Application of tri-axial accelerometer data to the interpretation of movement and behaviour of threatened black cockatoos
Lian Yeap A D , Kristin S. Warren A , Willem Bouten C , Rebecca Vaughan-Higgins A , Bethany Jackson A , Karen Riley B , Sam Rycken
A D , Kristin S. Warren A , Willem Bouten C , Rebecca Vaughan-Higgins A , Bethany Jackson A , Karen Riley B , Sam Rycken  B and Jill M. Shephard
B and Jill M. Shephard  A
A
A Harry Butler Institute, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
B Conservation Medicine Program, School of Veterinary Medicine, College of Science, Health, Engineering and Education, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
C Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Sciencepark 904, 1098 XH, Amsterdam, The Netherlands.
D Corresponding author. Email: L.Yeap@murdoch.edu.au
Wildlife Research 49(2) 100-110 https://doi.org/10.1071/WR20073
Submitted: 1 May 2020 Accepted: 8 June 2021 Published: 20 August 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC
Abstract
Context: Carnaby’s (Calyptorhychus latirostris), Baudin’s (Calyptorhynchus baudinii) and forest red-tailed black cockatoos (Calyptorhynchus banksii naso) are threatened parrot species endemic to south-western Australia. Behavioural monitoring has previously involved direct observation, which has proven challenging because of their cryptic nature, the type of habitat they move through and their speed of movement. The development of a model to accurately classify behaviour from tri-axial accelerometer data will provide greater insight into black cockatoo behaviour and ecology.
Aims: To develop an automated classifier model to classify accelerometer data from released black cockatoos to determine behaviour and activity budgets for three species of black cockatoo.
Methods: In the present study, we attached tri-axial accelerometers, housed in GPS tags, to four Carnaby’s cockatoos, three forest red-tailed black cockatoos and two Baudin’s cockatoos in captive care, undergoing rehabilitation for release back to the wild. Accelerometer data from these birds was coupled with 19 video files of the birds’ behaviour when flying, feeding and resting, to develop an automated behaviour classifier. The classifier was then used to annotate accelerometer data from 15 birds released after successful rehabilitation and to calculate activity budgets for these birds post-release.
Key results: We developed a classifier able to identify resting, flying and foraging behaviours from accelerometer data with 86% accuracy, as determined by the percentage of observed behaviours correctly identified by the classifier. The application of the classifier to accelerometer data from 15 released cockatoos enabled us to determine behaviours and activity budgets for all three species of black cockatoo. Black cockatoos spent most of their time at rest, followed by foraging with a short period of time flying.
Conclusions: Application of the classifier to data from released birds gives researchers the ability to remotely identify patterns of behaviour and calculate activity budgets.
Implications: Combining behaviour and activity budgets with location data provides useful insight into cockatoo movement, distribution, and habitat use. Such information is important for informing conservation efforts and addressing outstanding research objectives. Further studies including larger sample sizes of Baudin’s and forest red-tailed black cockatoos and comparing behaviour and activity between birds in breeding and non-breeding areas are warranted.
Keywords: black cockatoo, Calyptorhynchus spp., machine learning, behaviour, accelerometer, GPS.
Introduction
Accelerometers have been used in animal behavioural studies since the late 1990s (Brown et al. 2013). Accelerometers measure acceleration in three planes, namely dorso-ventral (up-down, z-axis), anterior-posterior (forward-back, x-axis) and lateral (side-side, y-axis), typically referred to as heave, surge and sway respectively (Shepard et al. 2008). When analysing behaviour, the accelerometer data measured must be translated into specific behaviours (Shamoun-Baranes et al. 2012). This is achieved through expert interpretation of sensor and video behavioural data collected in the field, automated clustering of sensor data without field observations, or automatic classification of sensor data in conjunction with behavioural observation (Shamoun-Baranes et al. 2012). Whereas the first two methods rely on inferences of animal behaviour, the third involves careful observation of animal behaviours, which are then matched to distinctive accelerometer signatures. This validated data can then be used to train machine-learning algorithms to recognise specific accelerometer signatures. These algorithms then automatically classify new accelerometer data into specific behaviour categories (Brown et al. 2013; Tatler et al. 2018).
The attachment of an accelerometer directly to an animal allows measurement of the body’s acceleration or change in velocity, fine movements and posture. Specific behaviours, and associated movements and postures, show distinct accelerometer signatures (Brown et al. 2013). Advances in technology, such as miniaturisation of components and solar recharging of batteries (Bouten et al. 2013), have resulted in a significant reduction in the size of tracking devices in recent years, enabling their use to study the movement and behaviours of a wider range of avian species, including small birds. Tracking devices can affect bird behaviour, such as flying ability, foraging and energy expenditure, thus researchers try to adhere to the ‘rule of thumb’ that the weight of a device should not exceed 3–5% of the animal’s bodyweight (Cochran 1980; Kenward 2001). This is easier to achieve with the smaller devices available today. For example, Bäckman (2017) used accelerometer data from a 1.2 g device to create annual actograms for the red-backed shrike (Lanius collurio), a small (25–35 g) migratory songbird. The study provided new insights into migratory behaviour and flight patterns. Arai et al. (2000), in a study of foraging behaviour of free-ranging Adélie penguins (Pygoscelis adeliae), attached two-direction accelerometers to determine swimming and diving activity. Shamoun-Baranes et al. (2016) described the use of tri-axial accelerometer data to determine body movement of lesser black-backed gulls (Larus fuscus), providing useful information on flying behaviour in different conditions and habitats, as well as the impact on foraging patterns and associated energy budgets. Accelerometers have even been placed into artificial eggs to study the egg turning behaviour, incubation temperature and the impact on hatching success of nesting Cassin’s auklets (Ptychoramphus aleuticus), western gulls (Larus occidentalis), and Laysan albatrosses (Phoebastria immutabilis) in California (Shaffer 2014).
Whereas animal movement can be determined by telemetry trackers, direct visual observation is often used to study activity and behaviour. However, direct observation is not always possible, owing to difficulties posed by the terrain (e.g. dense forest), lack of access (e.g. private land), or the distance to, and time taken to locate, animals. Furthermore, although direct observation has its advantages, an observer cannot easily watch a target animal continuously from morning to night and behaviours may be missed while the observer is not present (Altmann and Altmann 2003). The presence of an observer may also affect the animal’s behaviour (Lendvai et al. 2015). The amount of data that biologging devices, such as accelerometers, could provide, may offer useful insights into habitat-use and movement patterns that are otherwise difficult to acquire (Tatler et al. 2018).
Carnaby’s cockatoo (Calyptorhychus latirostris), Baudin’s cockatoo (Calyptorhynchus baudinii) and the forest red-tailed black cockatoo (Calyptorhynchus banksii naso) are iconic parrot species endemic to south-western Western Australia. All three species of black cockatoo are increasingly threatened by urban, agricultural and industrial development, habitat loss and fragmentation, competition with other bird species and feral bees for nest hollows, poaching, disease, and anthropogenic factors such as vehicle strike and illegal shooting (Saunders et al. 2011). Significant declines in black cockatoo numbers have been observed since the 1980s (Saunders and Ingram 1998; Williams et al. 2017) and all three species are classified as threatened under state and federal legislation and according to IUCN criteria (Department of Environment and Conservation 2008, 2012; IUCN 2020). Although there has been previous research on the ecology and breeding biology of black cockatoos, particularly of Carnaby’s cockatoos, these studies have been restricted to a small number of known breeding, foraging and roosting sites (Johnstone and Kirkby 2008; Johnstone et al. 2011; Johnstone and Kirkby 2017; Saunders and Dawson 2018). Little is known about flock behaviour and movement patterns more broadly across these species’ distribution ranges (Johnstone and Kirkby 2008; Saunders and Dawson 2018).
Animal tags containing tri-axial accelerometers can facilitate the collection of a large amount of behavioural data (Cooke et al. 2004). These data can be used to determine physiological measurements such as time–energy budgets, as well as an assessment of biotic and abiotic factors affecting on activity budgets. For example, Carnaby’s cockatoos tend to migrate to feed in the higher-rainfall coastal areas within their range in the non-breeding season (January–July), as food is more abundant (Department of Environment and Conservation 2012; Saunders and Dawson 2018; Stock et al. 2013). Accelerometer data can be used to quantify the extent to which energy budgets differ in different landscapes, enabling researchers to address ecological questions such as ‘how do activity demands differ for birds in urban environments (such as the Swan Coastal Plain) compared with those in the Wheatbelt or southern forest regions’, and ‘are the activity budgets similar across the three black cockatoo species, given the variation in preferred habitats’? This information can be used to guide conservation management decisions, such as protection of foraging, roosting and breeding habitat from development, determine the potential impact of proposed developments and identify suitable conservation offsets for developed land (Department of Environment and Conservation 2012).
Behavioural monitoring has previously been achieved through direct observation, which has proven difficult because of the cryptic nature of black cockatoos, the type of habitat that they move through and the speed with which they move. In the present study, we collected accelerometer data from wild black cockatoos undergoing rehabilitation for release back to the wild. These data were coupled with video footage of the birds’ behaviour in a flight aviary, before release. The aim of this project was to develop an automated classification model able to identify resting, flying and foraging behaviour from accelerometer data. This model was applied to data from released black cockatoos, allowing us to calculate activity budgets for three black cockatoo species, providing insight into their post-release behaviour and activity budgets.
Materials and methods
This project was completed under Murdoch University Animal Ethics Permit RW2576/13 and Department of Parks and Wildlife (Western Australia) Regulation 17 Licence To Take Fauna For Scientific Purposes SF010393 (Number 167484).
The study was conducted in two stages. Stage 1 included aviary-based filming of bird behaviour to train an automated classifier, and Stage 2 included application of the classifier to data retrieved from accelerometer-tagged birds in wild flocks.
Stage 1
Tag attachment
Nine black cockatoos, including four Carnaby’s cockatoos, three forest red-tailed black cockatoos and two Baudin’s cockatoos, were used to develop the classifier in this study. These were all injured wild birds that had been treated at Perth Zoo and undergone rehabilitation. The birds had no discernible differences in flight or activity from wild birds and were assessed as ready for release back into the wild by the Western Australian Department of Biodiversity, Conservation and Attractions. All birds were housed in a pre-release flight aviary (6 m × 64 m) to undergo flight conditioning at Kaarakin Black Cockatoo Conservation Centre (https://www.blackcockatoorecovery.com).
As the birds were destined for release, they had two tags attached. A Telonics TAV 2617 Platform Terminal Transmitter (PTT) ARGOS satellite tag was attached to the ventral base of the central tail feathers and a 7.5 g solar University of Amsterdam Bird Tracking System (UvA-BiTS) GPS tag incorporating a 20Hz tri-axial accelerometer was attached to the feathers between the wings, on the upper back as described in Yeap et al. (2017). Tags were attached under isoflurane anaesthesia to minimise stress.
To affix back-mounted accelerometer tags, we first fixed a flexible plastic backing plate (~1 mm thick) to the feathers by using adhesive cloth tape (Bear Black Gaffer Tape, Saint Gobain Abrasives Pty Ltd, Thomastown, Victoria, Australia; Yeap et al. 2017). Two strips of tape were used, one at the top of the back-plate and one at the bottom. The tape made several turns over the feathers and base-plate, so that tape adhered to tape. Three to four feathers were used to secure the back-plate. If possible, two separate rows of feathers were used to improve stability of the plate. The back-plate mirrored the shape of the tag and was a dark colour to reduce visual cues to the bird and conspecifics to reduce the likelihood of removal. Attachment holes were made to match the positioning of the eyelets on the tag to facilitate attachment of the tag to it. The tag was centred over the bird’s spine, 10 mm below the shoulders and 20 mm above the pelvis. The tag was then glued (Selleys Ultra Repair Glue; Selleys, Padstow, New South Wales, Australia) and tied through the tag eyelets to the back-plate with braided nylon fishing-line (Fireline®, Berkley®, Spirit Lake, Iowa, USA). Any feathers on the bird’s neck that might obscure the solar panel were trimmed. This attachment method allowed the tag to be shed with the feathers, or to be removed easily by the bird.
The satellite tags were attached to the ventral aspect of the base of the two central tail feathers, following the protocols of Le Souef et al. (2013) and Groom et al. (2014). The tag was secured using black braided fishing line (Fireline®, Berkley®, Spirit Lake, Iowa, USA) threaded through mounting holes along the sides of the tag and around the shaft of each central tail feather, tied with a surgeon’s knot. The tag antenna was laid along the shaft of one of the central tail feathers and secured with braid ties ~30 mm apart. A flexible, rapid setting glue (Selleys Ultra Repair Glue; Selleys, Padstow, New South Wales, Australia) was applied to each knot for additional security. The combined weight of the tags was less than 5% of the bird’s body mass, and met ethical requirements (Cochran 1980; Kenward 2001). Birds were under anaesthesia for an average of 35 min. Once recovered from anaesthesia, birds were returned to the flight aviary.
Satellite PTT tags provide an ARGOS satellite location (Argos CLS System 2018) to within 250–500 m accuracy. These locations were used to locate the study bird in the field during Stage 2 of the project, so as to facilitate accelerometer and GPS data downloads to a mobile base station.
Collection of accelerometer data and behavioural observations
For Carnaby’s cockatoos and forest red-tailed black cockatoos, each bird was banded with an Australian Bird and Bat Banding Scheme (ABBBS) metal numbered leg band on the right leg, just above the intertarsal joint. Two coloured metal leg bands were placed on the left leg, each colour combination corresponding to a specific bird. Placement was reversed for Baudin’s cockatoos, with the ABBBS band being placed on the left leg and colour bands on the right, to allow rapid differentiation from Carnaby’s cockatoo. The leg bands allowed identification of the bird from a distance in the field with binoculars or telephoto lens, or if recaptured. A spot of low-toxic (10-Free™ https://www.kesterblack.com/blog/edu/whats-the-deal-with-non-toxic-nail-polish) nail polish (Kester Black, Melbourne, Vic., Australia) was applied to the upper beak to allow quick identification of the study bird in the aviary because birds were housed in groups among non-study birds to facilitate normal activity.
Kaarakin staff observed that the black cockatoos in the pre-release flight aviary were most active in the morning, between 0900 hours and 1000 hours (Western Standard Time), after the aviary had been serviced and fresh food, water and native browse were supplied. Feeding, flying and resting behaviour were frequently observed; hence, filming was scheduled during this period.
The tags were programmed to collect 200 accelerometer samples every 2 s for 30 min. High frequency was required to capture fine-scale movement, but it depleted the tag battery. The program was limited to 30 min to ensure that the tag had sufficient power to function on release of the bird the following day. On the day following tag attachment, the study bird was observed in the pre-release flight aviary and its behaviour was filmed with a Sony HDR-PJ30VE Handycam (Sony Corporation, Tokyo, Japan) video camera. Filming commenced at the start of the accelerometer tag program and continued for 30 min, corresponding to the tag program. Footage was filmed in 720p high-definition resolution at 50 frames per second. The start time of filming was noted by recording UTC (Coordinated Universal Time) from an internet time server on a mobile phone. This allowed the synchronisation of accelerometer data and video footage during the annotation phase. Once saved, video footage was converted to AVI format for use with the annotation software, using Any Video Converter software (Anvsoft Inc., Shenzhen, Guangdong, China; www.anvsoft.com). Each study bird was filmed once.
At the completion of filming, the GPS and accelerometer data were remotely downloaded to the base-station netbook computer. Data were then transferred for processing to the UvA-BiTS e-infrastructure platform and permanently stored in a PostgreSQL spatial database and accessed through the Virtual Laboratory portal www.UvABiTS.nl/virtual-lab (Bouten et al. 2013). Within the Virtual Laboratory, the data from individual tags were selected (filtered by the tag identification number, date and time of filming) and downloaded for use within the annotation software.
Annotation of data and video footage
To annotate the accelerometer data, eight activities or behaviours of interest were determined and used to create a classification scheme (Table 1). The behaviours selected were resting, walking, feeding, drinking, flying – take-off, flying – flapping, landing and other behaviours (preening or aggressive interactions) that did not fit into those categories. Some behaviours could not be classified with sufficient reliability and were grouped within the classification of foraging. Foraging included walking, climbing, feeding behaviour and other unspecified behaviours such as preening or aggressive interactions, on the ground or on a perch. From viewing the video footage and personal observation of the cockatoos in the aviary and in the wild, walking behaviour mainly occurred while birds were eating or going to and from food sources; thus, grouping walking and feeding in the foraging category was considered appropriate. Similarly, aggressive interactions and preening behaviour often involved walking and feeding; hence, they were also grouped into the forage category. Flying included take-off, landing and flapping flying, so these behaviours were all grouped in the same class. There was insufficient video footage to reliably classify drinking, so it was excluded from the behaviour scheme. This resulted in three behaviour classes, namely, resting, foraging and flying, that were used for the final classification.

|
As outlined by de Bakker (2011), a software tool developed in the Matlab software package, version 2013a (The Mathworks Inc., Natick, Massachusetts, USA) provides a visual interface to view the accelerometer data, synchronised with associated video footage (hereafter this will be referred to as the Annotation Tool) as shown in Fig. 1. Access to this tool was provided by UvA-BiTS. The relevant movie file (.avi), corresponding accelerometer data file (.csv) and behavioural scheme were opened in the Annotation Tool and the timestamp at the start of the video was synchronised to the accelerometer data to facilitate annotation. The annotated data were saved as a.mat file for use in developing the classifier.

|
Additional accelerometer data from released birds, without accompanying video footage, were analysed and annotated on the basis of knowledge of the distinct accelerometer signatures generated by each behaviour (Meijer et al. 2016; Shamoun-Baranes et al. 2016). Examples of accelerometer signatures are included in supplementary material (Figs S1–S4, available as Supplementary material to this paper). As there was significantly less video footage of flying, this behaviour was targeted to increase the amount of flying accelerometer data used in the classifier to improve accuracy. Twelve data files consisting of 750 s of additional flying data were used. The addition of more flying data beyond this did not improve the classifier accuracy.
Classifier development
The classifier tool uses a decision tree machine-learning algorithm. There are 37 features or predictor variables (Shamoun-Baranes et al. 2012) that can be calculated for each 1-s segment (x-surge, y-sway, z-heave) to classify behaviours. We selected one feature, and then added additional features in a forward-stepwise approach to differentiate behaviour.
The dataset of 1881 data points was randomly split, with 40% used to train, 30% used to test and the remaining 30% used to validate the classification model (Vansteelant et al. 2016). Behaviours were visualised using scatterplots based on the features used in the classification software. Features were useful if behaviours were well differentiated (datapoints scattered), and not useful if behaviours were poorly differentiated (data points tightly clumped).
The features ‘mean pitch’, which measures forward–backward movement over all accelerometer points in the segment, and ‘mean of the absolute value of the derivative of z’, which measures up–down movement in the z-axis, produced the most accurate and consistent model for differentiating and classifying behaviours. Using more features did not improve accuracy of classification, so a simple model with two features was chosen. The model development process used was similar to that described by Camphuysen et al. (2012) (Shamoun-Baranes et al. 2012).
The modelling process resulted in a random forest classifier with 500 decision trees and two features. A confusion matrix was calculated to determine the number of correctly and incorrectly classified data points (Table 2). Accuracy was determined by the percentage of observed behaviours correctly identified by the classifier (Ladds et al. 2017).

|
Stage 2
Application to tracked birds in the wild
The nine birds that were used to develop the classifier in the aviary (Stage 1) were subsequently released to the wild with an additional nine Carnaby’s, two Baudin’s and two forest red-tailed black cockatoos (n = 22) as part of a large and ongoing movement-ecology study (Black Cockatoo Ecology Project, Murdoch University). The nine birds also had the same two tags attached as the birds in Stage 1. Tag attachment procedures were as described above; however, the GPS and accelerometer release program were adjusted to maximise battery life. Birds were released during non-breeding periods (Saunders 1980; Johnstone et al. 2011, 2013) into areas where wild flocks were known to be active or roosting. Carnaby’s cockatoos were released in May and June 2016 and March 2018; forest-red tailed black cockatoos in July 2016 and February 2017 and Baudin’s cockatoos in August 2015 and May 2017. During daylight hours (from 0530 hours), tags were scheduled to collect accelerometer readings and GPS location every 2.5 min when battery voltage was high, or every 15 min otherwise. During the night (from 1830 hours), GPS locations were collected at 30-min intervals. For both day and night programs, accelerometer readings and GPS locations were measured at a frequency of 20 Hz for 1 s.
Accelerometer data were successfully downloaded from 15 birds to the mobile base station (laptop and base antenna plus relay antenna) at night roosts and uploaded to the UvA-BiTS Virtual Laboratory for processing (classification) and activity budgets were determined.
Each bird was assigned an identifier on the basis of species, being BC for Baudin’s cockatoo, CC for Carnaby’s cockatoo, FRTBC for forest red-tailed black cockatoo, and UvA-BiTs four-digit tag number.
Results
Stage 1
Video footage and corresponding accelerometer data were successfully retrieved from four Carnaby’s cockatoos, three forest red-tailed black cockatoos and two Baudin’s cockatoos in the flight aviary. In total, 19 annotated data files were created from the video and accelerometer data.
The resulting classifier was able to distinguish behaviours that were grouped as flying, foraging, or resting with 86% accuracy. Misclassifications occurred most frequently with resting behaviour, as 195 of 1367 (14%) resting behaviours were incorrectly classified as foraging. Similarly, in regard to foraging behaviours, 57 of 460 (12%) were incorrectly classified as resting, 9 of 460 (2%) were incorrectly classified as flying, and 2 of 45 (4%) flying behaviours were incorrectly classified as foraging.
Stage 2
Activity budgets by species
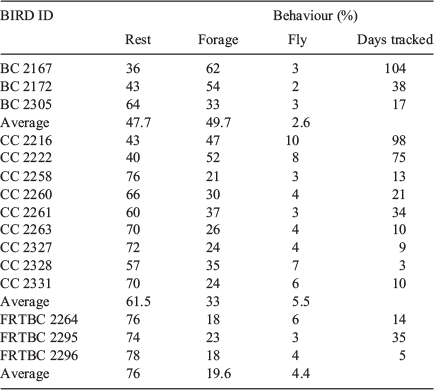
Despite the limited number of misclassifications that occurred, the classifier’s accuracy of 86% ensured that the activity budgets generated were a good indication of true activity. The activity budget for each of the released birds is shown in Table 3. CC 2330 retained the accelerometer tag only for 1 day and little data were retrieved. As such, results from this bird were excluded. During the tracking period, Baudin’s cockatoo behaviour was classified as 50% foraging, 3% flying, and the remainder as at rest. Carnaby’s cockatoo behaviour was 60% resting, 33% foraging and ~6% flying. Forest red-tailed black cockatoo behaviour was 75% resting, 20% foraging, and the remaining 4% flying.
|
Discussion
Understanding the movement of species through the landscape is a fundamental aspect of conservation management. South-west Western Australia is a biodiversity hotspot (Mittermeier et al. 2004), but is subject to widespread habitat modification through urban, industrial and agricultural development, which has resulted in a complex and continually evolving habitat matrix. Habitat modification, loss of transit corridors and anthropogenic threats, including vehicle strike, increase energy demands, highlighting the importance of understanding activity budgets relative to the activity cost of individual habitat types on black cockatoo species. As with other species where visual observation was both difficult or inaccurate (e.g. lesser black backed gulls (Larus fuscus; Shamoun-Baranes et al. 2016), griffon vultures (Gyps fulvus; Nathan et al. 2012), and, red-backed shrike (Lanius collurio; Bäckman 2017)), our indirect behaviour classification model was successful in capturing key rest, forage and flight behaviour classes that can be used to directly quantify the impact and activity cost of habitat type and structure on black cockatoo species. Our classification was 86% accurate for the three behavioural classes analysed and is comparable to the accuracy reported in Shamoun-Baranes et al. (2012), Sur et al. (2017) and Tatler et al. (2018).
Classifier development and performance
The original classification scheme had eight distinct behaviours we were interested in detecting in the wild-release birds; however, the classifier was unable to reliably distinguish some behaviours with the amount of data and video footage available. Although all types of flying behaviour were observed and filmed, there were both insufficient accelerometer data and video footage of take-off and landing behaviour to accurately train the classifier to distinguish between them and flapping flying. This was primarily due to the infrequent occurrence and very short duration (0.5–2 s) of each behaviour. Because we were interested in overall movement associated with flying behaviour, not including take-off and landing flight in the classifier was not a major concern. We will able to apply the model to quantify and compare the proportion of flight time between habitats that may be a result of flushing rates or other disturbance pressures that exert an energetic cost.
Activity budgets and behaviour
The three species of black cockatoo had quite similar activity budgets (Table 3), with birds spending most of their time at rest, interspersed with foraging activity throughout the day and some movement between roost sites or feeding habitat. Flying and foraging occurred most frequently around sunrise and sunset.
The Carnaby’s cockatoos in the present study were released on the Swan Coastal Plain, which is recognised as an important feeding ground for Carnaby’s cockatoos in the non-breeding season of February to June (Johnstone et al. 2011); thus, a high level of foraging was not unexpected. Adults and young birds feed in Banksia spp. woodlands (Saunders 1980, 1990), as well as pine plantations (Pinus spp.), which is recognised as an important food source (Stock et al. 2013).
The Baudin’s cockatoos in the present study appeared to spend almost equal amounts of time resting and foraging, with only a small amount of time spent flying. These birds were released in the Perth hills area in August, the start of the breeding season. Flocks have been observed gathering in traditional roosting areas on the Darling Scarp, –32°S, 115°E (Johnstone and Kirkby 2017) and southern Swan Coastal Plain, before flying south to their breeding grounds (Johnstone and Kirkby 2008). The limited flying activity captured at this time may be related to reduced activity in these pre-migration flocks. Tracking more Baudin’s cockatoos would undoubtedly show more detail about their activity and movement. Since this project began in 2015, only 16 Baudin’s cockatoos have been available for release post-rehabilitation. Given that they primarily occupy the southern forest, fewer injured Baudin’s cockatoos are presented for treatment and subsequent rehabilitation (Black Cockatoo Conservation Management Project, Murdoch University, unpubl. data; https://blackcockatooconservationwa.com).
Forest red-tailed black cockatoos spent more time resting than did Carnaby’s and Baudin’s cockatoos. Although one bird was released on the Swan Coastal Plain and two birds were released into a south-western forest area, all three birds exhibited similar behaviours during the period they were tracked. Forest red-tailed black cockatoos have a distinctive way of feeding. Birds have often been observed feeding in one part of a tree, eating all the nuts or fruit around them, and only moving to another part of the tree when all the food in their vicinity is exhausted. Birds will return to the same tree day after day until all the nuts or fruit are consumed (Johnstone and Kirkby 1999; Department of Environment and Conservation 2008; Johnstone et al. 2017).
Observations of black cockatoos foraging and feeding showed that they are often quite stationary, with only slight body movement (Black Cockatoo Conservation Management Project, Murdoch University, https://blackcockatooconservationwa.com, unpubl. data), such as, for example, lifting food items to the beak with a foot. In this position, there is little movement of the accelerometer tag in any axis. This may go some way to explaining the higher level of rest and lower levels of foraging in the black cockatoo activity budgets than with other black cockatoos of similar size.
Western Australian black cockatoos seem to spend less time foraging than do glossy black cockatoos (Calyptorhynchus lathami) found in eastern Australia. Pepper (1996) calculated activity budgets, showing that the birds spent almost 60% of their time foraging, 34% of their time at rest and less than 1% of their time flying. Feeding behaviour was similar to that of forest red-tailed black cockatoos, with birds tending to feed in one tree, eating everything within reach before moving to another feeding tree. Glossy black cockatoos feed predominantly on Allocasuarina seeds and have been observed to be highly selective when deciding where to feed. They tend to choose trees with abundant cones, then select cones of high seed weight (Cameron 2007). Studies by Cooper et al. (2003) suggested that marri seeds (Corymbia calophylla), which are the principle food source of forest red-tailed black cockatoos and Baudin’s cockatoos (Johnstone and Kirkby 2008), have a higher energy content than do Allocasuarina seeds; thus, birds feeding on marri would not need to forage for as long to meet their daily energy demands. Similarly, studies by Stock et al. (2013) showed many seeds eaten by Carnaby’s cockatoos were also much higher in energy. It is anticipated that planned removal of pine plantations on the Swan Coastal Plain will reduce food availability for Carnaby’s cockatoos (Williams et al. 2017). Pine seeds provide a readily available, high-energy food source in summer and autumn, when breeding birds are feeding fledgling chicks. Removal of pine plantations and loss of this food source may affect fledgling survival (Stock et al. 2013; Williams et al. 2017).
The daily pattern of activity of cockatoos is generally considered to be dictated by a bird’s energy requirements and the availability of food. If a bird cannot meet its daily energy requirements in one location, inevitably it will need to move to find another food source or risk starvation. Increased energy demands, such as during the breeding season, will also increase the length of foraging time required to meet those demands. The length of time a bird spends foraging is affected by its foraging strategy, supply of food and feeding technique. Time of year and weather will also have an impact on foraging ability. Cockatoos only forage in daylight hours; thus, time available for foraging is less in winter when daylength is reduced. Cockatoos need suitable foraging habitat and water close to (6–7 km) breeding and roosting sites (Saunders 1990; Groom 2015; Le Roux 2017). With land clearing and development resulting in habitat loss and fragmentation, loss of roosting trees and food resources, one would anticipate cockatoos will need to spend more time flying greater distances between diminishing resources. They will need to forage more to meet increasing energy demands. Numerous studies by Saunders (1982, 1990) and Saunders et al. (1985) have shown an association between longer foraging distances and poor chick health and reduced breeding success. In some locations, inadequate food resources have resulted in breeding areas being abandoned.
Although black cockatoos are capable of flying further afield to find food, water and roost sites, increased use of habitat in urbanised areas exposes them to numerous risks. Birds feeding and drinking on or by roadsides are at risk of being hit by motor vehicles. A total of 21.2% of Carnaby’s cockatoos that presented to Perth Zoo Veterinary department in 2017–2018 suffered from motor vehicle trauma (EPA 2019). Apples, pears and nut crops in south-western Western Australia have become a food source for Baudin’s cockatoos, particularly in years when marri (Corymbia calophylla) flowering is poor (Johnstone et al. 2011). Because of the damage caused to the crops, the birds are often considered a threat by growers and may be illegally shot and killed (Chapman 2007). Forest red-tailed black cockatoos are spending more time on the Swan Coastal Plain, utilising ornamental trees such as cape lilac (Melia azedarach) as a food source. Birds on the plain have also been observed foraging on the ground – this behaviour is uncommon in forest habitat (Johnstone et al. 2017). Movement into urban areas has also seen immature forest red-tailed black cockatoos being attacked and injured by Australian ravens (Corvus coronoides), a species that has become abundant in suburbia (Johnstone et al. 2017).
Although daily activity is primarily governed by individual energy requirements and food availability, environmental conditions also have a significant impact. Many cockatoo species have a recognised pattern of feeding in the early morning and late afternoon, with a rest period in the middle of the day to avoid higher temperatures (Cameron 2007; Shephard and Warren 2019). Recent studies by Shephard and Warren (2019) showed that black cockatoos rest, roost and forage in home-range areas that shift spatio-temporally in response to food availability and time of year.
Anthropogenic changes can have a significant impact on activity budgets. A recent study by Tucker et al. (2019) showed that birds living in homogenous environments, such as, for example, in crop lands and desert areas, can fly up to seven times further in search of resources (nesting sites, shelter, food) than do birds in heterogenous environments that provide a more diverse range of habitats and resources. Human-driven landscape disturbance and homogenisation result in resources being spread further apart; therefore, birds must fly further to access them and expend more energy doing so.
Cockatoos tend to forage in flocks, rather than individually, because it reduces the risk of predation (Westcott and Cockburn 1988); the collective knowledge of the flock is used to locate food sources and more than one bird can exploit a food source when in abundance (Cameron 2007). Carnaby’s cockatoos are recognised as a social species that forage in flocks by day and gather in groups to roost communally at night (Saunders 1977, 1980; Groom et al. 2017). Baudin’s cockatoos behave in a manner similar to Carnaby’s cockatoos, usually moving in family groups or small foraging flocks, which then form larger roosting flocks in the evening (Cameron 2007; Johnstone and Kirkby 2008). Studies by Johnstone and Kirkby (2017) indicated that forest red-tailed black cockatoos moved in smaller areas, tending to stay close to their home-range territory to feed and breed.
Knowledge of black cockatoo behaviour, such as daily routines and seasonal movements, provide insight into overall flock behaviour during specific time periods. Although not all birds will be engaged in the activity during a short period of data capture, it is likely that all birds will be engaged in the primary behavioural activity over time while at that site. For example, if a bird is classified as foraging at a location, it is highly likely that other birds in the flock will also be foraging around that time, at that particular location. The study of Johnstone and Kirkby (2008) of Baudin’s cockatoos, and our direct observations of study birds post-release (Black Cockatoo Conservation Management Project, Murdoch University, unpubl. data; https://blackcockatooconservationwa.com), showed that during the day, whereas many birds in a flock were engaged in a specific activity such as foraging, others would be resting, cleaning, preening and socialising during the same observation period. However, the primary purpose of the birds at this site was foraging. Accordingly, there is strong support for the application of the model data from a tagged flocked bird to the activity of the flock.
The difficulties faced by researchers using traditional methods of observation in the field further highlight the advantages of remote tracking devices. These data can provide specific insight into activity and behaviour for the duration of the transmitter recording period. Across the complete tracking period, this is much longer than is possible through direct observations. Researchers involved in the present study occasionally had difficulty accurately identifying individual birds in large flocks of birds, particularly in flight. Birds were often partially obscured by foliage, or high up in trees and difficult to observe clearly. On some occasions, birds were not accessible to researchers on foot or in vehicles due to fences, locked gates and poor track conditions. The use of remote tracking devices allowed researchers to track/follow birds over much greater distances and habitats without the physical limitations often faced by direct observation and tracking.
Application of the classifier tool to management issues and future research
From a management perspective, knowledge regarding the manner and speed at which black cockatoos move within urban or forest landscapes is valuable in planning the size, species composition and spacing of habitat resources. This tool is also valuable in assessing suboptimal habitats to guide revegetation and restoration activities to support species retention. Specifically, given the species activity bias towards foraging and resting, it is important that appropriate roost and forage trees are considered in restoration design. As the tool concentrates on key activities, the methodology will likely have strong transferability to other species, particularly birds.
In our future research, we will combine our classifier tool with behavioural change point analysis (Rycken et al. 2019) and species distribution modelling, to model the impacts and energetic cost of different habitat types on black cockatoos on the Swan Coastal Plain and more broadly across the south-west. As different habitats will incur different costs, the application of the tool to newly acquired accelerometer and location data will increase our ability to guide current and future conservation action, environmental offset priorities, and restoration activities, as we determine which habitats or corridor configurations retain functional landscape connectivity for these species.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This project was funded by Newmont Boddington Gold, South32, The Department of Housing (Government of Western Australia) and the Department of Biodiversity, Conservation and Attractions.
Acknowledgements
We thank the staff and volunteers from Kaarakin Black Cockatoo Conservation Centre and veterinary staff from the Conservation Medicine Program at Murdoch University for assistance. We also thank Karen Smith (Regional Wildlife Officer Nature Protection Branch, Department of Biodiversity, Conservation and Attractions Western Australia), Rick Dawson (Regional Wildlife Officer – retired, Nature Protection Branch, DBCA WA) and Dr Peter Mawson (Scientific Program Leader, Perth Zoo, DBCA WA). UvA-BiTS studies are facilitated by infrastructures for e-Ecology, developed with support of The Netherlands eScience Centre (NLeSC) and LifeWatch and carried out on the Dutch national e-infrastructure with the support of SURF Cooperative.
References
Altmann, S. A., and Altmann, J. (2003). The transformation of behaviour field studies. Animal Behaviour 65, 413–423.| The transformation of behaviour field studies.Crossref | GoogleScholarGoogle Scholar |
Arai, N., Kuroki, M., Sakamoto, W., and Naito, Y. (2000). Analysis of diving behavior of Adélie penguins using acceleration data logger. Polar Bioscience 13, 95–100.
Argos CLS System (2018). Available at https://argos-system.clsamerica.com/argos-cwi2/login.html [verified 8 May 2018].
Bäckman, J. (2017). Actogram analysis of free-flying migratory birds: new perspectives based on acceleration logging. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 203, 543–564.
| Actogram analysis of free-flying migratory birds: new perspectives based on acceleration logging.Crossref | GoogleScholarGoogle Scholar | 28343237PubMed |
Bouten, W., Baaij, E., Shamoun-Baranes, J., and Camphuysen, K. J. (2013). A flexible GPS tracking system for studying bird behaviour at multiple scales. Journal of Ornithology 154, 571–580.
| A flexible GPS tracking system for studying bird behaviour at multiple scales.Crossref | GoogleScholarGoogle Scholar |
Brown, D. D., Kays, R., Wikelski, M., Wilson, R., and Klimley, A. P. (2013). Observing the unwatchable through acceleration logging of animal behavior. Animal Biotelemetry 1, 20.
| Observing the unwatchable through acceleration logging of animal behavior.Crossref | GoogleScholarGoogle Scholar |
Cameron, M. (2007). ‘Cockatoos.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Camphuysen, K., Shamoun-Baranes, J., Bouten, W., and Garthe, S. (2012). Identifying ecologically important marine areas for seabirds using behavioural information in combination with distribution patterns. Biological Conservation 156, 22–29.
| Identifying ecologically important marine areas for seabirds using behavioural information in combination with distribution patterns.Crossref | GoogleScholarGoogle Scholar |
Chapman, T. F. (2007). An endangered species that is also a pest: a case study of Baudin’s Cockatoo Calyptorhynchus baudinii and the pome fruit industry in south-west Western Australia. Journal of the Royal Society of Western Australia 90, 33–40.
Cochran, W. W. (1980). Wildlife telemetry. In ‘Wildlife management techniques manual’. (Ed. S. D. Schemnitz) pp. 507–520. (The Wildlife Society: Washington, DC, USA.)
Cooke, S. J., Hinch, S. G., Wikelski, M., Andrews, R. D., Kuchel, L. J., Wolcott, T. G., and Butler, P. J. (2004). Biotelemetry: a mechanistic approach to ecology. Trends in Ecology & Evolution 19, 334–343.
| Biotelemetry: a mechanistic approach to ecology.Crossref | GoogleScholarGoogle Scholar |
Cooper, C. E., Withers, P. C., Mawson, P., Johnstone, R., Kirkby, T., Prince, J., Bradshaw, S., and Robertson, H. (2003). Characteristics of Marri (Corymbia calophylla) fruits in relation to the foraging behaviour of the Forest Red-tailed Black Cockatoo (Calyptorhynchus banksii naso). Journal of the Royal Society of Western Australia 86, 139–142.
de Bakker, M. (2011). Automatic Classification of Bird Behaviour on the basis of Accelerometer Data. B.Sc. – Artificial Intelligence Thesis. (University of Amsterdam.)
Department of Environment and Conservation (2008). ‘Forest Black Cockatoo (Baudin’s Cockatoo Calyptorrhynchus baudinii and Forest Red-tailed Black Cockatoo Calyptoryhnchus banksii naso) Recovery Plan 2007–2016.’ (Western Australian Government: Perth, WA, Australia.)
Department of Environment and Conservation (2012). ‘Carnaby’s Cockatoo (Calyptorhynchus latirostris) Recovery Plan.’ (Western Australian Government: Perth, WA, Australia.)
EPA (2019). Carnaby’s cockatoo in environmental impact assessment in the Perth and Peel Region. Advice of the Environmental Protection Authority under Section 16(j) of the Environmental Protection Act 1986. EPA Technical report. Government of Western Australia, Perth, WA, Australia. Available at https://www.epa.wa.gov.au/policies-guidance/carnaby%e2%80%99s-cockatoo-environmental-impact-assessment-perth-and-peel-region.
Groom, C. (2015). Roost site fidelity and resource use by Carnaby’s cockatoo, Calyptorhynchus latirostris, on the Swan coastal plain, Western Australia. Ph.D. Thesis, University of Western Australia, Perth, WA, Australia.
Groom, C., Warren, K., Le Souef, A., and Dawson, R. (2014). Attachment and performance of Argos satellite tracking devices fitted to black cockatoos (Calyptorhynchus spp.). Wildlife Research 41, 571–583.
| Attachment and performance of Argos satellite tracking devices fitted to black cockatoos (Calyptorhynchus spp.).Crossref | GoogleScholarGoogle Scholar |
Groom, C., White, N. E., Mitchell, N., Roberts, J. D., and Mawson, P. (2017). Assessing the spatial ecology and resource use of a mobile and endangered species in an urbanized landscape using satellite telemetry and DNA faecal metabarcoding. The Ibis 159, 390–405.
| Assessing the spatial ecology and resource use of a mobile and endangered species in an urbanized landscape using satellite telemetry and DNA faecal metabarcoding.Crossref | GoogleScholarGoogle Scholar |
IUCN (2020). The IUCN Red List of Threatened Species. Version 2020-2. Available at https://www.iucnredlist.org/.
Johnstone, R. E., and Kirkby, T. (1999). Food of the Forest Red-tailed Black Cockatoo Calyptorhynchus banksii naso in south-west Western Australia. Western Australian Naturalist (Perth) 22, 167–177.
Johnstone, R. E., and Kirkby, T. (2008). Distribution, status, social organisation, movements and conservation of Baudin’s Cockatoo (Calyptorhynchus baudinii) in South-west Western Australia. Records of the Western Australian Museum 25, 107–118.
| Distribution, status, social organisation, movements and conservation of Baudin’s Cockatoo (Calyptorhynchus baudinii) in South-west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Johnstone, R. E., and Kirkby, T. (2017). Black Cockatoo Research Project – Progress Report for Housing Authority 2017. Western Australian Museum, Perth, WA, Australia.
Johnstone, R. E., Johnstone, C., and Kirkby, T. (2011). Carnaby’s Cockatoo (Calyptorhynchus latirostris), Baudin’s Cockatoo (Calyptorhynchus baudinii) and the Forest Red-tailed Black Cockatoo (Calyptorhynchus banksii naso) on the Swan Coastal Plain (Lancelin–Dunsborough), Western Australia. Studies on distribution, status, breeding, food, movements and historical changes. Report for Department of Planning, Western Australia.
Johnstone, R. E., Kirkby, T., and Sarti, K. (2013). The breeding biology of the Forest Red-tailed Black Cockatoo Calyptorhynchus banksii naso Gould in south-western Australia. II. Breeding behaviour and diet. Pacific Conservation Biology 19, 143–155.
| The breeding biology of the Forest Red-tailed Black Cockatoo Calyptorhynchus banksii naso Gould in south-western Australia. II. Breeding behaviour and diet.Crossref | GoogleScholarGoogle Scholar |
Johnstone, R. E., Kirkby, T., and Sarti, K. (2017). The distribution, status movements and diet of the forest red-tailed black cockatoo in the south-west with emphasis on the Great Perth region, Western Australia. Western Australian Naturalist (Perth) 30, 193–219.
Kenward, R. (2001). ‘A manual for wildlife radio tagging.’ 2nd edn. (Academic Press: New York, NY, USA.)
Ladds, M. A., Thompson, A. P., Kadar, J.-P., Slip, D. J., Hocking, D. P., and Harcourt, R. G. (2017). Super machine learning: improving accuracy and reducing variance of behaviour classification from accelerometry. Animal Biotelemetry 5, 8.
| Super machine learning: improving accuracy and reducing variance of behaviour classification from accelerometry.Crossref | GoogleScholarGoogle Scholar |
Le Roux, C. (2017). Nocturnal roost tree, roost site and landscape characteristics of Carnaby’s Black-Cockatoo (Calyptorynchus latirostris) on the Swan Coastal Plain. M.Sc. Thesis, Edith Cowan University, Perth, WA, Australia.
Le Souef, A. T., Stojanovic, D., Burbidge, A. H., Vitali, S. D., Heinsohn, R., Dawson, R., and Warren, K. S. (2013). Retention of transmitter attachments on black cockatoos (Calyptorhynchus spp). Pacific Conservation Biology 19, 55–57.
| Retention of transmitter attachments on black cockatoos (Calyptorhynchus spp).Crossref | GoogleScholarGoogle Scholar |
Lendvai, Á. Z., Akçay, Ç., Ouyang, J. Q., Dakin, R., Domalik, A. D., St John, P. S., Stanback, M., Moore, I. T., and Bonier, F. (2015). Analysis of the optimal duration of behavioral observations based on an automated continuous monitoring system in tree swallows (Tachycineta bicolor): is one hour good enough? PLoS One 10, e0141194.
| Analysis of the optimal duration of behavioral observations based on an automated continuous monitoring system in tree swallows (Tachycineta bicolor): is one hour good enough?Crossref | GoogleScholarGoogle Scholar | 26559407PubMed |
Meijer, C., Verhoeven, S., Ranguelova, E., Camphuysen, C. J., Shamoun-Baranes, J., and Bouten, W. (2016). ‘Visually supported annotation of bird tracking data and subsequent classification of behaviour using machine learning. 2016, Helsinki, Finland.’ (Commission on Visual Analytics of the International Cartographic Association.)
Mittermeier, R., Gil, P., Hoffmann, M., Pilgrim, J., Brooks, T., Mittermeier, C., Lamoreux, J., and Fonseca, G. (2004). ‘Hotspots Revisited. Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions.’ (CEMEX: Monterrey, Mexico.)
Nathan, R., Spiegel, O., Fortmann-Roe, S., Harel, R., Wikelski, M., and Getz, W. M. (2012). Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: general concepts and tools illustrated for griffon vultures. Journal of Experimental Biology 215, .
| Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: general concepts and tools illustrated for griffon vultures.Crossref | GoogleScholarGoogle Scholar |
Pepper, J. W. (1996). ‘The Behavioral Ecology of the Glossy Black-Cockatoo Calyptorhynchus lathami halmaturinus.’ Order No. 9712059. (University of Michigan: Ann Arbor, MI, USA.)
Rycken, S., Warren, K., Yeap, L., Jackson, B., Riley, K., Page, M., Dawson, R., Smith, K., Mawson, P., and Shephard, J. M. (2019). Assessing integration of GPS-tagged black cockatoos using behavioural change point analysis. The Journal of Wildlife Management , .
| Assessing integration of GPS-tagged black cockatoos using behavioural change point analysis.Crossref | GoogleScholarGoogle Scholar |
Saunders, D. A. (1977). The effect of agricultural clearing on the breeding success of the White-tailed Black Cockatoo. Emu – Austral Ornithology 77, 180–184.
| The effect of agricultural clearing on the breeding success of the White-tailed Black Cockatoo.Crossref | GoogleScholarGoogle Scholar |
Saunders, D. (1980). Food and movements of the short-billed form of the White-tailed Black Cockatoo. Wildlife Research 7, 257–269.
| Food and movements of the short-billed form of the White-tailed Black Cockatoo.Crossref | GoogleScholarGoogle Scholar |
Saunders, D. A. (1982). The breeding behaviour and biology of the short-billed form of the White-tailed Black Cockatoo Calyptorhynchus funereus. The Ibis 124, 422–455.
| The breeding behaviour and biology of the short-billed form of the White-tailed Black Cockatoo Calyptorhynchus funereus.Crossref | GoogleScholarGoogle Scholar |
Saunders, D. A. (1990). Problems of survival in an extensively cultivated landscape: the case of Carnaby’s Cockatoo Calyptorhynchus funereus latirostris. Biological Conservation 54, 277–290.
| Problems of survival in an extensively cultivated landscape: the case of Carnaby’s Cockatoo Calyptorhynchus funereus latirostris.Crossref | GoogleScholarGoogle Scholar |
Saunders, D. A., and Dawson, R. (2018). Cumulative learnings and conservation implications of a long-term study of the endangered Carnaby’s Cockatoo Calyptorhynchus latirostris. Australian Zoologist 39, 591–609.
| Cumulative learnings and conservation implications of a long-term study of the endangered Carnaby’s Cockatoo Calyptorhynchus latirostris.Crossref | GoogleScholarGoogle Scholar |
Saunders, D. A., and Ingram, J. A. (1998). Twenty-eight years of monitoring a breeding population of Carnaby’s Cockatoo. Pacific Conservation Biology 4, 261–270.
| Twenty-eight years of monitoring a breeding population of Carnaby’s Cockatoo.Crossref | GoogleScholarGoogle Scholar |
Saunders, D., Rowley, I., and Smith, G. (1985). The effects of clearing for agriculture on the distribution of cockatoos in the south west of Western Australia. In ‘Birds of Eucalypt Forests and Woodlands: Ecology, Conservation, Management’. (Eds A. Keast, H. F. Recher, H. Ford, and D. Saunders.) pp. 309–321. (Royal Australasian Ornithologists Union & Surrey Beatty: Sydney, NSW, Australia.)
Saunders, D. A., Mawson, P., and Dawson, R. (2011). The impact of two extreme weather events and other causes of death on Carnaby’s black cockatoo: a promise of things to come for a threatened species? Pacific Conservation Biology 17, 141–148.
| The impact of two extreme weather events and other causes of death on Carnaby’s black cockatoo: a promise of things to come for a threatened species?Crossref | GoogleScholarGoogle Scholar |
Shaffer, S. A. (2014). As the egg turns: monitoring egg attendance behavior in wild birds using novel data logging technology. PLoS One 9, e97898.
| As the egg turns: monitoring egg attendance behavior in wild birds using novel data logging technology.Crossref | GoogleScholarGoogle Scholar | 24887441PubMed |
Shamoun-Baranes, J., Bom, R., van Loon, E. E., Ens, B. J., Oosterbeek, K., and Bouten, W. (2012). From sensor data to animal behaviour: an oystercatcher example. PLoS One 7, e37997.
| From sensor data to animal behaviour: an oystercatcher example.Crossref | GoogleScholarGoogle Scholar | 22693586PubMed |
Shamoun-Baranes, J., Bouten, W., van Loon, E. E., Meijer, C., and Camphuysen, C. J. (2016). Flap or soar? How a flight generalist responds to its aerial environment. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 371, 20150395.
| Flap or soar? How a flight generalist responds to its aerial environment.Crossref | GoogleScholarGoogle Scholar | 27528785PubMed |
Shepard, E. L. C., Wilson, R. P., Quintana, F., Gómez Laich, A., Liebsch, N., Albareda, D. A., Halsey, L. G., Gleiss, A., Morgan, D. T., Myers, A. E., Newman, C., and Macdonald, D. W. (2008). Identification of animal movement patterns using tri-axial accelerometry. Endangered Species Research 10, 47–60.
| Identification of animal movement patterns using tri-axial accelerometry.Crossref | GoogleScholarGoogle Scholar |
Shephard, J. M., and Warren, K. (2019). Conservation management of forest red-tailed black cockatoos associated with the Maddington-Kenwick Strategic Employment Area Precinct 3 (MKSEA P3) industrial development. Report for MKSEA Pty Ltd, Western Australia. Available at blackcockatooconservationwa.com.
Stock, W. D., Finn, H., Parker, J., and Dods, K. (2013). Pine as fast food: foraging ecology of an endangered cockatoo in a forestry landscape. PLoS One 8, e61145.
| Pine as fast food: foraging ecology of an endangered cockatoo in a forestry landscape.Crossref | GoogleScholarGoogle Scholar | 23593413PubMed |
Sur, M., Suffredini, T., Wessells, S. M., Bloom, P. H., Lanzone, M., Blackshire, S., Sridhar, S., and Katzner, T. (2017). Improved supervised classification of accelerometry data to distinguish behaviors of soaring birds. PLoS One 12, e0174785.
| Improved supervised classification of accelerometry data to distinguish behaviors of soaring birds.Crossref | GoogleScholarGoogle Scholar | 28403159PubMed |
Tatler, J., Cassey, P., and Prowse, T. A. A. (2018). High accuracy at low frequency: detailed behavioural classification from accelerometer data. The Journal of Experimental Biology 221, jeb184085.
| High accuracy at low frequency: detailed behavioural classification from accelerometer data.Crossref | GoogleScholarGoogle Scholar | 30322979PubMed |
Tucker, M. A., Alexandrou, O., Bierregaard Jr, R. O., Bildstein, K. L., Böhning-Gaese, K., Bracis, C., Brzorad, J. N., Buechley, E. R., Cabot, D., Calabrese, J. M., Carrapato, C., Chiaradia, A., Davenport, L. C., Davidson, S. C., Desholm, M., DeSorbo, C. R., Domenech, R., Enggist, P., Fagan, W. F., Farwig, N., Fiedler, W., Fleming, C. H., Franke, A., Fryxell, J. M., García-Ripollés, C., Grémillet, D., Griffin, L. R., Harel, R., Kane, A., Kays, R., Kleyheeg, E., Lacy, A. E., LaPoint, S., Limiñana, R., López-López, P., Maccarone, A. D., Mellone, U., Mojica, E. K., Nathan, R., Newman, S. H., Noonan, M. J., Oppel, S., Prostor, M., Rees, E. C., Ropert-Coudert, Y., Rösner, S., Sapir, N., Schabo, D., Schmidt, M., Schulz, H., Shariati, M., Shreading, A., Paulo Silva, J., Skov, H., Spiegel, O., Takekawa, J. Y., Teitelbaum, C. S., van Toor, M. L., Urios, V., Vidal-Mateo, J., Wang, Q., Watts, B. D., Wikelski, M., Wolter, K., Žydelis, R., and Mueller, T. (2019). Large birds travel farther in homogeneous environments. Global Ecology and Biogeography 28, 576–587.
| Large birds travel farther in homogeneous environments.Crossref | GoogleScholarGoogle Scholar |
Vansteelant, W., Lameris, T., and Bouten, W. (2016). Classification tool manual. Available at http://nlesc.github.io/eEcology-Classification/.
Westcott, D., and Cockburn, A. (1988). Flock Size and Vigilance in Parrots. Australian Journal of Zoology 36, 335–349.
| Flock Size and Vigilance in Parrots.Crossref | GoogleScholarGoogle Scholar |
Williams, M. R., Yates, C. J., Saunders, D. A., Dawson, R., and Barrett, G. W. (2017). Combined demographic and resource models quantify the effects of potential land-use change on the endangered Carnaby’s cockatoo (Calyptorhynchus latirostris). Biological Conservation 210, 8–15.
| Combined demographic and resource models quantify the effects of potential land-use change on the endangered Carnaby’s cockatoo (Calyptorhynchus latirostris).Crossref | GoogleScholarGoogle Scholar |
Yeap, L., Shephard, J. M., Bouten, W., Jackson, B., Vaughan-Higgins, R., and Warren, K. (2017). Development of a tag-attachment method to enable capture of fine- and landscape-scale movement in black-cockatoos. Australian Field Ornithology 34, 49–55.
| Development of a tag-attachment method to enable capture of fine- and landscape-scale movement in black-cockatoos.Crossref | GoogleScholarGoogle Scholar |


