Moving at the speed of flight: dabbling duck-movement rates and the relationship with electronic tracking interval
Fiona McDuie A B D , Michael L. Casazza A , David Keiter
A B D , Michael L. Casazza A , David Keiter  A , Cory T. Overton A , Mark P. Herzog A , Cliff L. Feldheim C and Joshua T. Ackerman A
A , Cory T. Overton A , Mark P. Herzog A , Cliff L. Feldheim C and Joshua T. Ackerman A
A US Geological Survey, Western Ecological Research Center, Dixon Field Station, 800 Business Park Drive, Suite D, Dixon, CA 95620, USA.
B San Jose State University Research Foundation, Moss Landing Marine Laboratories, 8272 Moss Landing Road, Moss Landing, CA 95039, USA.
C California Department of Water Resources, Suisun Marsh Program, West Sacramento, CA 95691, USA.
D Corresponding author. Email: fiona.mcduie@sjsu.edu
Wildlife Research 46(6) 533-543 https://doi.org/10.1071/WR19028
Submitted: 15 February 2019 Accepted: 22 June 2019 Published: 16 September 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Context: Effective wildlife management requires information on habitat and resource needs, which can be estimated with movement information and modelling energetics. One necessary component of avian models is flight speeds at multiple temporal scales. Technology has limited the ability to accurately assess flight speeds, leading to estimates of questionable accuracy, many of which have not been updated in almost a century.
Aims: We aimed to update flight speeds of ducks, and differentiate between migratory and non-migratory flight speeds, a detail that was unclear in previous estimates. We also analysed the difference in speeds of migratory and non-migratory flights, and quantified how data collected at different temporal intervals affected estimates of flight speed.
Methods: We tracked six California dabbling duck species with high spatio-temporal resolution GPS–GSM transmitters, calculated speeds of different flight types, and modelled how estimates varied by flight and data interval (30 min to 6 h).
Key results: Median migratory speeds were faster (but non-significant) for the larger mallard (Anas platyrhynchos; 82.5 km h–1), northern pintail (Anas acuta; 79.0 km h–1) and gadwall (Mareca strepera; 70.6 km h–1), than the smaller-bodied northern shoveler (Spatula clypeata; 65.7 km h–1), cinnamon teal (Spatula cyanoptera; 63.5 km h–1) and American wigeon (Mareca Americana; 52 km h–1). Migratory flights were faster than non-migratory flights for all species and speeds were consistently slower with an increasing data interval.
Implications: The need to balance time and energy requirements may drive different speeds for migratory and non-migratory flights. Lower speeds at longer intervals are likely to be due to a greater proportion of ‘loafing’ time included in flighted segments, demonstrating that data acquired at different intervals provide a means to evaluate and estimate behaviours that influence speed estimation. Shorter-interval data should be the most accurate, but longer-interval data may be easier to collect over lengthier timeframes, so it may be expedient to trade-off a degree of accuracy in broad-scale studies for the larger dataset. Our updated flight speeds for dabbling duck species can be used to parameterise and validate energetics models, guide management decisions regarding optimal habitat distribution, and, ultimately, improve conservation management of wetlands for waterfowl.
Additional keywords: data frequency, energetics, flight speed, GPS tracking, interval bias, migration, habitat management.
Introduction
To determine the food resources and habitat necessary to sustain animal populations, managers require a good understanding of animal movement and habitat use at different spatio-temporal scales (Central Valley Joint Venture 2006; Hays et al. 2019). Animals move through the landscape, obtaining resources necessary to fuel their movements (Pianka 1981), balancing energy requirements with energy intake (McNab 1980; Sapir et al. 2011). Animal movement can be quantified by speed and distance moved. Energetics modellers can use these parameters to estimate how much energy, in the form of food, is required for a particular population in a given area (Furness 1978; McNab 1980; Winship et al. 2002).
Animals performing lengthy migrations, such as humpback whales (Megaptera novaeangliae; Braithwaite et al. 2015), bats (Lasionycteris noctivagans; McGuire et al. 2014), wildebeest (Connochaetes taurinus), blue whales (Balaenoptera musculus) and bar‐tailed godwits (Limosa lapponica; Hein et al. 2012), there are trade-offs between the energy they can obtain en route and the speed and distance they can travel. For flighted animals such as waterfowl, flight speed is a measurable trait that can be used to evaluate the cost of movement, because it dictates the energetic consumption of that activity (Tucker 1971; Bruderer and Boldt 2001). Specifically, waterfowl-targeted energetic models such as spatially explicit waterbird agent-based model program (SWAMP) require accurate estimates of flight speed for multiple species, to inform managers about the optimal distribution of essential resources (Miller et al. 2014).
Few studies have accurately quantified waterfowl flight speeds (Table 1). The most current and accurate speeds were estimated for northern pintail (Anas acuta; hereafter pintail), using PTT satellite transmitters (Miller et al. 2005). However, estimates from other waterfowl species are lacking. Some estimates of flight speed have not been updated in many years, or are based on outdated approaches resulting in questionable accuracy. For example, early methods were based on observed velocities and included estimating flight speed with theodolites or radar (Tucker and Schmidt-Koenig 1971; Bruderer and Boldt 2001), or by chasing individuals or flocks with vehicles (Cooke 1933; Meinertzhagen 1955).
It is also generally unclear whether these prior speed estimates were from migratory or non-migratory flights, but because behaviour (distance, time and activity) varies according to the type of flight being conducted (Pennycuick 1975, 1978), this aspect must also be considered. Furthermore, according to optimal flight-speed theory, innate differences between flight type and speed used result in divergent energetic expenditure and intake (Alerstam and Lindström 1990; Hedenström 1993; Hedenström and Alerstam 1995; Alerstam 2011). For example, migratory flight has long been thought to be faster than non-migratory flight, and birds on extended, uninterrupted, and often more direct, flights maximise distance travelled for a given amount of fuel (Alerstam and Lindström 1990; Gudmundsson et al. 1992; Meinertzhagen 1955; Pennycuick 1969). By contrast, non-migratory flights are generally shorter, less directed and are thought to be conducted at a lower ‘cruising’ speed that involves a greater energy expenditure (Meinertzhagen 1955; Pennycuick 1969; Nudds and Bryant 2000; Alerstam et al. 2007). Therefore, to comprehensively assess energy expenditure, we also require accurate speed estimates of birds conducting different types of flight (Hedenström 1993; Hedenström and Alerstam 1995).
If energetic needs vary according to flight behaviour, resource management and conservation planning may, consequently, be affected. Migrating birds are constrained by en route fuel needs supplied in critical staging areas; so, effective location, size and distribution of these areas is essential to sustain migrating birds (Finger et al. 2016; Bartzen et al. 2017; Fronczak et al. 2017). Wintering areas that are subject to substantial periodic influxes of birds may require targeted management that provides sufficient resources to support these population increases (Alonso et al. 1994; Central Valley Joint Venture 2006). Equally, local or regional habitat juxtaposition may be optimised to improve the energy supply for birds conducting shorter, non-migratory flights. Equally, local or regional habitat management may have to optimise the scale and juxtaposition of ‘good’ habitat for birds conducting shorter, non-migratory flights.
Recent advances in technology mean that we can use high spatial- and temporal-resolution electronic devices to track larger-bodied birds over extended time periods. This has been achieved on a variety of other taxa to understand behavioural ecology, how behavioural states inform energetics, and identify ways of using movement data to support conservation and management (Kays et al. 2015; Hays et al. 2016, 2019; Wilmers et al. 2017). We tracked six species of dabbling ducks in California with high-resolution GPS–GSM transmitters to address four primary objectives in this understudied area of waterfowl research. We aimed to update flight-speed estimates by accurately quantifying speeds and distances moved in two different behavioural states, namely, migratory and non-migratory flight; calculating variation of speeds between flight types; identifying interspecific and/or intersexual divergence in flight speeds; and measuring the effect of the data-collection interval on flight speeds.
Materials and methods
Data collection and electronic tracking
We focussed our study on six species of ducks including gadwall (Mareca strepera), mallard (Anas platyrhynchos), northern pintail, northern shoveler (Spatula clypeata; hereafter, shoveler), cinnamon teal (Spatula cyanoptera; hereafter, teal) and American wigeon (Mareca americana; hereafter, wigeon), from January 2015 to October 2017. We captured males and females of all species except gadwall (females only) with baited funnel traps, rocket nets and hand-held dip nets within California’s Central Valley. Trapping was conducted in the Grizzly Island State Wildlife Area (SWA; 38.138306°, –121.978056°) and surrounding private properties within the Suisun Marsh Complex, and at Howard Slough SWA in the Sacramento Valley (39.467256°, –121.877411°). In addition, we captured teal at various locations within Oregon, Idaho, Colorado, Nevada, Washington and Utah. All birds were marked with individually numbered aluminium U.S. Geological Survey Bird Banding Laboratory leg bands, and we assessed size and weight with morphometric measurements (weight, wing, tarsus, bill and head lengths), to ensure electronic deployment package weights were within the accepted 3–5% bodyweight limit for birds (Cochran 1980; Drewien and Clegg 1992; Kenward et al. 2001; Phillips et al. 2003; Fair et al. 2010).
We deployed high-resolution Ecotone® GPS–GSM SAKER L series electronic transmitters (~5-m location accuracy) weighing 17 g and measuring 58 × 27 × 18 mm on all species except teal, on which smaller devices weighing 14 g and measured 30 × 20 × 14 mm were deployed. The transmitters had a foam base pad and were attached to adults on back-mounted body harnesses constructed of 5-mm automotive elastic, less likely to wick water to down feathers. Elastic ribbons fastened with crimps on early deployments were later modified to a simple double overhand knot affixed with cyanoacrylic glue, to hold the transmitter in place. Total deployment weights were 18–18.5 g for larger deployments and 14.2 g on teal. Each duck was released at the location of capture after a handling time of 20–30 min.
Location-data intervals varied on these transmitters according to battery-power levels, but, for the present study, we analysed data collected at the shortest interval that battery power allowed for lengthy deployments (30 min for all except teal migratory and non-migratory flights and wigeon migratory flights, which were at 1 h). Locations with date and time were transmitted to Ecotone (http://telemetry.ecotone.pl, accessed 28 September 2017) via cellular GSM text message when in network range. When out of range, data were stored on the device and backfilled from most recent to earliest, when ducks returned within range of a cell tower as battery power and GSM signal strength allowed. Handling during capture and electronic-device deployments are known to affect the behaviour of some species of birds (Pietz et al. 1993; Cox and Afton 1998; Phillips et al. 2003). Nevertheless, electronic tracking currently provides highly useful and accurate movement data. In field observations during our study, when behaviour was altered, this occurred primarily during the first 10 days after deployment (e.g. increased preening duration; USGS, unpubl. data); so, we conservatively excluded the first 14 days of data from all tracks.
Identifying flight type
California ducks perform breeding and molt migrations throughout the year; so as to identify and separate migratory from non-migratory movements, migration was classified as any movement of at least 200 km over three or more consecutive 30-min segments. We selected this distance because in California, pintails are known to perform 1500-km migrations with only a single stopover in southern Oregon, a distance of almost 500 km from Suisun Marsh where most birds were trapped (Miller et al. 2010). We applied the same distance criteria for all species because the smaller shoveler and wigeon conduct similarly lengthy migrations as does the pintail. While some teal migrations were slightly shorter, migratory flights were of similar speed and distance to be comparable (USGS, unpubl. data).
Estimating flight speeds
Flight speed was calculated from the distance travelled between two consecutive locations in our dataset (flight segment) at a variety of temporal intervals (30 min, 1 h, 2 h, 3 h and 6 h). To produce the most accurate speed estimates, we always used the shortest interval data at which ducks had been tracked, which, in most cases, was 30 min, but in the case of teal and wigeon migratory data, this was 1 h. To accurately identify flighted segments, we filtered these GPS data with minimum and maximum speed thresholds. The minimum speed was 20 km h–1, a distance that a duck is unlikely to be able to travel on foot or by swimming. The maximum speed used was 160 km h–1, the same used by Miller et al. (2005), in the only previous study of speed in this population. Ducks are not thought to be capable of these speeds, so any flights in excess of this speed are more likely to reflect GPS error.
Without data from activity sensors, it was not possible to be certain a bird was in flight for the entire duration of any identified flight segment (i.e. to determine precisely when a bird took off or landed during any 30-min interval). As such, any segment could potentially include some portion of time on the ground (not flight), which would lead to underestimating the actual flight speed. If a bird is in flight for three or more consecutive segments, take-off and landing are more likely to occur during the first and final segments respectively. As such, those segments would be the most likely to include non-flight periods. The middle segments, by contrast, should consist entirely of flight. Therefore, for all species migratory and non-migratory datasets with sufficient data, we extracted the middle segments from any trajectory of three or more consecutive 30-min flights, producing a ‘conservative’ dataset with which to calculate the best estimates of flight speed.
Comparing GPS-location intervals
To understand whether estimated flight speeds varied according to the interval between GPS locations, we first extracted 30-min location data only from ‘bird days’ (24-h periods) that included a complete set of 46–49 high-frequency (short-interval) GPS locations (i.e. no missing locations) by species. From these high-frequency datasets, we subsampled locations at 1-, 2-, 3- and 6-hourly intervals (equal to the intervals with which the GPS transmitters can be programmed), producing a categorical data interval (‘rescat’). We then re-assessed migratory versus non-migratory flight status for each segment at all intervals, applying the same distance and speed thresholds as previously noted, and estimated speeds at each interval. To calculate predicted values ± standard errors, 95% confidence limits and the equations representing the slopes of the relationships between interval and speed, we created a continuous (‘rescon’) GPS-data interval variable at 1-min intervals between 30 and 360 min (60–360 min for teal). Wigeon were excluded from these evaluations because of the different minimum intervals of migratory and non-migratory data. Finally, we averaged daily flight speeds to evaluate whether flight-speed estimates at lower intervals (1 h, 2 h, 3 h, 6 h) were predicted to be different from those estimated at the highest interval of 30 min. Teal and wigeon were excluded from these analyses because of lack of data at 30-min intervals.
Statistical analysis
We identified and classified flight types with the AdehabitatLT package (Calenge 2006) in R (R Core Team 2016), which we used to perform all statistical analyses. We calculated mean flight speeds for each species’ migratory and non-migratory flights, with 95% confidence intervals (95% CI), for every 30-min flight segment in the data and, where possible, for the most conservative flight groupings. Migratory data for wigeon, and data for both flight types for teal, did not exist at 30-min intervals, so we used the shortest interval, which was hourly.
To quantify differences between migratory and non-migratory flights, we ran sets of linear mixed-effect (LME) models, for each species independently, using lme4 and gamlss (Stasinopoulos and Rigby 2007; Bates et al. 2015) packages. Flight speed (log-transformed) was our response variable in all models. We used the Satterthwaite approximation to estimate ‘effective degrees of freedom’ when our probability distributions were formed from several independent normal distributions for which we had only estimates of the variance (Satterthwaite 1946). We ran interactive and additive models to analyse flight speed as a function of migratory status and data interval, testing data interval as both a continuous (to represent a linear relationship we extrapolated the data to 1-min intervals from 30 to 360 min) and categorical (30 min, 1 h, 2 h, 3 h and 6 h) variable. Bird ID nested within date was included as a random effect. We tested the slope of the relationships between speed and data interval, and the differences between migratory and non-migratory flights, with the ANOVA function in R.
We investigated interspecific flight-speed responses with additive and interactive LME models for gadwall, mallard, pintail and shoveler from 30-min data, with species and migratory status as fixed effects and bird ID as the random effect. We did the same to investigate inter-sexual responses for teal with sex and migratory status as fixed effects and bird ID as the random effect, with their hourly data. In all model sets using the same datasets, we assessed the support of models using Akaike’s information criterion (AIC) values, identifying the most parsimonious model as that which had the lowest AIC value. We then compared their relative strength with the AICcmodavg (Mazerolle and Mazerolle 2017) package, using the ΔAIC values to determine whether the model with the lowest AIC was significantly better than the next-best model. A ΔAIC value of <2 indicates model uncertainty, namely, that the model with the lowest AIC value is not significantly better than the next-lowest model (Burnham and Anderson 2003).
Finally, to determine how speed estimates varied as the GPS data interval increased, we modelled flight speeds estimated at each of the lower frequencies (1 h, 2 hr, 3 h and 6 h), with speeds from the 30-min data for gadwall, mallard, pintail and shoveler. Linear mixed-effects models performed with the R packages lme4, lsmeans and lmerTest (Bates et al. 2015; Kuznetsova et al. 2015; Lenth 2016) used flight speeds averaged over an entire bird day by individual, and we compared the relative bias of speed estimates among temporal data intervals with contrasts using the general linear hypothesis (glht) function in R. If there were no bias owing to the data interval, the speed at any longer interval would be the same as the speed at 30 min, a ratio of 1 : 1, so we offset the model by 1 to test whether the slopes of the lines varied from this ratio.
Results
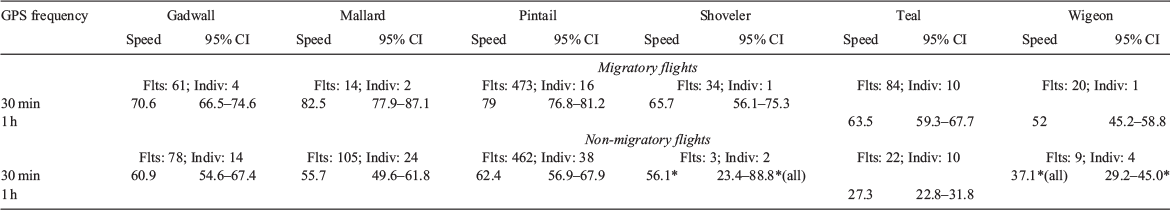
There were too few data for shoveler, teal and wigeon to produce robust ‘conservative’ migratory and non-migratory data subsets, so those speeds are calculated from ‘all’ identified flight segments (Table 2). Less than 0.1% of GPS points necessitated removal owing to erroneous locations.
Migratory flight speed
Median migratory flight speeds varied among species from 52 to 82.5 km h–1. The fastest were those of mallards at 82.5 km h–1 (95% CI: 77.9–87.0; Fig. 1, Table 2). However, as few post-nesting mallards perform any kind of migration, this was from only 10 flights by two individuals. Our other breeding residents, gadwalls, provided 47 migratory flights at 30-min intervals on four individuals, with a median speed of 70.6 km h–1 (95% CI: 66.5–74.6; Table 2).
Of our long-distance migratory species, pintail provided the most data, including 417 migratory flight segments from 15 individuals, and had the fastest average speed of 79.0 km h–1 (95% CI: 76.8–81.1; Table 2). Shoveler average flight speed from 26 flights made by a single individual was 65.7 km h–1 (95% CI: 56.1–75.3). Ten teal individuals provided 80 hourly flights at a median speed of 63.5 km h–1 (95% CI: 59.3–67.7; Table 2). A single wigeon migrated at 52 km h–1 (95% CI: 45.2–58.8; Table 2) over 20 hourly flight segments.
Non-migratory flight speeds
Flight speeds classified as non-migratory were slower than migratory flight speeds for all species except shoveler (Fig. 2, Table 3), and averaged between 36.5 and 62.4 km h–1 (Table 2). Shoveler could not be accurately quantified because of excessive variance. Non-migratory flights were more difficult to classify and separate into conservative subsets because, by definition, they were limited to less than 200 km of any continuous flight path. Therefore, we had sufficient data to produce the 30-min conservative subsets only for gadwall, mallard and pintail and a 1-h dataset for teal (Table 2). The small datasets for shoveler, teal and wigeon were insufficient for robust conservative subsets, so speeds were estimated from all flights (3 flights from 2 shovelers and 9 flights from 4 wigeons). Of the locally resident breeders, gadwall median non-migratory flight speeds were 60.9 km h–1 (95% CI: 54.5–67.4) derived from 25 flights from seven individuals. The large majority of identified 30-min-interval flights for mallards (Table 2) were non-migratory and the conservative dataset produced 24 flights on seven individuals and a median speed of 55.7 km h–1 (95% CI: 49.6–61.8; Fig. 1, Table 2).

|
Again, pintail provided the most flight data of the long-distance migrants, with 78 flights on 16 individuals demonstrating a median flight speed of 62.4 km h–1 (95% CI: 56.9–67.9; Fig. 1, Table 2). Shoveler median flight speed was 56.1 km h–1 (95% CI: 23.4–88.8), whereas wigeon median speed was 37.1 km h–1 (95% CI: 29.2–45.0; Fig. 1, Table 2). From 10 teal individuals, we identified 22 non-migratory flights at hourly intervals, for a median speed of 36.5 km h–1 (95% CI: 31.9–41.1; Fig. 1, Table 2).
Inter-specific and inter-sexual variation
We found no differences in migratory speeds among the larger-bodied gadwall, mallard and pintail (LME: F2,22.21 = 0.27, P = 0.77), or among non-migratory flight speeds of gadwall, mallard, pintail and wigeon (LME: F3,73.71 = 2.41, P = 0.07). A series of nested LME models that assessed speed as a function of species and migratory status, demonstrated that the most parsimonious was the model that included only the migratory status as a fixed effect (AIC 1035.3; Table 3). However, this model with only the migratory status as a fixed effect was not significantly better than the next-best model that included species as an additional fixed effect (ΔAIC = 0.5; Table 3).
We modelled teal separately from the other species because the fastest interval of GPS data collected on teal was hourly. Female migratory speeds were ~50.2 km h–1, compared with male migratory speeds at 48.2 km h–1, and female non-migratory flight speeds were 32.1 km h–1 and male migratory speeds were 30.8 km h–1. The most supported model was that which included only the migratory status as a predictor variable. The model with sex as an additional fixed effect had a higher AIC value but, as the difference was smaller than the minimum ΔAIC threshold of 2 (Burnham and Anderson 2003), that model was not significantly less informative (ΔAIC = 1.48; Table 3).
Migratory versus non-migratory flights
We assessed how flight speeds varied by GPS data interval in separate sets of models for each species (gadwall, mallard, pintail, shoveler and teal). Migratory and non-migratory flight-speed estimates were consistently negatively influenced by the interval of the GPS data, and, for all species, the best model (lowest AIC) was the additive model with migratory status and data interval as a continuous variable, as the fixed effects (Fig. 2, Table 4). That is, flight speeds estimated from 30-min-interval GPS data were always faster than speeds at longer intervals (30-min data subset to 1-h, 2-h, 3-h and 6-h intervals). The top two models for pintail, shoveler and teal indicated some uncertainty (because of ΔAICc of <2, Burnham and Anderson 2003). The top model was not significantly better than the subsequent models (ΔAICc = 1.62, 1.94 and 1.02 respectively; Table 4). In these cases, the next-best model was the nested interactive model of the continuous resolution variable with migration, which potentially indicates a differential effect of the data resolution on migratory versus non-migratory flight speeds. These models demonstrated that, for each species, (1) estimated speeds were predicted to be significantly slower with longer-interval data and (2) non-migratory flight-speed estimates were always predicted to be significantly slower than were migratory speed estimates, at all temporal intervals (Fig. 2, Table 4).

|
Comparison of GPS location intervals
Finally, daily average speeds across species (excluding teal and wigeon) were always predicted to be slower as the interval of the data increased (LME: F4,347 = 1337, P < 0.0001; Table 5). If there were no biases in flight speeds estimated from data collected at different temporal intervals, we would see a relationship of 1 : 1 when comparing these speeds. However, as the temporal interval increased, the lines produced from our model deviated increasingly farther from the expectation (Fig. 3). For example, at a nominal flight speed of 75 km h–1 at the 30-min interval, 1-h speed estimates were 15% slower (t840 = –8.274, P < 0.0001), 2-h estimates were 24% slower (t840 = –12.26, P < 0.0001), 3-h estimates were 26% slower (t840 = –12.56, P < 0.0001) and 6-h estimates were 47% slower (t847 = –19.43, P < 0.0001). With the exception of the decline from 2-h-interval to 3-h-interval data, all other speed estimates decreased significantly as the data interval increased (see Table 5 for results).

|
Discussion
Many of the flight-speed estimates presented in our study are the first ones published in almost a century (Table 1). Our estimates were produced from the largest datasets at the fastest temporal interval to date, and our study is the first to use high-resolution GPS to do so. The only previous study that used electronic tracking technology to estimate flight speeds of any dabbling duck employed PTT satellite telemetry, to estimate pintail migratory flight speed (Miller et al. 2005). This lower spatial- and temporal-resolution technology noted a speed of 77 km h–1 (90% CI = 69–84), which was not dissimilar to our result (79 km h–1; 95% CI: 76.8–81.1). By contrast, earlier estimates produced with outdated methodologies, such as observed velocities from chase, radar or theodolite data (Cooke 1933; Meinertzhagen 1955; Tucker and Schmidt-Koenig 1971; Bruderer and Boldt 2001), often presented a wide range of estimates (see Tables 1, 2), affording less confidence in their accuracy. This suggests that measuring speed by displacement with very high-resolution technology provides estimates that are more accurate.
In the present study, migratory flights were consistently faster with lower variance, than were flights classified as non-migratory for all species, and migratory status was the strongest model predictor. This may be explained by innate behavioural differences involved in migration. Migratory flight, a seasonal relocation between breeding grounds and non-breeding areas, often covers large distances efficiently and rapidly, is more direct, with fewer stops and is thought to be faster, than are briefer, shorter non-migratory flights (Meinertzhagen 1955; Pennycuick 1969; Alerstam and Lindström 1990; Gudmundsson et al. 1992; Greenberg and Marra 2005). Because flights with different objectives could involve distinct flight speeds and movement patterns, with concomitant implications for assumptions regarding energy use (Alerstam and Lindström 1990; Hedenström and Alerstam 1995), speed measurements of birds conducting different types of flight can help determine flight time and energy constraints (Hedenström 1993; Hedenström and Alerstam 1995). These data can then be used to parameterise and validate energetics models such as the agent-based ‘SWAMP’ model, and better understand how ducks use the landscape, habitat and food available to them (Miller et al. 2014). Moreover, outcomes from these models allow managers to estimate the amount of food required to support populations, as well as their resource and habitat needs.
Although this is beneficial for managing multi-species populations, such as that of California’s Central Valley, species may differ in the habitat they utilise because of divergent movement patterns (McDuie et al. 2019), which regulates energy expenditure and intake through the habitat they use (Fox and Abraham 2017). Therefore, developing species-specific management plans also requires an understanding of interspecific differences in flight and speeds. Our results do not rule out potential interspecific differences in flight speeds, despite inconclusive evidence from the current data, for two reasons. First, our models that included species were nearly as informative as models that did not, suggesting that, if larger sample sizes reduced variance in the less represented species, an effect may become apparent. Second, we observed a general divergence between the speeds of larger- and smaller-bodied ducks. Mallards, previously noted as among the fastest flying ducks (Bellrose and Crompton 1981), displayed some of the fastest speeds, together with gadwall and pintail, our largest species (Baldassarre 2014). However, discernible distinctions among the smaller shoveler, teal and wigeon suggest that speed may not be entirely regulated by body size. Instead, it may also be influenced by behavioural, life-history or physiological divergences such as wing size and shape (Raikow 1973; Hedenström and Alerstam 1995; Alerstam et al. 2007). For example, wigeon has a more slotted wing morphology that favours slower flight, reduces minimum flight speed, and increases efficiency at a lower speed (Savile 1957; Raikow 1973). Nevertheless, we require more data to unequivocally determine how flight speeds vary by species and what the biological or ecological significance of those differences may be.
The lower flight speeds estimated from longer GPS intervals are likely to be due to bias resulting from a reduced ability of the longer-interval data to accurately reflect the actual flight behaviour. Measuring speeds from the distance flown between two points assumes a direct flight path. That is, if flight was not linear, the actual distance flown would be greater than the measured distance between the first and last locations would reflect. This would result in a spuriously slow speed that reflects the actual (greater) distance flown, a bias that is likely to be exacerbated as the interval lengthens. However, as migratory flights are generally directed with low turn angles, which would make distances (and, hence, speed) equivalent regardless of interval. Therefore, we would expect to see a greater discrepancy between the shortest and longer intervals of non-migratory than migratory flights, but both flight types indicated proportionally similar speed reductions across the GPS intervals. Alternatively, the discrepancy may be explained by the inclusion of some portion of stoppage or ‘loafing’ time, rather than flight, within longer segments. In this case, speed would be underestimated, with the effect being more enhanced the greater the interval. Nevertheless, we expect speed estimates from the highest-interval data to be most accurate because to have no bias in the estimates across intervals would require some systematic source of error that was equally distributed across time (e.g. loafing would be equally proportioned at every data interval). This seems unlikely but could be tested with accelerometer or shorter-interval data.
Further support for loafing came from the faster speeds estimated with our most conservative datasets, compared with the least conservative ones, which were also those most likely to include some proportion of loafing behaviour. Consequently, we have gained evidence of divergent behaviours that may influence flight, speed and energy expenditure that may be prudent to account for in energetics models for management. In addition, we have demonstrated the increasing bias shown in estimates produced from longer-interval data. Shorter-interval data better depict movements at finer spatio-temporal scales, beneficial in fine-scale and behavioural research, whereas longer-interval data, which produce less drain on batteries, can be collected over longer time periods, and may be more useful to identify and describe activity on broader scales. For example, slower estimates of migratory flight speeds may not be the chief concern if the research is querying the average rate of movement across the duration of the migration path. On the contrary, underestimating flight speeds or misinterpreting the flight type could result in inaccurate energetics assumptions for modelling. Nevertheless, the relationship between the accuracy of information produced and the interval at which data are acquired highlights the trade-offs inherent in making research decisions about the data that can be realistically collected, and the questions those data can be used to address. Finally, it is possible that device effects could have influenced flight speeds and results in the present study. However, we consistently observed that device-influenced behavioural adjustments, such as increased preening, ceased ~10 days after deployment. After this period, birds appeared to adapt well to the GPS. By conservatively excluding the first 2 weeks of each track, we believe these data represent behaviour undisturbed by device deployments.
Conclusions
Our results are the most accurate and up-to-date estimates of flight speed for these six duck species, and demonstrated how higher spatial- and temporal-resolution tracking data can detect distinct behaviours that may affect flight, speed and energy expenditure. Because our results did not meaningfully alter estimates produced in the only other higher-resolution tracking study that obtained locations at intervals that were hourly at best (Miller et al. 2005), further increasing the temporal data interval may not further change our estimates. Consequently, our combined results can be incorporated into current energetics models to directly inform decision-making in the conservation and management of waterfowl wetlands, and generate accurate insights into the impact of environmental changes in wetlands, on population foraging success and survival.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the following for contributions to the completion of this research: Suisun Resources Conservation District (SRCD), California Waterfowl Association (CWA), California Department of Fish and Wildlife, USA Fish and Wildlife, California Department of Water Resources, Colorado Parks Wildlife, Ducks Unlimited, Nevada Department of Wildlife, Oregon Fish & Wildlife, Utah DNR Wildlife Resources and Washington Department of Fish and Wildlife. Field work, capture, trapping, handling and tagging of ducks was completed by Caroline Brady, Michael Carpenter, Steve Chappell, Katharine Cody, Breanne Cooney, Michael D’Errico, Alex Dopkin, Tim Edmunds, Daniel Essert, Matthew Falcon Brady Fettig, Katharine Fielding, Michael Fontana, Patrick Graham, Jacob Gray, Andy Greenawalt, Rich Hansen, Clint Helms, Brian Huber, Melissa Hunt, Jeff Knetter, Jeffrey Kohl, Nathan LaShomb, Desmond Mackell, Elliott Matchett, Andrea Mott, Chris Nicolai, Dave Olson, Dean Podolsky, Matthew Prinzing, Brandon S. Reishus, Orlando Rocha, Ivonne Romero, Casey Setash, Shannon Skalos, Kyle Spragens, Casey Stemler, Blair Stringham, Jeff Taylor, David Van de Riet, Matt Pieron, Bruce Wickland and Matthew Wilson. We thank the Grizzly Island Wildlife Area and California Department of Fish and Wildlife staff for assistance with all aspects of the field data collection. Julie Yee provided assistance with data analyses and Lisa Parker administrative support. Funding for this study was provided by the California Department of Water Resources and the USA Geological Survey. The datasets used and analysed are available from the corresponding author on reasonable request. This study was approved by the USA Geological Survey Western Ecological Research Center Animal Care and Use Committee and was conducted under Federal Banding Permit #21142 and state SC permit #SC-8090. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the USA Government.
References
Alerstam, T. (2011). Optimal bird migration revisited. Journal of Ornithology 152, 5–23.| Optimal bird migration revisited.Crossref | GoogleScholarGoogle Scholar |
Alerstam, T., and Lindström, Å. (1990). Optimal bird migration: the relative importance of time, energy, and safety. In ‘Bird Migration’. (Ed. E. Gwinner.) pp. 331–351. (Springer: Berlin, Heidelberg, Germany.)
Alerstam, T., Rosén, M., Bäckman, J., Ericson, P. G., and Hellgren, O. (2007). Flight speeds among bird species: allometric and phylogenetic effects. PLoS Biology 5, e197.
| Flight speeds among bird species: allometric and phylogenetic effects.Crossref | GoogleScholarGoogle Scholar | 17645390PubMed |
Alonso, J. C., Alonso, J. A., and Bautista, L. M. (1994). Carrying capacity of staging areas and facultative migration extension in common cranes. Journal of Applied Ecology 31, 212–222.
| Carrying capacity of staging areas and facultative migration extension in common cranes.Crossref | GoogleScholarGoogle Scholar |
Baldassarre, G. A. (2014) ‘Ducks, Geese, and Swans of North America.’ (Johns Hopkins University Press: Baltimore, MD, USA.)
Bartzen, B. A., Dickson, D. L., and Bowman, T. D. (2017). Migration characteristics of long-tailed ducks (Clangula hyemalis) from the western Canadian Arctic. Polar Biology 40, 1085–1099.
| Migration characteristics of long-tailed ducks (Clangula hyemalis) from the western Canadian Arctic.Crossref | GoogleScholarGoogle Scholar |
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bellrose, F. C., and Crompton, R. C. (1981). Migration speeds of three waterfowl species. The Wilson Bulletin 93, 121–124.
Braithwaite, J. E., Meeuwig, J. J., and Hipsey, M. R. (2015). Optimal migration energetics of humpback whales and the implications of disturbance. Conservation Physiology 3, cov001.
| Optimal migration energetics of humpback whales and the implications of disturbance.Crossref | GoogleScholarGoogle Scholar | 27293686PubMed |
Bruderer, B., and Boldt, A. (2001). Flight characteristics of birds. The Ibis 143, 178–204.
| Flight characteristics of birds.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P., and Anderson, D. R. (2003) ‘Model Selection and Multimodel Inference: a Practical Information-theoretic Approach.’ (Springer Science & Business Media: New York, NY, USA.)
Calenge, C. (2006). The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197, 516–519.
| The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals.Crossref | GoogleScholarGoogle Scholar |
Central Valley Joint Venture (2006). ‘Central Valley Joint Venture 2006 Implementation Plan: Conserving Bird Habitat.’ Central Valley Joint Venture [CVJV]. (US Fish and Wildlife Service: Sacramento, CA, USA.)
Cochran, W. W. (1980). Willdife telemetry. In ‘Willdife Management Techniques Manual’. (Ed. S. Schemnitz.) pp. 507–520. (The Wildlife Society: Washington, DC, USA.)
Cooke, M. T. (1933). Speed of bird flight. The Auk 50, 309–316.
| Speed of bird flight.Crossref | GoogleScholarGoogle Scholar |
Cox, R. R., and Afton, A. D. (1998). Effects of capture and handling on survival of female northern pintails (Efectos de la captura y la manipulación en la supervivencias de Anas acuta). Journal of Field Ornithology 69, 276–287.
Drewien, R. C., and Clegg, K. R. (1992). Capturing whooping cranes and sandhill cranes by night-lighting. In ‘Proceedings of the 6th North American Crane Workshop’, 1992, Grand Island, Nebraska, USA. (Ed. D. W. Stahlecker.) pp. 43–49. (North American Crane Working Group, University of Nebraska: Lincoln, NE, USA.)
Fair, J., Paul, E., and Jones, J. (2010). ‘Guidelines to the Use of Wild Birds in Research.’ (Ornithological Council: Washington, DC, USA.)
Finger, T. A., Afton, A. D., Schummer, M. L., Petrie, S. A., Badzinski, S. S., Johnson, M. A., Szymanski, M. L., Jacobs, K. J., Olsen, G. H., and Mitchell, M. A. (2016). Environmental factors influence lesser scaup migration chronology and population monitoring. The Journal of Wildlife Management 80, 1437–1449.
| Environmental factors influence lesser scaup migration chronology and population monitoring.Crossref | GoogleScholarGoogle Scholar |
Fox, A. D., and Abraham, K. F. (2017). Why geese benefit from the transition from natural vegetation to agriculture. Ambio 46, 188–197.
| Why geese benefit from the transition from natural vegetation to agriculture.Crossref | GoogleScholarGoogle Scholar | 28215009PubMed |
Fronczak, D. L., Andersen, D. E., Hanna, E. E., and Cooper, T. R. (2017). Distribution and migration chronology of eastern population sandhill cranes. The Journal of Wildlife Management 81, 1021–1032.
| Distribution and migration chronology of eastern population sandhill cranes.Crossref | GoogleScholarGoogle Scholar |
Furness, R. (1978). Energy requirements of seabird communities: a bioenergetics model. Journal of Animal Ecology 47, 39–53.
| Energy requirements of seabird communities: a bioenergetics model.Crossref | GoogleScholarGoogle Scholar |
Greenberg, R., and Marra, P. P. (2005) ‘Birds of Two Worlds: the Ecology and Evolution of Migration.’ (Johns Hopkins University Press: Baltimore, MD, USA.)
Gudmundsson, G. A., Alerstam, T., and Larsson, B. (1992). Radar observations of northbound migration of the Arctic tern, Sterna paradisaea, at the Antarctic Peninsula. Antarctic Science 4, 163–170.
| Radar observations of northbound migration of the Arctic tern, Sterna paradisaea, at the Antarctic Peninsula.Crossref | GoogleScholarGoogle Scholar |
Hays, G. C., Ferreira, L. C., Sequeira, A. M., Meekan, M. G., Duarte, C. M., Bailey, H., Bailleul, F., Bowen, W. D., Caley, M. J., and Costa, D. P. (2016). Key questions in marine megafauna movement ecology. Trends in Ecology & Evolution 31, 463–475.
| Key questions in marine megafauna movement ecology.Crossref | GoogleScholarGoogle Scholar |
Hays, G. C., Bailey, H., Bograd, S. J., Bowen, W. D., Campagna, C., Carmichael, R. H., Casale, P., Chiaradia, A., Costa, D. P., and Cuevas, E. (2019). Translating marine animal tracking data into conservation policy and management. Trends in Ecology & Evolution 34, 459–473.
| Translating marine animal tracking data into conservation policy and management.Crossref | GoogleScholarGoogle Scholar |
Hedenström, A. (1993). Migration by soaring or flapping flight in birds: the relative importance of energy cost and speed. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 342, 353–361.
| Migration by soaring or flapping flight in birds: the relative importance of energy cost and speed.Crossref | GoogleScholarGoogle Scholar |
Hedenström, A., and Alerstam, T. (1995). Optimal flight speed of birds. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 348, 471–487.
| Optimal flight speed of birds.Crossref | GoogleScholarGoogle Scholar |
Hein, A. M., Hou, C., and Gillooly, J. F. (2012). Energetic and biomechanical constraints on animal migration distance. Ecology Letters 15, 104–110.
| Energetic and biomechanical constraints on animal migration distance.Crossref | GoogleScholarGoogle Scholar | 22093885PubMed |
Kays, R., Crofoot, M. C., Jetz, W., and Wikelski, M. (2015). Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478.
| Terrestrial animal tracking as an eye on life and planet.Crossref | GoogleScholarGoogle Scholar | 26068858PubMed |
Kenward, R. E., Clarke, R. T., Hodder, K. H., and Walls, S. S. (2001). Density and linkage estimators of home range: nearest‐neighbor clustering defines multinuclear cores. Ecology 82, 1905–1920.
| Density and linkage estimators of home range: nearest‐neighbor clustering defines multinuclear cores.Crossref | GoogleScholarGoogle Scholar |
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2015). Package ‘lmerTest’. Test fpr random and fixed effects for linear mixed effect models (lmer objects of lme4 package) R package version 2 software.
Lenth, R. V. (2016). Least-squares means: the R package lsmeans. Journal of Statistical Software 69, 1–33.
| Least-squares means: the R package lsmeans.Crossref | GoogleScholarGoogle Scholar |
Mazerolle, M. J., and Mazerolle, M. M. J. (2017). ‘Package ‘AICcmodavg’: Model Selection and Multimodel Inference Based on (Q)AIC(c).’ Available at https://cran.r-project.org/web/packages/AICcmodavg/index.html [verified 27 July 2018].
McDuie, F., Casazza, M. L., Overton, C. T., Herzog, M. P., Hartman, C. A., Peterson, S. H., Feldheim, C. L., and Ackerman, J. T. (2019). GPS tracking data reveals daily spatio-temporal movement patterns of waterfowl. Movement Ecology 7, 6.
| GPS tracking data reveals daily spatio-temporal movement patterns of waterfowl.Crossref | GoogleScholarGoogle Scholar | 30834128PubMed |
McGuire, L. P., Jonasson, K. A., and Guglielmo, C. G. (2014). Bats on a budget: torpor-assisted migration saves time and energy. PLoS One 9, e115724.
| Bats on a budget: torpor-assisted migration saves time and energy.Crossref | GoogleScholarGoogle Scholar | 25551615PubMed |
McNab, B. K. (1980). Food habits, energetics, and the population biology of mammals. American Naturalist 116, 106–124.
| Food habits, energetics, and the population biology of mammals.Crossref | GoogleScholarGoogle Scholar |
Meinertzhagen, R. (1955). The speed and altitude of bird flight (with notes on other animals). The Ibis 97, 81–117.
| The speed and altitude of bird flight (with notes on other animals).Crossref | GoogleScholarGoogle Scholar |
Miller, M. R., Takekawa, J. Y., Fleskes, J. P., Orthmeyer, D. L., Casazza, M. L., Haukos, D. A., and Perry, W. M. (2005). Flight speeds of northern pintails during migration determined using satellite telemetry. The Wilson Bulletin 117, 364–374.
| Flight speeds of northern pintails during migration determined using satellite telemetry.Crossref | GoogleScholarGoogle Scholar |
Miller, M. R., Takekawa, J. Y., Battaglia, D. S., Golightly, R. T., and Perry, W. M. (2010). Spring migration and summer destinations of northern pintails from the coast of southern California. The Southwestern Naturalist 55, 501–509.
| Spring migration and summer destinations of northern pintails from the coast of southern California.Crossref | GoogleScholarGoogle Scholar |
Miller, M. L., Ringelman, K. M., Schank, J. C., and Eadie, J. M. (2014). SWAMP: an agent-based model for wetland and waterfowl conservation management. Simulation 90, 52–68.
| SWAMP: an agent-based model for wetland and waterfowl conservation management.Crossref | GoogleScholarGoogle Scholar |
Nudds, R. L., and Bryant, D. M. (2000). The energetic cost of short flights in birds. The Journal of Experimental Biology 203, 1561–1572.
| 10769218PubMed |
Pennycuick, C. J. (1969). The mechanics of bird migration. The Ibis 111, 525–556.
| The mechanics of bird migration.Crossref | GoogleScholarGoogle Scholar |
Pennycuick, C. J. (1975). Mechanics of flight. Avian Biology 5, 1–73.
Pennycuick, C. J. (1978). Fifteen testable predictions about bird flight. Oikos 30, 165–176.
| Fifteen testable predictions about bird flight.Crossref | GoogleScholarGoogle Scholar |
Phillips, R. A., Jose, C. X., and Croxall, J. P. (2003). Effects of satellite transmitters on albatrosses and petrels. The Auk 120, 1082–1090.
| Effects of satellite transmitters on albatrosses and petrels.Crossref | GoogleScholarGoogle Scholar |
Pianka, E. R. (1981). Resource acquisition and allocation among animals. In ‘Physiological Ecology: an Evolutionary Approach to Resource Use’. (Eds C. R. Townsend, and P. Calow.) pp. 300–314. (Blackwell Scientific Publishers: Oxford, UK.)
Pietz, P. J., Krapu, G. L., Greenwood, R. J., and Lokemoen, J. T. (1993). Effects of harness transmitters on behavior and reproduction of wild mallards. The Journal of Wildlife Management 57, 696–703.
| Effects of harness transmitters on behavior and reproduction of wild mallards.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2016). ‘R: a Language and Environment for Statistical Computing.’ (R: Foundation for Statistical Computing: Vienna, Austria.)
Raikow, R. J. (1973). Locomotor mechanisms in North American ducks. The Wilson Bulletin 85, 295–307.
Sapir, N., Butler, P. J., Hedenström, A., and Wikelski, M. (2011). Energy gain and use during animal migration. In: ‘Animal Migration A Synthesis’. (Eds E. J. Milner-Gulland, J. M. Fryxell, and A. R. E. Sinclair.) pp. 52–67. (Oxford University Press: Oxford, UK.)
Satterthwaite, F. E. (1946). An approximate distribution of estimates of variance components. Biometrics Bulletin 2, 110–114.
| An approximate distribution of estimates of variance components.Crossref | GoogleScholarGoogle Scholar | 20287815PubMed |
Savile, O. (1957). Adaptive evolution in the avian wing. Evolution 11, 212–224.
| Adaptive evolution in the avian wing.Crossref | GoogleScholarGoogle Scholar |
Stasinopoulos, D. M., and Rigby, R. A. (2007). Generalized additive models for location scale and shape (GAMLSS) in R. Journal of Statistical Software 23, 1–46.
| Generalized additive models for location scale and shape (GAMLSS) in R.Crossref | GoogleScholarGoogle Scholar |
Tucker, V. A. (1971). Flight energetics in birds. American Zoologist 11, 115–124.
| Flight energetics in birds.Crossref | GoogleScholarGoogle Scholar |
Tucker, V. A., and Schmidt-Koenig, K. (1971). Flight speeds of birds in relation to energetics and wind directions. The Auk 88, 97–107.
| Flight speeds of birds in relation to energetics and wind directions.Crossref | GoogleScholarGoogle Scholar |
Wilmers, C. C., Isbell, L. A., Suraci, J. P., and Williams, T. M. (2017). Energetics‐informed behavioral states reveal the drive to kill in African leopards. Ecosphere 8, e01850.
| Energetics‐informed behavioral states reveal the drive to kill in African leopards.Crossref | GoogleScholarGoogle Scholar |
Winship, A. J., Trites, A. W., and Rosen, D. A. (2002). A bioenergetic model for estimating the food requirements of Steller sea lions Eumetopias jubatus in Alaska, USA. Marine Ecology Progress Series 229, 291–312.
| A bioenergetic model for estimating the food requirements of Steller sea lions Eumetopias jubatus in Alaska, USA.Crossref | GoogleScholarGoogle Scholar |