Hose C and Kristine French A
A Centre for Sustainable Ecosystem Solutions, School of Earth, Atmospheric and Life Sciences, University of Wollongong, Wollongong, NSW 2522, Australia.
B Australian Plague Locust Commission, Fyshwick, ACT 2601, Australia.
C Department of Biological Sciences, Macquarie University, Sydney, NSW 2109, Australia.
Wildlife Research 49(6) 557-570 https://doi.org/10.1071/WR20152
Submitted: 2 September 2020 Accepted: 22 January 2022 Published: 27 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Quantifying the space use and movement patterns of animals is important to understand other aspects of a species ecology, such as habitat use and social systems. However, basic data on space use and movement patterns, and how they are influenced by biotic or abiotic factors, are lacking for many species.
Aims: We identified the space use and movement patterns of the central bearded dragon (Pogona vitticeps), and assessed how external factors (environmental conditions) and internal factors (sex and morphology) shape these patterns.
Methods: We tracked 36 P. vitticeps individuals over three seasons from 2017 to 2018. Animals carried tags with a Global Positioning Systems (GPS) device to collect spatial data and an accelerometer to collect movement data in far western New South Wales, Australia. Measurements of body morphology were taken for each individual and ambient temperatures were recorded. Space use was analysed by calculating minimum convex polygons (MCPs) and kernel density estimates (KDEs) using the spatial data. Movement data were analysed to determine whether dragons had moved during 10-min periods.
Results: Twenty-three out of 36 individuals held defined activity areas. Males with wider tails were most likely to be floaters (i.e. not restricted to specific areas). Evidence of floater behaviour was shown by 45% of females and 20% of males, though both sexes often roamed over distances greater than 1 km. Air temperatures strongly influenced movement rates in both sexes. Movement rate was significantly higher for males than females during late-spring, but not mid-summer, and was inversely related to head sizes and body mass during mid-summer. Interestingly, although there was no correlation observed between daily movement rates and size of MCPs calculated, the movement rates of residents were significantly different from floaters for each season.
Conclusions: These results confirm that wild P. vitticeps movement patterns are driven by temperature, though space-use patterns vary from previously studied agamids, with high rates of nomadism, possibly due to drought conditions. Individuals varied widely in their space-use tactics, which seem unrelated to size, a potential proxy for social status or age.
Implications: Our study provides baseline information on a common arid zone agamid that is lacking research in the wild. More complex studies on the ecology of P. vitticeps can build on the findings of this study.
Keywords: accelerometer, agamid lizard, floater, GIS, home range, movement patterns, Pogona vitticeps, space use, telemetry, temperature.
Introduction
The movement of individuals to specific locations, i.e. their space use, is driven by a combination of internal and external factors (Nathan et al. 2008). For example, the physiological requirement for an ectotherm to regulate its body temperature (internal) drives that ectotherm to move into a more appropriate location based on the current environmental conditions (external). However, because suitable basking or shelter sites may be limiting, social interactions can influence the movement and space-use patterns of individual of different social ranks (e.g. Sinervo and Lively 1996; Baird et al. 2012).
External factors that influence physiological function, such as ambient temperature, are particularly important for ectotherms, such as lizards. A lizard’s daily activities such as foraging or seeking mates, and the maximum performance level of these activities, are directly driven by ambient temperatures (Huey 1982; Adolph and Porter 1993; Angilletta et al. 2002). Therefore, to successfully survive and reproduce, lizards must keep their body temperature at an optimum, meaning that certain activities can only take place in a small range of ambient temperatures. This is shown in Tiliqua rugosa, which has activity periods in spring from 0800 to 1700 hours, but shifts to a bimodal activity pattern in summer, with a peak of activity occurring at 0800 hours and a secondary peak at 1700 hours, in order to avoid the intense heat in the middle of the day in the Australian arid zone (Kerr and Bull 2006). Furthermore, lizards are able to reduce their metabolic rate for extended periods, which can allow for substantial savings in energy and water during times of drought (Guppy and Withers 1999; Kerr and Bull 2006).
Predation is another factor that influences the space use and time of activity in lizards. Lizards are faced with a trade-off of being more active and moving further to feed on more resources (Guarino 2001) or increase mating opportunities (Alberts et al. 2002), at the cost of increased predation risk (Schwarzkopf and Shine 1992). Strategies to avoid predation include reducing activity when predators are nearby (Steinberg et al. 2014), choosing a home range with cover from possible predators, i.e. high shrub cover or rock crevices (Downes 2001), as well as camouflage (Schwarzkopf and Shine 1992). The strategies adopted by lizard individuals or species will also depend on the need for that individual to move to access resources as well as the benefit of the resource to the lizard (Downes 2001).
Resource availability and distribution changes seasonally, which in turn influences the timing and rate of lizard activity. In the tropics, many lizards reduce their activity in the dry season, even though ambient temperatures are suitable for activity (Christian et al. 1996; Fitch et al. 2005; García et al. 2010). This reduction in activity is likely to represent an energy-saving tactic in response to there being fewer resources in the dry season (Christian et al. 1996; Price-Rees et al. 2014). In the arid zone, temporal variation in resources is largely driven by patterns in rainfall and drought (Morton et al. 2011; Maute et al. 2019), and lizard activity reflects this by being higher in periods of rainfall (Kerr and Bull 2006).
As isolated rainfall events in the arid zone drive the availability of food resources (Morton et al. 2011), arid zone animals must have space use and movement strategies that locate and exploit resources when they become available. A high-mobility strategy is used by some species, such as Australian arid zone mammals, in which individuals move over large distances to locate resources rather than stay in a defined area to defend resources (Dickman et al. 1995). Furthermore, the arid zone skink Tiliqua rugosa, has home ranges that are on average almost four times as large as similar body-sized congeners from the tropics (Kerr and Bull 2006; Price-Rees et al. 2013). This is in contrast to other arid zone species such as T. adelaidensis and Egernia stokesii, which rely on shelter sites that are limiting but not transient and thus have small home ranges (Gardner et al. 2007; Fenner and Bull 2011). This suggests that there is variation in the space-use strategies used by lizards in the arid zone, which in part is driven by the variation in resource availability and type.
The variation in space-use strategies among lizard species is also driven by individual traits, such as body size, sex, and sociality. Larger lizards have higher food requirements (Nagy et al. 1999), and thus need to forage more extensively, and often have larger home ranges to cover more food resources to meet energy demands (Guarino 2001; Perry and Garland 2002). This is seen in varanid lizards, in which species with larger body mass have larger home ranges, and the relationship between home range size and mass among species is linear (Guarino 2001). However, individual lizards may show different movement and space-use patterns based on their social rank and/or social system. Visual observations of iguanid and agamid lizards suggest that to defend their territories, dominant individuals are more vigilant and engage in aggressive displays more often than subordinate non-territorial lizards (Alberts et al. 2002; Baird et al. 2012). Dominant male lizards are rewarded for defending the territory by gaining more mating opportunities than subordinates (Alberts et al. 2002), or by gaining higher quality food resources (Lattanzio and Miles 2014). However, for females, holding territories to gain mating opportunities may not be as important and other factors may drive their space-use patterns. Certain social systems may also change the direction in the relationship between body size and home range area, as dominant lizards are often larger than subordinates, though subordinates usually have larger home ranges due to them floating widely around the landscape rather than defending a defined territory (Alberts et al. 2002). However, although the subordinate lizards move longer distances, they do not necessarily spend more time moving than dominants, who put a lot of energy into defending their territory. Therefore, combining data on space use with movement patterns of individuals within a population provides insights into the social and mating system of that species.
The central bearded dragon (Pogona vitticeps) is a large, common, conspicuous agamid found in central Australia (Cogger 2014). It is a semiarboreal omnivore that breeds from September to December and ranges in size from 15 to 25 cm snout–vent length (SVL), with a body mass of 150–600 g (Cogger 2014). Despite being often used in laboratory studies (over 120 publications listed in Scopus from 2008 to 2018), there is almost no published research on the behaviour of P. vitticeps in the wild (Cogger 2014; Smith et al. 2016a). The overall aim of this study was to identify patterns of space use and movement rates of arid zone P. vitticeps and to determine how these patterns are influenced by individual traits and environmental conditions. The following hypotheses were tested: (1) sex and morphological characteristics influence space use and movement patterns; and (2) seasonal (climate) changes in environmental conditions influence space use and movement patterns. Based on data on other Australian Agamid lizards, we expected that P. vitticeps would show a range of social tactics, with the majority of individuals restricting movement to a defined area or territory, and small proportion of the population (younger or smaller individuals) would show evidence of non-territoriality or floater behaviour (Kamath and Losos 2017; Strickland and Frère 2018). Additionally, we predicted that the social status of individuals would influence timing and rate of movement patterns, with physical traits being used as a proxy for social status.
Materials and methods
Study site
This study took place at Fowler’s Gap Arid Zone Research Station, in far western New South Wales, Australia (31°20′28.50″S, 141°44′33.18″E). Fowler’s Gap has an arid climate with a mean annual rainfall of 255.3 mm and monthly average maximum temperatures ranging from 17.0 to 36.4°C (Bureau of Meteorology 2018). The annual rainfall for the year leading up to the study was 84.4 mm, indicating that this study took place during drought (Bureau of Meteorology 2018). All applicable international, national, and institutional guidelines for the care and use of animals were followed and the methods used were approved by the University of Wollongong Animal Ethics Committee authority AE17/19.
Capture and tracking of animals
Bearded dragons were studied over three tracking periods: mid-spring (26 September–13 October 2017; late-spring (21 November–9 December 2017); and mid-summer (19 January–12 February 2018). Dragons were hand caught by searching under shrubs or in burrows. Upon capture, measurements were taken of SVL, tail length and width, head length and width, and body mass. Sex was determined by raising the tail to see hemipene eversion. Each dragon was toe-clipped for permanent identification.
In total, 36 P. vitticeps were caught and tracked for 3–20 days (Supplementary Table S1). Twelve dragons (nine male, three female) were tracked in mid-spring for an average of 11 days. In late-spring, 13 dragons (eight male, five female) were tracked for an average of 12 days. Thirteen dragons (seven male, six female) were also tracked in mid-summer for an average of 14 days. Two dragons that were tracked in mid-spring were recaptured in late-spring, and all other dragons were tracked for one period. The variation in total tracking times was due to staggered tag deployment schedules, tag failure events and the rare occurrence of an individual/tag not being relocated.
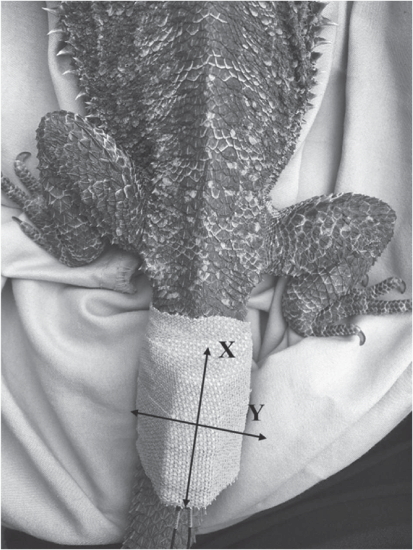
We attached Pinpoint Beacon 120 or 240 (Sirtrack Ltd, Havelock North, New Zealand) GPS trackers to the dorsal surface of each dragon’s tail, using surgical tape (Fig. 1). The GPS tags were set to record latitude, longitude, and Dilution of Precision (DOP; a measure of the precision of a GPS reading) locational data. In mid-spring and late-spring, GPS tags were scheduled to take locational fixes every 40 min from 0600 or 0800 to 2100 hours. Pilot studies showed that dragons did not often move overnight during these periods. In the mid-summer period, the occurrence of movement in overnight periods increased (A. Bernich and K. Maute, pers. obs.), likely in response to temperature increases. Therefore tags were rescheduled to take fixes every 2 h all day and all night in mid-summer to allow for a longer battery life and to accommodate this change in behaviour. A VHF beacon enabled relocation of the individual (VHF receiver and antennae; Telonics TR5). We relocated each dragon every 3–11 days to download GPS data and replace tags, with recaptures ranging from 1 to 5 times. Location data with a DOP greater than 5 was excluded from further analysis, because the probability of those fixes being an accurate representation of the dragon’s location was low (Recio et al. 2011). At the end of the study, we removed all tags from the animals that could be relocated.
|
For all dragons tracked in late-spring and mid-summer, but only three individuals from mid-spring, we attached accelerometers (HOBO Pendant G) underneath the GPS tags (Fig. 1) to measure activity rates. Accelerometers are sensors that measure gravitational and inertial acceleration (g) of three axes (X, Y, and Z) caused by movement (see Brown et al. (2013) for a review on their application in studies of the movement of free-ranging animals), thus a change in acceleration indicates a movement performed by the animal. To measure activity, accelerometers were set to record acceleration (from −3 g to +3 g) on the X and Y axes (Fig. 1) every 30 s. Not all animals were fitted with accelerometers in mid-spring, because this was a trial period undertaken early in our research.
Space-use patterns
The tracking periods (3–20 days) were unlikely to capture the entire home range of the animals; therefore, we estimated a subset of their home range, called an activity area (Thompson et al. 1999). To calculate activity areas, we used the minimum convex polygon (MCP) method, using the 100% isopleth. This was calculated by using the adehabitatHR package (Calenge 2006) in R v4.0.2 (R Core Team 2020). Cumulative MCP areas were created for successive tracking days for each individual and areas were plotted against days tracked to determine if individuals remained in a defined area, and if repeated patterns of movement were evident in the two individuals that were recaptured in multiple trapping sessions (see Supplementary Fig. S2). Dragons for which the cumulative MCP area curve was asymptotic were regarded as ‘residents’, most likely occupying a home range or a territory. Dragons that did not have an asymptotic plot were called ‘floaters’. We use these terms as relative characterisation labels for individuals, not absolute definitions of resident or nomadic behaviour, due to the short time periods of measurement. Images of the dragon’s location on cumulative days were also visually inspected in QGIS (QGIS Development Team 2017, QGIS Geographic Information System; http://qgis.osgeo.org) to ensure that the dragon revisited core areas, as a pattern of movement in a single direction, followed by a lack of movement at the end of tracking also resulted in an asymptotic curve. Five individuals that showed asymptotic area vs days tracked curves were considered as floaters due to this movement pattern. Therefore, all residents were dragons that held defined activity areas over the time they were tracked and were used in the analysis of MCPs, whereas MCPs were not calculated for floaters.
The 95% kernel density estimate (KDE) method was used to determine areas of more intense use (the utilisation distribution; Worton 1989). We did not use KDEs as estimates of home range size or activity areas because they have been shown to produce inaccurate estimates in reptiles (Row and Blouin-Demers 2006), and all animals were tracked for <20 days. Instead, KDEs were used as a visual tool representing the core area of usage and compared among individuals to determine areas of overlapping and more concentrated usage, rather than for comparison of core area sizes among individuals. The KDE search radius (sometimes referred to as bandwidth, smoothing parameter, or h) was calculated using the ad hoc method (had hoc) following Kie (2013). To determine what percentage of the activity area represents the core areas of activity, the isopleth of KDE that bounds the core area was calculated following Vander Wal and Rodgers (2012). The adehabitatHR package (Calenge 2006) was used in R v4.0.2 (R Core Team 2020) to calculate MCPs, 95% KDEs and core areas. Only animals with sufficient data were included in KDE calculations, based on recommendations from Stone and Baird (2002) and Rose (1982). We found that roughly 8 days described 80% of the average MCP of resident dragons and thus was used as the minimum sample size. Individuals that were tracked for fewer than 8 days were removed from KDE analyses. The distribution of capture sites of individual dragons limited visualisation of space-use overlap to six dragons in mid-spring and five dragons in late-spring. Individuals tracked in late-summer were spread across 14 km2 of area on the property, as opposed to 4 km2 in other seasons. Due to this small sample size and short temporal scale of tracking, we were only able to report whether home range overlap occurs in P. vitticeps, rather than perform a statistical analysis of overlap differences.
Analysis of movement
A moving individual is represented by large fluctuations in acceleration recorded by that animal’s accelerometer (Brown et al. 2013). Therefore, we used the variance statistic for the raw acceleration data (recorded in g units; 1 g = 9.80665 m s−2) measured on the X- and Y-axis by the accelerometer to define when an animal was moving. Because the accelerometers used in this study could only record when an individual is moving and not when it is active (as activity may include basking individuals that do not move for extended periods of times), we refer to measurements taken by the accelerometers as movement rather than activity. To determine what amount of variance constituted movement, a GPS tag was set up on a single individual in spring to take locational fixes every 20 min, and the accelerometer was set up as above. Accelerometer data was split into 10-min intervals and was matched with the GPS data to see if movements shown represented an actual change in location. The smallest variance for a 10-min period where movement occurred was 0.0268 g2, and the largest variance for a 10- min period without movement was 0.0159 g2. Based on this, 0.0199 g2 was taken as the smallest amount of variance that constituted movement and 10-min variance subtotals were calculated for each individual using 0.0199 g2 variance as the movement threshold. Data that were recorded 10 min before recapture, and 20 min after returning the dragon to the point of capture were removed from analysis to reduce the impact of human disturbance on the acceleration readings. Although we removed data as a precaution, it is unknown whether capture stress impacts behaviour in this species for any length of time.
Two types of movement rates were calculated:– total average movement per hour (for all dragons pooled) and average daily movement (per individual). Total average movement per hour was calculated by first summing 10 min moving periods for every hour to determine hourly movements (i.e. 0, 10, 20, 30, 40, 50 or 60 min) for each individual. Next, the average minutes of movement for each hour of the day was calculated by dividing the summed minutes of movement over all days of recording that hour by the number of days that hour was recorded for the focal ndividual. The total average movement per hour of the day was calculated by averaging data from all individual dragons. These data were used to analyse the patterns in timing of diel movement during the late-spring and mid-summer tracking periods, the mid-spring tracking period was not used as only three dragons carried accelerometers during this period. The total minutes that a dragon moved was divided by the total number of full days the dragon was tracked to determine the average daily movement rates per individual.
Air temperature and movement
Air temperature data was accessed from Fowlers Gap Weather Station (UNSW 2018) (31°4′35.54″S, 141°44′2.40″E), which recorded air temperature (°C) every 10 min. To determine how fine-scale air temperature patterns influenced movement, 10 min temperature data were converted into integer classes (i.e. 30°C represents values from 30.00 to 30.99°C). The occurrence of movement within each integer class was calculated by summing the number of times a dragon was recorded moving within a specific integer class. The number of times the dragon was not moving in each integer class was also summed for use in analysis.
Statistical analysis
For the analysis of space-use patterns we used logistic regressions to see if more individuals from one sex were residents, and to determine if body measurements were related to the probability of being a resident. Activity area data were tested with separate t-tests to determine if MCP area differed between resident males and females. Analysis of variance (ANOVA) was used to test for differences in MCP area among seasons. We also used linear regression to see if body measurement characteristics influenced MCP area.
We used t-tests to compare individual dragon average daily movement data between sexes and between the two tracking periods. A t-test was used to compare the MCP size of residents and floaters within each season separately. Linear regressions were used to explore relationships between body measurements and daily movement rates. Logistic regression was used to determine if there was a relationship between numbers of captures (i.e. capture stress) and the probability that a dragon was a resident or floater.
We used a generalised additive model (GAM) with a logit link to test how ambient temperature influenced the occurrence of individual dragon movement. Temperature was used as the explanatory variable and response was a two-column matrix of the number of times a dragon moved at a temperature and the number of times it did not move at that temperature. Dragon identity was added as a random effect to account for repeated sampling of each individual dragon being used in the dataset. The two tracking periods with movement data, late-spring and mid-summer were analysed separately. This test was undertaken in R v4.0.2 (R Core Team 2020) using the mgcv package (Wood 2017).
All tests on differences between a response variable and sex were run for each tracking period and all periods combined, and all tests between tracking periods and a response variable were run separately for each sex and both sexes combined. All tests on body measurement variables were run for each tracking period and all tracking periods combined, as well as separate tests for each sex and both sexes. Because six body measurement variables were being tested on the same set of activity area and movement data, the significance level (α, nominally 0.05) was adjusted following the sequential Bonferroni method (Holm 1979) for these tests. All data were explored to ensure assumptions of statistical analyses were met by following the protocols in Zuur et al. (2010). Statistical analyses were undertaken using JMP Pro (version 11.0, 2013; SAS Institue Inc., Cary, NC, USA) and all averages are quoted as mean ± standard deviations (s.d.).
Results
Space-use patterns
Twenty-three of the P. vitticeps tracked held a defined activity area and were labelled as residents, and nine did not meet our criteria for resident behaviour and were labelled floaters. The remaining four dragons were only tracked for 4 days or less, which was insufficient to determine whether they were residents. The limited tracking periods for these dragons were due to us being unable to relocate them, or on one occasion the individual died from predation (dragon was found deceased with only the skin remaining). These dragons were removed from further analyses. All other dragons were tracked for 8 or more days.
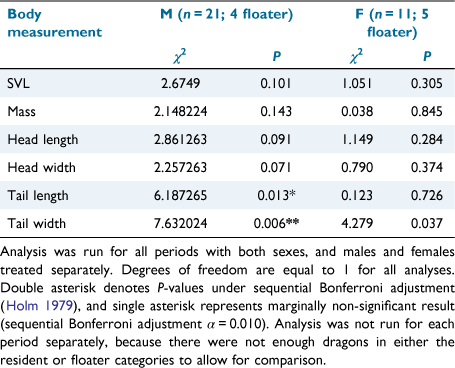
Tracking resulted in 6849 total successful GPS fixes being taken, an average of 190 (±135) fixes per dragon (Supplementary Table S1). The average fixes per dragon for each tracking period was 210 (±146) for mid-spring, 264 (±113) for late-spring and 77 (±36) for mid-summer. Approximately, 80% of males and 55% of females were residents, but logistic regression showed that this difference was not significant (χ21 = 2.42, P = 0.12). The number of times a dragon was recaptured to change GPS and accelerometer devices did not influence whether they would be a resident or not (χ21 = 1.44, P = 0.23). Male dragons with wider tails were significantly more likely to be a floater, with the average tail width for floaters 12% wider than residents. Males with longer tails were more likely to be a resident, with tails of residents on average 16% longer than those of floaters, though this trend was not significant (Table 1). There was also a non-significant trend for females with wider tails being more likely to be a resident, with residents having tails 12% wider on average (Table 1).
|
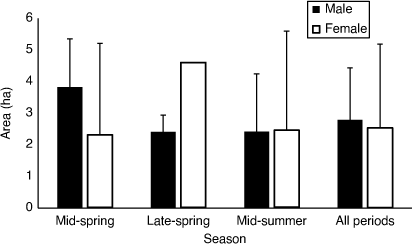
The average MCP activity area of residents was 2.89 ± 1.82 ha, and there was no difference in the activity area sizes of males and females over all seasons (Fig. 2; t-test: t7 = 0.429, P = 0.682), though male MCPs were, on average, 25% larger. There was no significant difference between the size of male and female activity areas within seasons (mid-spring: t3 = 1.131, P = 0.346; mid-summer: t5 = 0.639, P = 0.550) or for the total average activity areas among seasons when both sexes were pooled (ANOVA: F2,20 = 1.40, P = 0.270). Linear regression suggested that the size of MCPs was not influenced by the number of times dragons were recaptured for tag replacement when either all dragons were analysed together or each period was analysed separately (mid-spring: F1,8 = 1.48, R2 = 0.157, P = 0.259; late-spring: F1,4 = 3.34, R2 = 0.455, P = 0.142; mid-summer: F1,7 = 1.83, R2 = 0.207, P = 0.218; all periods: F1,23 = 0.106, R2 = 0.005, P = 0.747). No significant relationships were seen between body measurements and MCP area when all resident individuals were analysed for all tracking periods, or when each sex was analysed separately for each period (based on sequential Bonferroni adjustment; Supplementary Table S2).
|
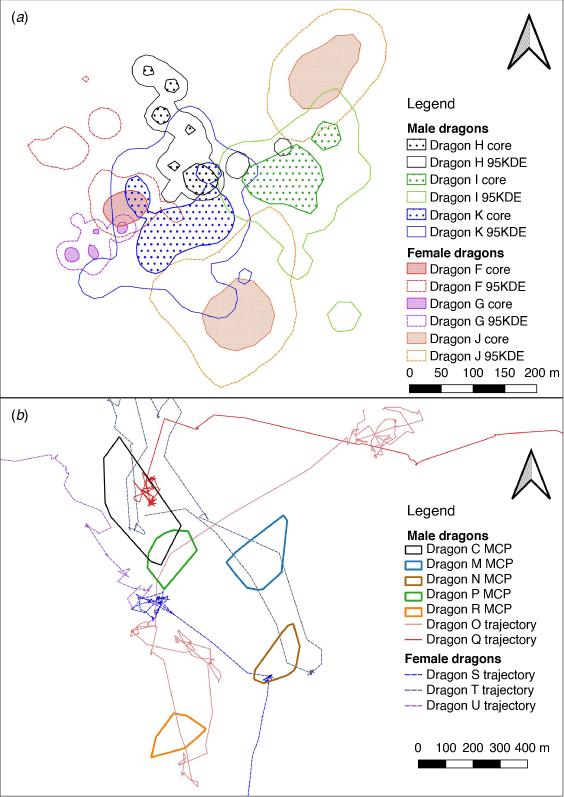
Across all tracking periods, core area size ranged from 0.029 ha to 1.13 ha and occupied an average of 22.6% (±2.4%) of the size of the 95% KDE. No resident P. vitticeps used space uniformly, and 19 out of 23 individuals revisited core areas (Fig. 3a). Three of the four individuals who did not revisit core areas were females. For floater P. vitticeps, six individuals continuously moved away from the site of capture roughly in the same direction (Fig. 3c), whereas three individuals (two males, one female) roamed in varied directions and returned to previously used areas (Fig. 3d).
In mid-spring, all tracked dragons had areas of space-use overlap with at least one other individual (Fig. 4a). Overlap among residents occurred only once in late-spring, between male dragons C and P (Fig. 4b, Supplementary Table S3). Other resident dragons only had overlap occurring with floaters, who all crossed through at least one resident’s area (Fig. 4b, Supplementary Table S3).
|
Daily movement: late-spring and summer only
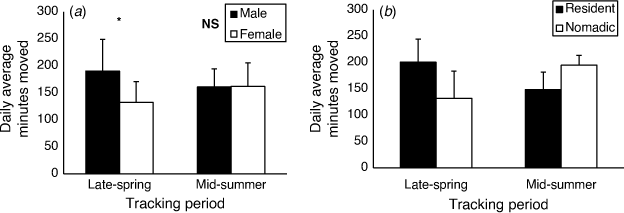
The total average daily movement per individual was 162 (±49) min moving per day. Males had significantly higher daily average movement than females during the late-spring period (Fig. 5a; t9 = 1.44, P = 0.0403). However, daily movement rates were very similar between the sexes for mid-summer (t9 = 0.48, P = 0.322). There was no significant difference in movement among the seasons when each sex was analysed separately (males: t10 = 1.1311, P = 0.142; females: t7 = 1.07, P = 0.160).
|
Resident dragons, regardless of sex, moved significantly more each day than floaters during late-spring (Fig. 5b; t8 = 2.34, P = 0.0237). However, the opposite trend was seen in mid-summer (Fig. 5b; t7 = 2.97, P = 0.0109). Linear regression suggested that the size of the MCP or core area was not correlated with the daily average movement (MCP: F1,12 = 0.103, R2 = 0.009, P = 0.753; core: F1,12 = 0.5104, R2 = 0.041, P = 0.489). Daily average movement rates per individual showed significant negative relationships with head length and width, and body mass when data from both sexes from mid-summer were pooled (Table 2). When analysed separately, there was a negative, approaching significant, relationship between movement and tail length in males, and movement and tail width in females in mid-summer (Table 2).

|
Influence of temperature on occurrence of movement
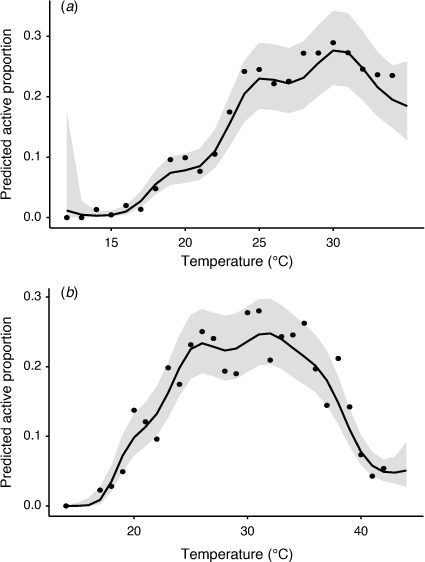
The GAM model showed that temperature influenced the occurrence of movement in both tracking periods, with the variance explained by the model being 64.5 and 48.9% in late-spring and mid-summer, respectively (Supplementary Table S4). In late-spring, a peak in the predicted mean movement rate occurred at around 30–31°C, though levels were similar between 25 and 35°C. Below 25°C the activity rate dropped linearly until dragons were mostly inactive around 15°C (Fig. 6a). Similar results were seen in mid-summer, with the highest rates of movement occurring between 25 and 35°C, and a negative linear trend below 25°C to inactivity around 15°C. However, there were warmer temperatures in the mid-summer period and the trend shows a sharp decline in predicted movement above 35°C, with only low predicted movement at the highest temperatures, 42°C and above (Fig. 6b).
|
Diel timing of movement
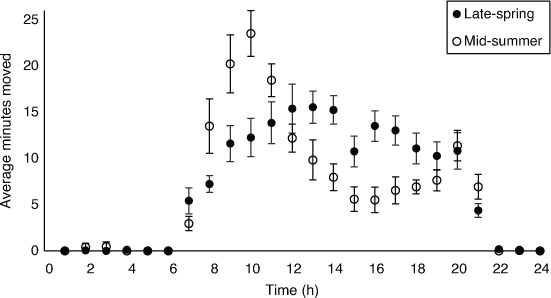
Total average movement per hour (pooled data) showed that P. vitticeps is mainly diurnal, with movements rarely recorded in the late-night hours (2200–0600 hours). In late-spring, movement showed a unimodal distribution, with the most movement occurring during the middle of the day (Fig. 7). However, the movement pattern of the mid-summer tracking period had a bimodal distribution, with the highest amounts of movement occurring at 1000 hours, followed by a large reduction in movement during the middle of the day and then a secondary, smaller peak at 2000 hours (Fig. 7), around local sunset at 2040 hours AEDT. Hourly temperatures in mid-summer were about 6°C higher than those in late-spring (Supplementary Fig. S1).
|
Discussion
The data collected provide information regarding the space use and movement patterns of P. vitticeps, as well as insights into the possible social system. Two-thirds of individuals remained in defined activity areas, whereas the remaining individuals were wide-roaming floaters, often moving distances greater than 1 km. Although no significant difference was found, the high proportion of female floaters contrasts with a lower proportion of male floaters and suggests that sexes may have differing space-use tactics. Overall individual movement rates coincided with optimal temperatures as expected (Cadena and Tattersall 2009). We also found seasonal differences in movement rates; males and residents moved more in late-spring, but floaters moved more mid-summer, possibly indicative of seasonal changes in feeding resources, breeding or territorial behaviours. Findings of the current study corroborate other research that suggests wild P. vitticeps movement patterns may fit well into expected models of ectotherm behaviour associated with optimising movement to preferred temperatures (Cadena and Tattersall 2009; Smith et al. 2016b), but space-use patterns suggest an unexpected lack of site fidelity and large movement distances, particularly for females. These patterns may be driven by variation in resources or social factors.
The large activity areas and high proportion of floaters set P. vitticeps apart from other studied Australian agamids. The activity areas recorded do not necessarily represent the actual home range of P. vitticeps, but average activity areas (2.9 ha) for P. vitticeps tracked from 3 to 20 days were still larger than average home ranges recorded for other Australian agamids. The Australian agamid with the closest recorded home range size is Chlamydosaurus kingii (1.9 ha), which was tracked over several years in monsoonal tropics (Griffiths 1999). The proportion of floaters found in this study has not been observed in large agamids previously (Griffiths 1999; Baird et al. 2012; Ujvari et al. 2015), including the closely related P. barbata (Wotherspoon 2007). The proportion of territorial males in smaller agamid species ranges widely (<10 to >80%), but females are commonly sedentary (Olsson 1995; Lebas 2001; Watt et al. 2003; Olsson et al. 2007; Stevens et al. 2010). Our results suggesting a large proportion of females do not remain in a defined activity area were therefore unexpected.
Environmental factors may drive the large activity areas and wide-roaming behaviour of both males and females of P. vitticeps. This study occurred during a very dry year (2017 annual rainfall was 170 mm below average) and may have driven a switch to wider-ranging behaviours to increase the probability of encountering scarce resources in the landscape. Similarly, other species of lizards have been observed to switch space-use tactics towards greater nomadism during drought (Knapp et al. 2003). Floaters moving more than resident dragons during mid-summer (when resources would be rarer and breeding behaviour would be less likely) supports this hypothesis. Drought effects and grazing pressure from kangaroos, sheep and goats substantially reduced the shrub cover at the site, and may have increased exposure to predators, such as birds of prey, foxes, pigs and feral cats, which would force individuals to alter their behaviour to find areas with suitable shelter and resources (Downes 2001). Further investigations of lizard movement behaviour during periods of higher rainfall and vegetation cover would be needed to test these hypotheses.
Although P. vitticeps males with wider tails were significantly less likely to show resident behaviour, there was also a trend towards shorter-tailed males to be floaters. Tail autotomy does not occur in agamids, and many species use their tails to display and communicate to conspecifics, with tail flicks forming parts of aggressive displays (Antonio and Anthony 2016). Thus, territorial individuals may use their longer tails to aid in defending their territories either through tail flicks or as a social status badge. Removal of lizard tails can reduce social status (Fox et al. 1990), and the two largest P. vitticeps individuals tracked in late-spring were each missing a large part of their tail (Dragons O and Q; Supplementary Table S1), and showed floater behaviour. This suggests that many P. vitticeps males defend territories, and is supported by the finding that overlap of MCPs only occurred once among males during the late-spring tracking period, which is likely when breeding, and therefore territorial behaviours were at their most intense. Conversely, the high rates of overlap between floaters and residents could suggest a dominance hierarchy in which only dominant males are excluded from other dominant males' territories, as seen in Intellagama lesueurii and Cyclura nubila (Alberts et al. 2002; Baird et al. 2012). However, not enough dragons were tracked to fully understand the social system of P. vitticeps, and further study is needed.
The wider tails, yet similar movement levels, of these floater males may drive them to expend more energy on foraging than energetically costly mating or territorial behaviours. This would allow for floaters to maintain body condition at cost of reduced breeding success, a scenario that occurs in C. nubila (Alberts et al. 2002). Increased storage of tail fat may allow these individuals to roam more widely in search of transient resources, which is a tactic used by small mammal species in the arid zone (Dickman et al. 1995), and may result in improved chances of survival during drought. In contrast, heavier females with wider tails showed a trend towards reduced movement and resident behaviour after the breeding season, suggesting females in better body condition remain in areas, possibly to revisit nest sites. However, we did not see clear evidence of gravid females during our monitoring. Our findings could instead suggest heavier females are able to reduce both the amount of time moving and distance travelled due to larger energy stores or higher social status. The opposite direction of influence that tail width has on space use and movement between the sexes suggests that males and females have different space-use and movement tactics. This is also supported by the high number of females that showed floater behaviour compared with males across all seasons. However, more study is needed to fully understand the range of social and spatial strategies of P. vitticeps, and the consequences of those strategies on body condition and survival.
P. vitticeps males moved more than females in late-spring during breeding, perhaps to maximise mating success, as is seen in T. rugosa (Kerr and Bull 2006) and Iberolacerta cyreni (Salvador et al. 2008). However, enhanced activity rates can impose survival costs, and lower daily activity is likely to be selected when not essential (Marler et al. 1995), resulting in the lower movement seen in P. vitticeps females during breeding (late-spring), resident males after breeding (mid-summer), and heavier individuals compared with lighter individuals in mid-summer. Similarly, dominant C. nubila males relaxed their territorial behaviour outside of the breeding season, yet little change was seen in subordinate individuals (Alberts et al. 2002). The higher movement of males than females in the breeding season may suggest that males are expending more energy in social displays that attract females or for competition with rival males, which may be another factor that explains why resident males had thinner tails (potentially less fat storage) after the breeding season. This, coupled with the floater behaviour of females, may suggest both sexes are maximising mating opportunities and could indicate that P. vitticeps has a promiscuous mating system, similar to at least one other agamid (I. lesueurii; Frere et al. 2015). Our observed high rates of home range overlap and non-exclusive core areas support this, and may suggest that alternative mating strategies may be occurring (Sinervo and Lively 1996; Baird et al. 2012). However, although a promiscuous mating system is likely, it cannot be confirmed without genetic parentage studies.
The lack of a relationship between movement and activity area measures was not expected, because a larger home range size is often used as an indirect measure of higher activity in lizards (Guarino 2001; Stone and Baird 2002). However, this assumption is often based on consecutive radio tracking fixes that measure straight line distances between two points, and therefore may miss patterns of higher or lower movement between these two points. In this study, movements recorded by the accelerometer were not always from a change in horizontal location; rather they could have been from climbing, feeding, burrowing and displaying to conspecifics. Therefore, individuals that had higher rates of movement in this study may have engaged in these behaviours more often while still occupying home ranges of similar sizes as individuals that moved less. In addition, a large home range size does not always correlate with dominance, though high activity levels do correlate with dominance in some species (Alberts et al. 2002; Baird et al. 2012). Therefore, the absence of a relationship between activity areas with movement levels in P. vitticeps was likely due to individuals moving more by feeding, burrowing or social signalling for territorial defence or mating opportunities. Nevertheless, how social or resource pressure drive the space-use tactics of P. vitticeps is unclear, and greater knowledge of the resource use and social and breeding systems for this species are needed to make strong conclusions.
Our study differed from the previous studies tracking agamid home ranges in that we used GPS devices rather than VHF methods to track individuals. Our method required multiple recaptures of dragons to replace GPS units before their batteries expired. It could be argued that the multiple recaptures could lead to larger activity areas due to individuals moving to new areas after the stress of being captured. Indeed, Kerr et al. (2004) found that T. rugosa individuals altered their movement behaviour more when being handled compared to just being observed. However, we did not find that the number of recaptures, which ranged from one to five (including the initial capture), did not influence MCP; nor did more recaptures make it more likely for individuals to be floaters. Supporting this, the two dragons that were tracked in two separate tracking periods, and thus recaptured twice as much as other dragons, had core areas and MCPs in very similar locations in both periods (see Supplementary Fig. S2). Furthermore, the ability of the GPS devices to record data remotely removes the need for observers to track down an individual (sometimes multiple times a day) (Guarino 2001; Osterwalder et al. 2004; Smith and Griffiths 2009) to take a location, which also alters their behaviour (Kerr et al. 2004). Therefore, although it is likely that recapturing dragons to replace GPS tags does alter their behaviour for a period after handling, it is unlikely that it leads to the large activity areas recorded for some individuals.
Conclusions
It was found that P. vitticeps individuals exhibited a variety of space-use and movement tactics over short periods, in which most males show resident behaviours and females less commonly so. Ambient temperatures had a strong influence on movement rates, but relationships were also seen among movement and individual morphological traits that may reflect social status or body condition. With further study, these patterns may provide insights into the resource use, ecology and sociality of this species. Wide-ranging, floater behaviour appeared to be a relatively common tactic for females, and the lack of strong differences in activity area size between sexes suggests that space-use and movement tactics within activity areas differ. In contrast, the high rates of wide-ranging behaviour recorded may be a response to reduced resources, because this study took place during a drought. Future surveys may determine whether these patterns in space use and movement are conserved under better climate conditions. This is the first study looking at the space-use patterns of this species in the wild, despite P. vitticeps being a dominant species in Australia’s semiarid to arid environments, and a commonly used laboratory model. This study shows how P. vitticeps utility in scientific research extends with free-ranging individuals and provides baseline information that more complex studies on the space-use tactics, sociality and mating systems of P. vitticeps can build upon.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Funding for this project was provided by the Australian Research Council (ARC) and the Australian Plague Locust Commission (APLC) through an ARC Linkage Grant (LP160100686) administered through the University of Wollongong in collaboration with Macquarie University.
Supplementary material
Supplementary material is available online.
Acknowledgements
Many thanks to all the enthusiastic University of Wollongong, Macquarie University and University of Canberra volunteers and staff that helped capture and radio track dragons. We thank the Australian Plague Locust Commission (APLC) staff for field support, and the staff at Fowler’s Gap (Keith Leggett, Gary and Vicki Dowling), for access to experimental sites, accommodation and assistance in the field. We also thank Michael Bedward for assistance in statistical analysis and three anonymous reviewers for their advice on the manuscript. All applicable international, national, and institutional guidelines for the care and use of animals were followed. This study was undertaken under the University of Wollongong Animal Ethics Committee authority AE17/19, and the NSW National Parks and Wildlife Service Scientific License (SL100109).
References
Adolph, SC, and Porter, WP (1993). Temperature, activity, and lizard life histories. The American Naturalist 142, 273–295.| Temperature, activity, and lizard life histories.Crossref | GoogleScholarGoogle Scholar | 19425979PubMed |
Alberts, AC, Lemm, JM, Perry, AM, Morici, LA, and Phillips, JA (2002). Temporary alteration of local social structure in a threatened population of Cuban iguanas (Cyclura nubila). Behavioral Ecology and Sociobiology 51, 324–335.
| Temporary alteration of local social structure in a threatened population of Cuban iguanas (Cyclura nubila).Crossref | GoogleScholarGoogle Scholar |
Angilletta, MJ, Cooper, BS, Schuler, MS, and Boyles, JG (2002). The evolution of thermal physiology in endotherms. Journal of Thermal Biology 2, 249–268.
| The evolution of thermal physiology in endotherms.Crossref | GoogleScholarGoogle Scholar |
Antonio, RJ, and Anthony, PR (2016). Dragon wars: movement‐based signalling by Australian agamid lizards in relation to species ecology. Austral Ecology 41, 302–315.
| Dragon wars: movement‐based signalling by Australian agamid lizards in relation to species ecology.Crossref | GoogleScholarGoogle Scholar |
Baird, TA, Baird, TD, and Shine, R (2012). Aggressive transition between alternative male social tactics in a long-lived Australian dragon (Physignathus lesueurii) living at high density. PLoS One 7, e41819.
| Aggressive transition between alternative male social tactics in a long-lived Australian dragon (Physignathus lesueurii) living at high density.Crossref | GoogleScholarGoogle Scholar | 22905109PubMed |
Brown, DD, Kays, R, Wikelski, M, Wilson, R, and Klimley, AP (2013). Observing the unwatchable through acceleration logging of animal behavior. Animal Biotelemetry 20, 16.
Bureau of Meteorology (2018) Climate statistics for Australian locations. Available at http://www.bom.gov.au/climate/averages/tables/cw_046128.shtml [Accessed 27 February 2018]
Cadena, V, and Tattersall, GJ (2009). The effect of thermal quality on the thermoregulatory behavior of the bearded dragon Pogona vitticeps: influences of methodological assessment. Physiological and Biochemical Zoology 82, 203–217.
| The effect of thermal quality on the thermoregulatory behavior of the bearded dragon Pogona vitticeps: influences of methodological assessment.Crossref | GoogleScholarGoogle Scholar | 19323642PubMed |
Calenge, C (2006). The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197, 516–519.
Christian, KA, Griffiths, AD, and Bedford, GS (1996). Physiological ecology of frillneck lizards in a seasonal tropical environment. Oecologia 106, 49–56.
| Physiological ecology of frillneck lizards in a seasonal tropical environment.Crossref | GoogleScholarGoogle Scholar | 28307156PubMed |
Cogger H (2014) ‘Reptiles and amphibians of Australia.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Dickman, CR, Predavec, M, and Downey, FJ (1995). Long-range movements of small mammals in arid Australia: implications for land management. Journal of Arid Environments 31, 441–452.
| Long-range movements of small mammals in arid Australia: implications for land management.Crossref | GoogleScholarGoogle Scholar |
Downes, S (2001). Trading heat and food for safety: costs of predator avoidance in a lizard. Ecology 82, 2870–2881.
| Trading heat and food for safety: costs of predator avoidance in a lizard.Crossref | GoogleScholarGoogle Scholar |
Fenner, AL, and Bull, CM (2011). Central-place territorial defence in a burrow-dwelling skink: aggressive responses to conspecific models in pygmy bluetongue lizards. Journal of Zoology 283, 45–51.
| Central-place territorial defence in a burrow-dwelling skink: aggressive responses to conspecific models in pygmy bluetongue lizards.Crossref | GoogleScholarGoogle Scholar |
Fitch, AJ, Goodman, AE, and Donnellan, SC (2005). Isolation and characterisation of microsatellite markers for the Australian monitor lizard, Varanus acanthurus (Squamata: Varanidae) and their utility in other selected varanid species. Molecular Ecology Notes 5, 521–523.
| Isolation and characterisation of microsatellite markers for the Australian monitor lizard, Varanus acanthurus (Squamata: Varanidae) and their utility in other selected varanid species.Crossref | GoogleScholarGoogle Scholar |
Fox, SF, Heger, NA, and Delay, LS (1990). Social cost of tail loss in Uta stansburiana: lizard tails as status-signalling badges. Animal Behaviour 39, 549–554.
| Social cost of tail loss in Uta stansburiana: lizard tails as status-signalling badges.Crossref | GoogleScholarGoogle Scholar |
Frere, CH, Chandrasoma, D, and Whiting, MJ (2015). Polyandry in dragon lizards: inbred paternal genotypes sire fewer offspring. Ecology and Evolution 5, 1686–1692.
| 25937911PubMed |
García, A, Valtierra-Azotla, M, and Lister, BC (2010). Behavioral responses to seasonality by two sceloporine lizard species from a tropical dry forest. Animal Biology 60, 97–113.
| Behavioral responses to seasonality by two sceloporine lizard species from a tropical dry forest.Crossref | GoogleScholarGoogle Scholar |
Gardner, MG, Bull, CM, Fenner, A, Murray, K, and Donnellan, SC (2007). Consistent social structure within aggregations of the Australian lizard, Egernia stokesii across seven disconnected rocky outcrops. Journal of Ethology 25, 263–270.
| Consistent social structure within aggregations of the Australian lizard, Egernia stokesii across seven disconnected rocky outcrops.Crossref | GoogleScholarGoogle Scholar |
Griffiths, AD (1999). Demography and home range of the frillneck lizard, Chlamydosaurus kingii (Agamidae), in northern Australia. Copeia 1999, 1089–1096.
| Demography and home range of the frillneck lizard, Chlamydosaurus kingii (Agamidae), in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Guarino, F (2001). Spatial ecology of a large carnivorous lizard, Varanus varius (Squamata: Varanidae). Wildlife Research 28, 627–630.
| Spatial ecology of a large carnivorous lizard, Varanus varius (Squamata: Varanidae).Crossref | GoogleScholarGoogle Scholar |
Guppy, M, and Withers, P (1999). Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biological Reviews 74, 1–40.
| Metabolic depression in animals: physiological perspectives and biochemical generalizations.Crossref | GoogleScholarGoogle Scholar | 10396183PubMed |
Holm, S (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6, 65–70.
| A simple sequentially rejective multiple test procedure.Crossref | GoogleScholarGoogle Scholar |
Huey RB (1982) Temperature, physiology, and the ecology of reptiles. In ‘Biology of the Reptilia’, Vol. 12, Physiology. (Eds C Gans, FH Pough) pp. 25–91. (Academic Press: London)
| Crossref |
Kamath, A, and Losos, J (2017). The erratic and contingent progression of research on territoriality: a case study. Behavioral Ecology and Sociobiology 71, 1–13.
| The erratic and contingent progression of research on territoriality: a case study.Crossref | GoogleScholarGoogle Scholar |
Kerr, GD, and Bull, CM (2006). Movement patterns in the monogamous sleepy lizard (Tiliqua rugosa): effects of gender, drought, time of year and time of day. Journal of Zoology 269, 137–147.
| Movement patterns in the monogamous sleepy lizard (Tiliqua rugosa): effects of gender, drought, time of year and time of day.Crossref | GoogleScholarGoogle Scholar |
Kerr, GD, Bull, CM, and Mackay, D (2004). Human disturbance and stride frequency in the sleepy lizard (Tiliqua rugosa): implications for behavioral studies. Journal of Herpetology 38, 519–526.
Kie, JG (2013). A rule-based ad hoc method for selecting a bandwidth in kernel home-range analyses. Animal Biotelemetry 1, 13.
| A rule-based ad hoc method for selecting a bandwidth in kernel home-range analyses.Crossref | GoogleScholarGoogle Scholar |
Knapp, R, Hews, DK, Thompson, CW, Ray, LE, and Moore, MC (2003). Environmental and endocrine correlates of tactic switching by nonterritorial male tree lizards (Urosaurus ornatus). Hormones and Behavior 43, 83–92.
| 12614637PubMed |
Lattanzio, MS, and Miles, DB (2014). Ecological divergence among colour morphs mediated by changes in spatial network structure associated with disturbance. Journal of Animal Ecology 83, 1490–1500.
| Ecological divergence among colour morphs mediated by changes in spatial network structure associated with disturbance.Crossref | GoogleScholarGoogle Scholar |
Lebas, NR (2001). Microsatellite determination of male reproductive success in a natural population of the territorial ornate dragon lizard, Ctenophorus ornatus. Molecular Ecology 10, 193–203.
| Microsatellite determination of male reproductive success in a natural population of the territorial ornate dragon lizard, Ctenophorus ornatus.Crossref | GoogleScholarGoogle Scholar | 11251798PubMed |
Marler, CA, Walsberg, G, White, ML, Moore, M, and Marler, CA (1995). Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behavioral Ecology and Sociobiology 37, 225–231.
| Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation.Crossref | GoogleScholarGoogle Scholar |
Maute, K, Hose, GC, Story, P, Bull, CM, and French, K (2019). Surviving drought: a framework for understanding animal responses to small rain events in the arid zone. Ecology 100, 1–9.
| Surviving drought: a framework for understanding animal responses to small rain events in the arid zone.Crossref | GoogleScholarGoogle Scholar |
Morton, SR, Stafford Smith, DM, Dickman, CR, Dunkerley, DL, Friedel, MH, McAllister, RRJ, Reid, JRW, Roshier, DA, Smith, MA, Walsh, FJ, Wardle, GM, Watson, IW, and Westoby, M (2011). A fresh framework for the ecology of arid Australia. Journal of Arid Environments 75, 313–329.
| A fresh framework for the ecology of arid Australia.Crossref | GoogleScholarGoogle Scholar |
Nagy, KA, Girard, IA, and Brown, TK (1999). Energetics of free-ranging mammals, reptiles, and birds. Annual Review of Nutrition 19, 247–277.
| Energetics of free-ranging mammals, reptiles, and birds.Crossref | GoogleScholarGoogle Scholar | 10448524PubMed |
Nathan, R, Getz, WM, Revilla, E, Holyoak, M, Kadmon, R, Saltz, D, and Smouse, PE (2008). A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences 105, 19052–19059.
| A movement ecology paradigm for unifying organismal movement research.Crossref | GoogleScholarGoogle Scholar |
Olsson, M (1995). Territoriality in Lake Eyre dragons Ctenophorus maculosus: are males ‘superterritorial’? Ethology 101, 222–227.
| Territoriality in Lake Eyre dragons Ctenophorus maculosus: are males ‘superterritorial’?Crossref | GoogleScholarGoogle Scholar |
Olsson, M, Healey, M, Wapstra, E, Schwartz, T, Lebas, N, and Uller, T (2007). Mating system variation and morph fluctuations in a polymorphic lizard. Molecular Ecology 16, 5307–5315.
| Mating system variation and morph fluctuations in a polymorphic lizard.Crossref | GoogleScholarGoogle Scholar | 18092994PubMed |
Osterwalder, K, Klingenböck, A, and Shine, R (2004). Field studies on a social lizard: home range and social organization in an Australian skink, Egernia major. Austral Ecology 29, 241–249.
| Field studies on a social lizard: home range and social organization in an Australian skink, Egernia major.Crossref | GoogleScholarGoogle Scholar |
Perry, G, and Garland, TJ (2002). Lizard home ranges revisited: effects of sex, body size, diet, habitat, and phylogeny. Ecology 83, 1870–1885.
Price-Rees, SJ, Brown, GP, and Shine, R (2013). Spatial ecology of bluetongue lizards (Tiliqua spp.) in the australian wet–dry tropics. Austral Ecology 38, 493–503.
| Spatial ecology of bluetongue lizards (Tiliqua spp.) in the australian wet–dry tropics.Crossref | GoogleScholarGoogle Scholar |
Price-Rees, SJ, Brown, GP, and Shine, R (2014). Activity patterns and movements of free-ranging bluetongue lizards (Tiliqua scincoides intermedia and Tiliqua multifasciata) in the Australian wet–dry tropics. Journal of Herpetology 48, 298–305.
| Activity patterns and movements of free-ranging bluetongue lizards (Tiliqua scincoides intermedia and Tiliqua multifasciata) in the Australian wet–dry tropics.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2020) R: A language and environment for statistical computing. (R Foundation for Statistical Computing) Available at https://www.r‐project.org/
Recio, MR, Mathieu, R, Denys, P, Sirguey, P, and Seddon, PJ (2011). Lightweight GPS-tags, one giant leap for wildlife tracking? an assessment approach. PLoS One 6, .
| Lightweight GPS-tags, one giant leap for wildlife tracking? an assessment approach.Crossref | GoogleScholarGoogle Scholar | 22163286PubMed |
Rose, B (1982). Lizard home ranges: methodology and functions. Journal of Herpetology 16, 253–269.
Row, JR, and Blouin-Demers, G (2006). Kernels are not accurate estimators of home-range size for herpetofauna. Copeia 2006, 797–802.
| Kernels are not accurate estimators of home-range size for herpetofauna.Crossref | GoogleScholarGoogle Scholar |
Salvador, A, Díaz, JA, Veiga, JP, Bloor, P, and Brown, RP (2008). Correlates of reproductive success in male lizards of the alpine species Iberolacerta cyreni. Behavioral Ecology 19, 169–176.
| Correlates of reproductive success in male lizards of the alpine species Iberolacerta cyreni.Crossref | GoogleScholarGoogle Scholar |
Schwarzkopf, L, and Shine, R (1992). Costs of reproduction in lizards: escape tactics and susceptibility to predation. Behavioral Ecology and Sociobiology 31, 17–25.
| Costs of reproduction in lizards: escape tactics and susceptibility to predation.Crossref | GoogleScholarGoogle Scholar |
Sinervo, B, and Lively, CM (1996). The rock–paper–scissors game and the evolution of alternative male strategies. Nature 380, 240–243.
| The rock–paper–scissors game and the evolution of alternative male strategies.Crossref | GoogleScholarGoogle Scholar |
Smith, JG, and Griffiths, AD (2009). Determinants of home range and activity in two semi-aquatic lizards. Journal of Zoology 279, 349–357.
| Determinants of home range and activity in two semi-aquatic lizards.Crossref | GoogleScholarGoogle Scholar |
Smith, KR, Cadena, V, Endler, JA, Kearney, MR, Porter, WP, and Stuart-Fox, D (2016a). Color change for thermoregulation versus camouflage in free-ranging lizards. The American Naturalist 188, 668–678.
| Color change for thermoregulation versus camouflage in free-ranging lizards.Crossref | GoogleScholarGoogle Scholar | 27860512PubMed |
Smith, KR, Cadena, V, Endler, JA, Porter, WP, Kearney, MR, and Stuart-Fox, D (2016b). Colour change on different body regions provides thermal and signalling advantages in bearded dragon lizards. Proceedings of the Royal Society B: Biological Sciences 283, 20160626.
| Colour change on different body regions provides thermal and signalling advantages in bearded dragon lizards.Crossref | GoogleScholarGoogle Scholar |
Steinberg, DS, Losos, JB, Schoener, TW, Spiller, DA, Kolbe, JJ, and Leal, M (2014). Predation-associated modulation of movement-based signals by a Bahamian lizard. Proceedings of the National Academy of Sciences of the United States of America 111, 9187–9192.
| Predation-associated modulation of movement-based signals by a Bahamian lizard.Crossref | GoogleScholarGoogle Scholar | 24843163PubMed |
Stevens, TA, Evans, MC, Osborne, WS, and Sarre, SD (2010). Home ranges of, and habitat use by, the grassland earless dragon (Tympanocryptis pinguicolla) in remnant native grasslands near Canberra. Australian Journal of Zoology 58, 76–84.
| Home ranges of, and habitat use by, the grassland earless dragon (Tympanocryptis pinguicolla) in remnant native grasslands near Canberra.Crossref | GoogleScholarGoogle Scholar |
Stone, PA, and Baird, TA (2002). Estimating lizard home range: the Rose model revisited. Journal of Herpetology 36, 427–436.
| Estimating lizard home range: the Rose model revisited.Crossref | GoogleScholarGoogle Scholar |
Strickland, K, and Frère, CH (2018). Predictable males and unpredictable females: repeatability of sociability in eastern water dragons. Behavioral Ecology 29, 236–243.
| Predictable males and unpredictable females: repeatability of sociability in eastern water dragons.Crossref | GoogleScholarGoogle Scholar |
Thompson, GG, Boer, Mde, and Pianka, ER (1999). Activity areas and daily movements of an arboreal monitor lizard, Varanus tristis (Squamata: Varanidae) during the breeding season. Australian Journal of Ecology 24, 117–122.
| Activity areas and daily movements of an arboreal monitor lizard, Varanus tristis (Squamata: Varanidae) during the breeding season.Crossref | GoogleScholarGoogle Scholar |
Ujvari, B, Fisher, P, Rydell, J, Wahlgren, R, Wright, B, and Madsen, T (2015). Population demography of frillneck lizards (Chlamydosaurus kingii, Gray 1825) in the wet–dry tropics of Australia. Austral Ecology 40, 60–66.
UNSW (2018) WRL: Datawarehouse. Available at http://datawarehouse.wrl.unsw.edu.au/ [Accessed 13 March 2018]
Vander Wal, E, and Rodgers, AR (2012). An individual-based quantitative approach for delineating core areas of animal space use. Ecological Modelling 224, 48–53.
| An individual-based quantitative approach for delineating core areas of animal space use.Crossref | GoogleScholarGoogle Scholar |
Watt, MJ, Forster, GL, and Joss, JMP (2003). Steroid correlates of territorial behavior in male jacky dragons, Amphibolurus muricatus. Brain, Behavior and Evolution 61, 184–194.
| 12784056PubMed |
Wood SN (2017) ‘Generalized additive models: an introduction with R’. 2nd edn. (Chapman and Hall/CRC: London, UK)
Worton, BJ (1989). Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168.
| Kernel methods for estimating the utilization distribution in home-range studies.Crossref | GoogleScholarGoogle Scholar |
Wotherspoon AD (2007) Ecology and management of eastern bearded dragon: Pogona barbata. PhD Thesis, University of Western Sydney, Sydney, NSW, Australia.
Zuur, AF, Ieno, EN, and Elphick, CS (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14.
| A protocol for data exploration to avoid common statistical problems.Crossref | GoogleScholarGoogle Scholar |
† A pre-print of this article is available on research square: https://www.researchsquare.com/article/rs-9293/v1.



