Sympathy for the devil: captive-management style did not influence survival, body-mass change or diet of Tasmanian devils 1 year after wild release
Tracey Rogers A C , Samantha Fox B , David Pemberton B and Phil Wise BA Evolution and Ecology Research Centre, School of BEES, The University of New South Wales, Sydney, NSW 2052, Australia.
B Wildlife Monitoring and Management Section, Wildlife Management Branch, Department of Primary Industries, Parks, Water and Environment, GPO Box 44, Hobart, Tas. 7001, Australia.
C Corresponding author. Email: tracey.rogers@unsw.edu.au
Wildlife Research 43(7) 544-552 https://doi.org/10.1071/WR15221
Submitted: 3 December 2015 Accepted: 2 September 2016 Published: 2 November 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Context: The value of captive breeding for recovery programs of endangered carnivorous mammals is often questioned because of low post-release survival reported for founder animals following translocation.
Aims: The aim of the present study was to test the effect of rearing method on survival and body mass of captive-raised Tasmanian devils (Sarcophilus harrisii) following release on an offshore island. We also compared the post-release diet of these devils with the diet of wild devils on mainland Tasmania, where a similar array of diet items is available.
Methods: Twenty-eight captive-raised devils were released onto the island; 19 had been raised in intensive captive-management facilities (IC) and nine in free-range (22 ha) enclosures (FRE). Survival and body-mass change were compared between IC and FRE for up to 440 days post-release. Devil diet was assessed via scat analysis.
Key results: A high proportion (96%) of the founders survived 1 year post-release. Pre-release captive-rearing method had no effect. Released devils gained an average of 14% of their original body mass, irrespective of captive-rearing method. There was very little difference in the diet of captive-reared devils released onto Maria Island relative to wild mainland devils: Tasmanian pademelon, Thylogale billardierii, was the primary food item for both.
Conclusions: The intensity of captive rearing did not affect the survival of devils released onto Maria Island. This suggests that even devils held in IC facilities retain the innate behaviour required to scavenge and hunt prey, and therefore maintain bodyweight post-release. The lack of any threatening processes on the island is also likely to have contributed to the high survival rate 2 years post-release.
Implications: Our study provided preliminary evidence that the release of captive-raised Tasmanian devils onto off-shore islands is a viable conservation action. Captive-breeding programs and captive-raised founders can play a viable and valuable role in the conservation action plans for recovery programs of endangered carnivorous mammals.
Additional keywords: applied ecology, conservation biology, endangered species, foraging, vertebrates, wildlife management.
Introduction
Translocation programs for threatened species have had variable but often low success, especially those involving carnivores (Griffith et al. 1989; Wolf et al. 1996; Fischer and Lindenmayer 2000; Soorae 2013; Tarszisz et al. 2014), and survival rates decrease when captive-raised animals are used (Mathews et al. 2005; Jule et al. 2008), although the reasons for this are not always clear. In certain taxa (e.g. fish), rapid evolution after a single generation in captivity can result in substantial selection for traits that are beneficial in captivity but reduce the fitness of captive-raised individuals when they are reintroduced into the wild (Christie et al. 2012). Captive animals can lose natural behaviours associated with wild fitness (Snyder et al. 1996; Rabin 2003), particularly behaviours specifically associated with socialisation, foraging and hunting in birds (van Heezik and Ostrowski 2001; Vickery and Mason 2003) and mammals (Stoinski et al. 2003; Mathews et al. 2005). One of the primary underlying causes of translocation failures in captive-raised mammalian carnivores has been starvation (Jule et al. 2008) because of the absence of foraging behaviours. This raises important animal-welfare concerns (Mathews et al. 2005; Tarszisz et al. 2014). Thus, it is critical to examine how captive-bred marsupial carnivores will behave in the wild post-release, particularly at the initial stages of a program.
The Tasmanian devil (Sarcophilus harrisii) is a carnivorous marsupial that scavenges on carcasses and hunts for small prey such as insects, lizards, frogs, birds and small mammals (Pemberton and Renouf 1993; Pemberton et al. 2008). It was listed as endangered under the Environment Protection and Biodiversity Conservation Act (Hawkins et al. 2008) and, across its Tasmanian distribution, it has experienced population declines of 60–80% over a 10-year period (Hawkins et al. 2006; McCallum et al. 2009; DPIPWE, unpubl. data). The reduced population numbers are largely due to devil facial-tumour disease (DFTD; McCallum et al. 2009), which is an infectious fatal cancer (Pearse and Swift 2006). Disease transmission occurs when live tumour cells pass between animals during aggressive interactions over food or during mating (Hamede et al. 2013). The disease is spreading west across Tasmania at a rate of ~5–10 km per year, and has now been detected across the majority of the devil’s geographic range (DPIPWE, unpubl. data). Recovery of individuals after clinical symptoms present has not been documented (Hamede et al. 2013). Alongside DFTD, the synergistic effects of habitat loss, human-related factors (e.g. roadkill) and the increase in introduced predators, such as cats and foxes, are thought to be important in the observed decline. As part of a conservation effort, DFTD-free devils are being bred in captivity in Tasmania and on mainland Australia. This insurance population was established for use in population supplementation projects to boost the genetic diversity and environmental functionality of wild devil populations.
Captive-reared Tasmanian devils released onto the Tasmanian mainland had moderate survival (~42%) 2–8 months post-release (Sinn et al. 2014). Twenty-eight DFTD-free captive devils have been released onto Maria Island (a devil-free Tasmanian island), and the present study examines their survival and diet. Maria Island is ~12 km off the south-eastern coast of Tasmania. Although devils have not been recorded there (Hope 1972), the island has a range of the devil’s prey species and is large enough to theoretically support a large devil population. Ongoing management is required, through additional releases, to maintain genetic diversity, and to make removals once carrying capacity has been reached (DPIPWE 2011). Maria Island was connected to mainland Tasmania as recently as 5000–8000 years ago; thus, it is likely that devils were on Maria Island in the past during glacial periods.
Tasmanian devils are being bred at 35 captive-management institutions using two different methodologies, namely, free-range enclosures (FRE) (22-ha pens) and intensively managed captive facilities (IC; e.g. zoos, sanctuaries, hand-rearing). We predict that the intensity of captive-rearing will influence survival of released animals because the free-range pens provide opportunities for captive devils to hunt and forage, which is not possible in intensively managed facilities. Any loss of natural hunting behaviours is likely to be greatest among animals that have been raised in IC facilities. Reduced hunting abilities for these animals would mean that they would lose more body mass post-release than do animals raised in FRE facilities.
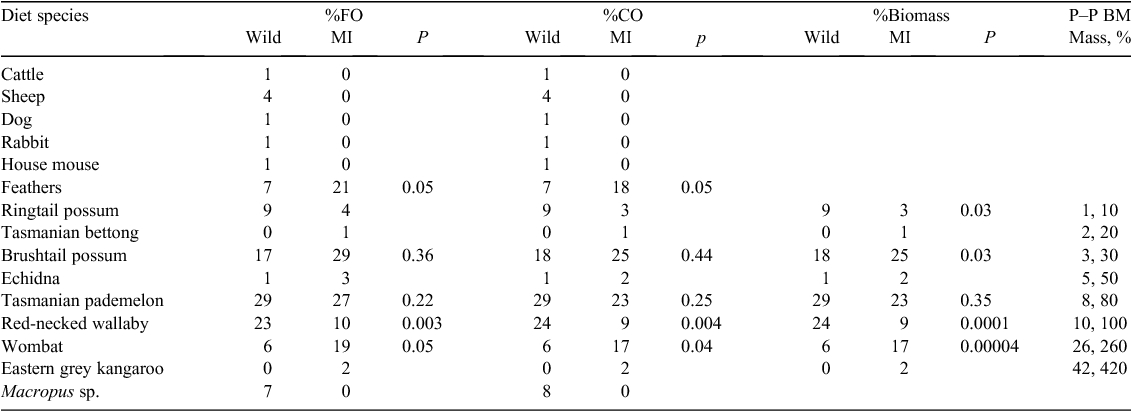
We further predict that captive-rearing will influence the ability of devils to forage in the wild; thus, captive-raised and released devils will have a diet different from that of wild devils. As there are no natural devil populations on other offshore islands, we compared the diet of Maria Island devils to that of animals on mainland Tasmania. Nonetheless, the main diet items recorded for mainland devils (e.g. ringtail possum (Pseudocheirus peregrinus), Tasmanian bettong (Bettongia gaimardi), Tasmanian pademelon, common brushtail possum (Trichosurus vulpecula), short-beaked echidna (Tachyglossus aculeatus), red-necked wallaby (Macropus rufogriseus), common wombat (Vombatus ursinus) and eastern grey kangaroo (Macropus giganteus)) are known to occur on Maria Island, so prey availability is not likely to differ substantially.
The devil takes a broad range of prey, from as small as 0.01 kg (e.g. frogs and moths) to large macropods (Pemberton and Renouf 1993; Pemberton et al. 2008). Compared with other terrestrial mammalian carnivores (Carbone et al. 1999; Tucker and Rogers 2014a, 2014b), the devil, with a body mass of ~8–10 kg for a male, consumes relatively large species compared with its body size. For example, the Tasmanian pademelon, red-necked wallaby, common wombat and eastern grey kangaroo are ~80%, 100%, 260% and 420% of the devil’s body mass respectively. These large species are available as roadkill on the mainland and the devil presumably acts as a scavenger when it feeds on them. Maria Island is a national park, with little road traffic. The translocated Maria Island devils are less likely to use these large species because they may not be available as carrion. We cannot determine whether the method of captive management (IC or FRE) influences the diversity of prey species because we cannot attribute wild-collected scat to an individual devil.
We propose the following three hypotheses: (1) animals that have been raised in intensively managed facilities will have lower survivorship than do animals raised in free-range pens; (2) animals that have been raised in intensively managed facilities will have greater body-mass losses; and (3) captive-raised devils will have a diet different from that of wild devils on the mainland.
Release of captive-raised animals, particularly mammalian carnivores, for conservation programs is risky; however, it is important to make decisions and implement programs while opportunities to act still remain (Martin et al. 2012). In the situation described herein, inaction could result in the loss of the world’s largest extant marsupial carnivore, the Tasmanian devil.
Materials and methods
Animal release
Twenty-eight adult (~1 year of age) Tasmanian devils (13 females and 15 males) were released onto devil-free Maria Island. Nineteen of these animals were reared in IC management facility and nine were reared in 22-ha FRE facilities (Table 1). The release site at Four Mile Creek has a clear flowing stream. The release strategy for both groups was the same. There were two releases, with the first 15 animals released on 12 November 2012 and an additional 13 animals released about 1 year later (between 25 October and 1 November 2013; Table 1). Fifteen animals were selected for the first release because this number allowed for adequate post-release monitoring. The number of IC-raised (n = 7) and FRE-raised (n = 8) animals was similar. The second release was predominantly of IC (n = 12) animals (n = 1, FRE).
Survivorship and body-mass change
The mean life expectancy of wild Tasmanian devils is 5 years (Jones 2001). A baited-trapping and weighing program was conducted monthly for the first 4 months following each release, and every 3 months thereafter. During each trapping session, up to 40 traps were deployed over seven consecutive nights across accessible areas of Maria Island. All activities were conducted in accordance with the standard operating procedures for trapping and handling wild Tasmanian devils (DPIPWE 2011) approved under the Animal Care and Ethics Committee of the Department of Primary Industries, Parks, Water and Environment (DPIPWE 2011), Hobart. The traps were placed in areas considered conducive to regular devil movement (i.e. the intersection of two or more tracks or beside a track where a culvert/drainage/creek crosses underneath) and located in shaded quiet sites. The traps were custom-designed (N. J. M. Mooney and D. Ralph, unpubl. data) PVC pipe traps ~315 mm in diameter and 875 mm long. The traps were inspected throughout the night as well as in the early morning hours. The animals were individually identified by scanning for subcutaneous microchip transponders (Trovan, Microchips Australia, Melbourne, Victoria, Australia) and then weighed.
Diet
Following release, carcasses in variable quantities were used as lure as part of the ongoing monitoring program; an average of 124 kg (s.d. 124 kg) was used per trapping event following the first release and an average of 32 kg (s.d. 29 kg) per trapping event following the second release, for three months after each release event. Trapping schedule is outlined above in ‘Survivorship and body-mass change’. Scat was not collected during the 3 weeks following carcass provisioning. In total, 105 devil-scat samples were collected over 662 days from Maria Island between 19 November 2012 and 12 November 2014. Samples collected in 2014 may have included scats from juvenile animals because young were born on the island. To ensure representative sampling of wild-devil diet, 161 Tasmanian devil-scat samples were collected from five disparate Tasmanian mainland locations, including Fentonbury (n = 14, October to December 2004), Bronte (n = 45, October 2004 to March 2005), Mersey Forest (n = 28, May 2012), Waratah (n = 15, November 2011) and Woolnorth (n = 46, December 2012); scats with unknown locations were also included (n = 13, June 2005 to August 2008).
It was not possible to distinguish between diet items that were prey (known or likely to have been predated on) and those that had been scavenged from carcasses; thus, we refer to all species detected in the scat as diet items. We attempted to identify diet items in the Tasmanian devil scat to the species level. However, bird species were not distinguishable from the feather remains and were considered a single diet-item group, namely ‘feathers’.
To evaluate our prediction that captive-raised devils will have a diet different from that of wild (mainland) devils, we used a range of diversity indices, including the following: species richness (S; Whittaker 1972); Shannon–Wiener diversity (H′) where the H′ index measures the value of any species as a diet item as a function of their frequency in the community; effective number of species (ENS); and Brillouin’s (HB) index where a high HB value represents a high dietary diversity, suggesting that many different species are in the diet and that they are used more evenly (Birillouin 1956). Indices were calculated as follows:
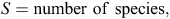

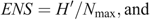
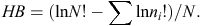
where n = the total number of individual diet items recorded in scat samples, ni = the number of individual dietary items in the ith category, Nmax = the number of individuals in the most abundant species and pi = ni/N.
The frequency of occurrence (FO) of a diet item (species or species group) was expressed as a percentage and calculated as the number of occurrences of a diet item in the scat samples, divided by the total number of scat samples collected. The results were expressed as the percentage occurrence. The CO, the ‘diet item composition’ was the number of occurrences of a species or species group, calculated by dividing the number of times a particular diet item was identified by the total number of occurrences for all diet items.
To convert the FO data to biomass consumption, we multiplied the FO by a linear function (Ackerman’s equation) for each diet species (Ackerman et al. 1984), as follows:
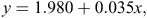
where y = the biomass consumed (kg) that produced a single field-collectable scat sample and x = the average bodyweight of the diet species (kg; Table 2).
|
Ackerman’s equation was developed for felids (specifically cougar (Felis concolor); Ackerman et al. 1984), but has been used for a range of mammalian carnivores, including the crab-eating fox (Cerdocyon thous), ocelot (Leopardus pardalus), puma (Puma concolor) and jaguar (Panthera onca; Farrell et al. 2000). Although the digestive physiology of the Tasmanian devil is likely to differ from that of eutherian carnivores, this equation was used as an approximation.
Statistics
We used a mixed-model ANOVA to examine the mean change in BM (relative to BM at release) over 440 days post-release of 26 of the captive-raised animals (Table 1). Two of the original 28 released animals were excluded from the BM-change analysis, because there were fewer than 4 monthly trapping weights available post-release (trap-shy animals). The animals excluded from analysis were both from the first release, one was an IC and one FRE reared.
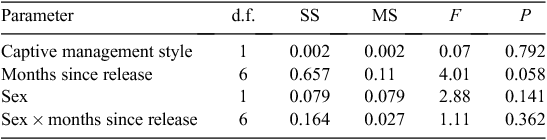
The factors for the mixed-model ANOVA corresponded to a hierarchy of levels, with correlated measurements of the dependent variable (relative BM change) occurring at each level. The fixed factors were assumed to be (1) sex and (2) the rearing style (assuming that the animals were not randomly selected from a larger population of captive animals). Time (months) was assumed to be a random factor, because the trapping of the animals was not a fixed event occurring at regular time intervals, with no missing values. The results of the mixed-model ANOVA were not compromised by violations of the assumptions of normality, because the residuals were normally distributed, or by heteroskedacity, because the variances were homogeneous across the levels (Levene’s test P = 0.376).
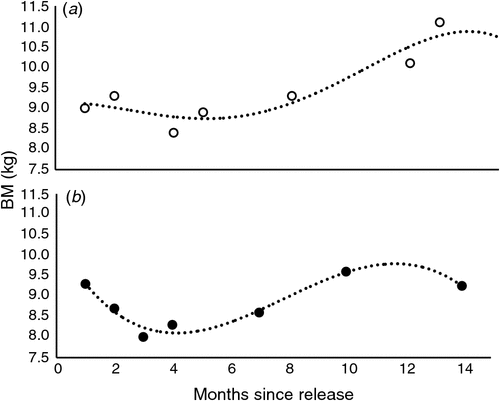
Cubic polynomial regression equations (examples for two devils, Fig. 1a, b) of the following form were computed to describe the patterns of BM change (kg) of each of 26 animals against time (months) post-release:
|
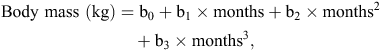
where b0, b1, b2 and b3 are polynomial regression coefficients. The constant b0 is a function of the BM of the devils at the time of release. The first-order regression coefficient b1 is a function of the initial change in BM, immediately following release. The second-order regression coefficient b2 is a function of a subsequent change in BM, several months after release. The third-order regression coefficient b3 is a function of a subsequent change in BM towards the end of the study. For the devils in the first release, in 2012, this is at 825 days post-release, whereas for the devils in the second release, in 2013, this is at 440 days post-release (Table 1). The data were good fits to the polynomial model (indicated by R2-values up to 99.9%).
The polynomial equations defined above do not describe the growth of the animals, but, rather, reflect temporal fluctuations in the BM. The devils were released as adults, so it is presumed that BM change reflects a change in body condition (e.g. loss or gain of soft tissue such as reserves of adipose and muscle tissue) rather than growth. BM gain is presumed to reflect successful foraging, whereas BM loss reflects unsuccessful foraging.
To classify the 26 devils into groups according to their BM-change patterns, we used a hierarchical agglomerative cluster analysis that used the coefficients of the polynomial regression equations (b0, b1, b2 and b3). The clusters were linked using Ward’s method and separated using squared Euclidean distances. A dendrogram was constructed, in which, the further apart the clusters were, the greater were the differences between the patterns of BM change of the animals in each cluster.
Diet
Species diversity curves (Shannon–Wiener (H′) index (Fig. 2a), representing diversity; Brillouin’s (HB) index (Fig. 2b), representing dominant species) showed that the number of diet items in the scat reached an asymptote after analysing ~40 scat samples. This result indicated that the diets of Maria Island (n = 105) and mainland (n = 161) devils were adequately sampled (Fig. 2).
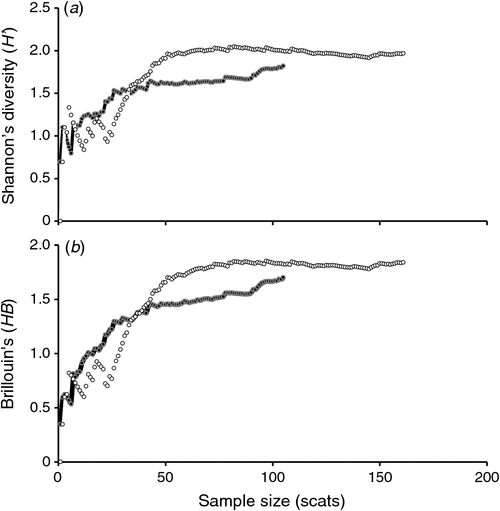
|
Results
Survival
Of the 15 animals in the first release, most (87%, 13 of 15) were alive 825 days post-release (Table 1). One animal, a 2-year-old male (Manny) reared in an IC-management facility, died within the first month after release. A second animal, a 5-year-old male (Jimmy) reared in an FRE facility, was presumed dead because he had not been observed in the trapping program after 460 days. Of the 28 captive-raised devils that were released, 96% (27 of 28) were alive 365 days later (Table 1). The rearing style (IC or FRE) did not influence survival at least up to the time of this report. The mean age of the surviving founders was 3 years (0.9 s.d., n = 26).
Body-mass change
The released devils had an average proportional BM gain of 0.14 (0.17 s.d., range 0.22–0.78, n = 150) over the post-release period of 440 days. The mixed-model ANOVA indicated that none of the factors significantly (at the 0.05 α-level) influenced the mean BM change (relative to BM at release) of the captive-reared devils over the first 440 days post-release (Table 3). However, the effect size was very small (adjusted R2 = 11.85%).
|
The BM changes in each individual animal over time were explored using non-linear regression analysis. Polynomial regression coefficients defined mostly sigmoid-shaped trajectories to explain BM-change patterns (Fig. 1a, b). A dendrogram of a hierarchical cluster analyses, which used the coefficients of the polynomial regression equations (b0, b1, b2 and b3), displayed that one major dichotomy divided the devils into two groups labelled Clusters A and B. The Clusters A and B were separated predominantly according to release date (Table 1), but not according to any of the other factors (e.g. sex, birth year or rearing style). Most animals in Cluster A were from the first group of devils released in 2012. Only the one devil in Cluster A, namely Dylan, had been from the second group released in 2013. All but the one animal in Cluster B were released in 2013, as part of the second release. Jimmy, the oldest devil, a 3-year-old male at the time of release, was the only animal in Cluster B from the first release.
The values of coefficient b0, a function of the body mass at release, was lowest in a group of females in Cluster A, and highest in males cluster within Clusters B and A (Table 1). This is unsurprising because the Tasmanian devil is sexually dimorphic.
Diet
The Maria Island devils consumed significantly (χ2: FO, P = 0.0007; CO, P = 0.001; biomass, P < 0.1000; d.f. = 4; Table 2) different proportions of some species as diet items. They consumed significantly greater proportions of wombat (by FO, CO and biomass), bird (by FO and CO) and brushtail possum (by biomass), but significantly smaller proportions of red-necked wallaby and ringtail possum (by biomass; Table 2) than did the devils from the wild mainland population.
The H′ index was lower for the Maria Island population (H′ = 1.82), indicating that 6.2 species was the effective number of species (ENS) used, compared with an ENS of 7.2 species for the mainland population (H′ = 1.97; Table 2). The HB from all mainland sites (HB = 1.84, n = 161) was slightly higher than that for the Maria Island (HB = 1.70, n = 105) devils. Wombat and bird were important for Maria Island devils, whereas red-necked (Bennet’s) wallaby and ringtail possum were used by mainland devils (Table 2). The captive-raised devils and their offspring did not consume significantly (χ2 = 0.7, d.f. = 1, P = 0.4; Table 2) different numbers of species (S = 9) compared with the wild mainland devils (S = 11).
Discussion
Survival
The method of captive-rearing did not influence survivorship post-release. In contrast to our prediction, Tasmanian devils raised in IC facilities did not experience lower survivorship. The proportion of captive-bred founders surviving on Maria Island was higher (~96% over 2 years) than that observed for animals released on the mainland (~42% over 2–8 months) in the study of Sinn et al. (2014).
A review of 45 carnivore reintroduction programs (17 species across 5 families) that used captive-raised animals as founders found a low (~30%) founder survival success 12 months post release (Jule et al. 2008). More recent translocation programs have reported higher post-release founder success for captive-raised mammalian carnivores than in the studies reviewed by Jule et al. (2008), namely, 61% for the Vancouver Island marmot (Marmota vancouverensis; Aaltonen et al. 2009), 50% for the European mink (Mustela lutreola; Maran et al. 2009) and 53–67% for the Iberian lynx (Lynx pardinus; Simón et al. 2013). Largely because of this low survival of released captive-bred animals, a great deal of debate has surrounded the value of captive-raised founders for conservation programs (Armstrong and Seddon 2008; Jule et al. 2008). The generally low success rate in releases of captive-raised founders, including devils (Sinn et al. 2014), has been a concern for managers of the Tasmanian devil program. Over the long-term, captive-raised devils are an important part of the conservation strategy to maintain devil insurance populations, while DFTD moves through the wild population. Thus, the survivability of captive-raised devils in the wild post-release must be maximised. The Maria Island population, including the devils released, and subsequently born, has risen to more than 90 Tasmanian devils (DPIPWE, unpubl. data, part of a further study) in the 2.5 years since the commencement of the release program. The high post-release survival of the captive-raised devils, which showed little influence from pre-release management methods, is promising; however, several favourable factors influenced the outcome of this translocation program. Human-related activities (shooting, poisoning and car collisions) have directly caused the death of captive-raised founders in over 50% of all translocation programs for mammalian carnivores (Jule et al. 2008) and roadway-related mortalities are significant for devils on the Tasmanian mainland; however, Maria Island has little vehicular traffic. Predation and inter-specific competition and aggression have also reduced founder survivorship in other mammalian carnivore translocations, with ~50% of captive-raised European mink killed by other carnivores and raptors within the first few months following release (Maran et al. 2009), and newly released captive-raised African wild dogs (Lyacon pictus) killed by lions (Moehrenschlager and Somers 2004). Although young devils, less than 12 months old, are vulnerable to predation, adult Tasmanian devils have few natural predators in Tasmania. Because the devils that were released on Maria Island were at least 12 months old, they were less vulnerable to predation.
Body-mass change
In contrast to our prediction, rearing style (intensive or free-range management) did not influence the body condition of devils post-release. On average, the captive-raised devils gained weight during the release period. Although rearing style did not influence BM change, the year of release did. Although devils in the first release gained mass immediately following release, the devils in the second release, 1 year later, lost weight immediately following release. Releases occurred at the same time, in the austral spring (October 2012 and 2013), timed to maximise food availability for newly released devils. The differences in BM change following release between years may have occurred because of a shift in species availability or climatic variability.
Diet
In contrast to our prediction, the captive-raised devils did not have a diet substantially different from that of wild mainland devils. The captive-raised founders and their offspring used a similar broad diversity of diet species and consumed the same dominant food items typical of wild devils from five different Tasmanian locations. The Tasmanian pademelon was the primary food item used by both Maria Island and mainland devils. The Maria Island devils consumed different proportions of some species, such as, for example, greater quantities of wombat, bird and brushtail possum and lower proportions of red-necked wallaby and ringtail possum. This may reflect differences in species availability on the island and the mainland. Although red-necked (Bennett’s) wallaby and ringtail possum are present on the island (DPIPWE data) and important in the diet of mainland devils, their remains were less important in the diet of Maria Island devils.
Bird feathers were found more frequently in and represented a greater proportion of the scats of the Maria Island population than on the mainland. Maria Island is an important nesting site for ground-nesting birds, including short-tailed shearwaters (Puffinus tenuirostris), little penguins (Eudyptula minor) and Cape Barren geese (Cereopsis novaehollandiae). Unfortunately, because the majority of bird bones and feathers were small, they could not be identified to the species level. However, devils on the island were observed by the authors to prey on all three of these ground-nesting bird species.
Devils are considered scavengers that hunt (Owen and Pemberton 2005), and their massive skull can produce high bite forces for their size (Attard et al. 2011), thus allowing them to crack open bones. A large proportion of the diet items used by devils on mainland Tasmania are believed to be carrion. The current Tasmanian mainland landscape has been greatly altered, and a large quantity of available carrion is created by anthropogenic activity, such as road mortality or farmland-generated waste (Owen and Pemberton 2005). Although Maria Island is heavily modified, it does not have these anthropogenically derived resources. As less carrion is available, devils have less opportunity to scavenge on Maria Island than on the mainland, and yet, the larger-sized species, such as the Tasmanian pademelon, common wombat and eastern grey kangaroo, remain important diet items. Thus, it is likely that Maria devils hunt large prey.
Conclusions
Although the survivorship of released captive-raised endangered carnivores has traditionally been low for translocation programs, our study indicated high founder survivorship following the first stage of a release program that employed captive-raised Tasmanian devils. The high post-release survival of the captive-raised devils was irrespective of pre-release rearing style (intensive or free-range). The rearing method did not affect the body-condition change, and captive-raised devils maintained and further increased their weight post-release. The captive-raised founders were not susceptible to starvation as a result of a lack of foraging skills; they had a broad diet similar to the diet of wild mainland devils. Although the program is at an early stage (approximately 2 years post-release), the preliminary indicators are that captive propagation with the subsequent release of captive-reared individuals on devil-free islands can play a viable role in the conservation action for the Tasmanian devil. In specific contexts, captive-breeding programs, including both intensive and free-range management styles, are valuable for recovery programs of endangered carnivorous mammals.
Acknowledgements
Funding was provided by Australian Federal Government to the Save the Tasmanian Devil Program. Phil Iles, Nick Mooney and Oliver Gales are thanked for scat collections and Barbara Triggs for her scat analysis. Oliver and Nick are also thanked for discussions on diet of Tasmanian fauna. T. R. was supported by a grant from Australian Research Council (LP0989933).
References
Aaltonen, K., Bryant, A. A., Hostetler, J. A., and Oli, M. K. (2009). Reintroducing endangered Vancouver Island marmots: survival and cause-specific mortality rates of captive-born versus wild-born individuals. Biological Conservation 142, 2181–2190.| Reintroducing endangered Vancouver Island marmots: survival and cause-specific mortality rates of captive-born versus wild-born individuals.Crossref | GoogleScholarGoogle Scholar |
Ackerman, B. B., Lindzey, F. G., and Hemker, T. P. (1984). Cougar food habits in southern Utah. The Journal of Wildlife Management 48, 147–155.
| Cougar food habits in southern Utah.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P., and Seddon, P. J. (2008). Directions in reintroduction biology. Trends in Ecology & Evolution 23, 20–25.
| Directions in reintroduction biology.Crossref | GoogleScholarGoogle Scholar |
Attard, M. R. G., Chamoli, U., Ferrara, T. L., Rogers, T. L., and Wroe, S. (2011). Skull mechanics and implications for feeding behaviour in a large marsupial carnivore guild: the thylacine, Tasmanian devil and spotted-tailed quoll. Journal of Zoology 285, 292–300.
| Skull mechanics and implications for feeding behaviour in a large marsupial carnivore guild: the thylacine, Tasmanian devil and spotted-tailed quoll.Crossref | GoogleScholarGoogle Scholar |
Birillouin, L. (Ed.) (1956). ‘Science and Information Theory.’ (Academic Press: New York.)
Carbone, C., Mace, G. M., Roberts, S. C., and Macdonald, D. W. (1999). Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288.
| Energetic constraints on the diet of terrestrial carnivores.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnvFSiu70%3D&md5=ffb5edba908a8ff7805d53bfa635f182CAS | 10580498PubMed |
Christie, M. R., Marine, M. L., French, R. A., and Blouin, M. S. (2012). Genetic adaptation to captivity can occur in a single generation. Proceedings of the National Academy of Sciences, USA 109, 238–242.
| Genetic adaptation to captivity can occur in a single generation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVehtr0%3D&md5=f651ee280149883669227d7832646285CAS |
DPIPWE (2011). Proposal to establish a managed population of Devil facial tumour disease (DFTD)-free Tasmanian devils (Sarcophilus harrisii) on Maria Island. Department of Primary Industries, Parks, Water and Environment Internal Document, Tasmania. Available at http://www.parks.tas.gov.au/file.aspx?id=26885 [Accessed: 18 August 2016].
Farrell, L. E., Roman, J., and Sunquist, M. E. (2000). Dietary separation of sympatric carnivores identified by molecular analysis of scats. Molecular Ecology 9, 1583–1590.
| Dietary separation of sympatric carnivores identified by molecular analysis of scats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3crgt1Cmtg%3D%3D&md5=80883747d16f9ec41f3b797974022451CAS | 11050553PubMed |
Fischer, J., and Lindenmayer, D. B. (2000). An assessment of the published results of animal relocations. Biological Conservation 96, 1–11.
| An assessment of the published results of animal relocations.Crossref | GoogleScholarGoogle Scholar |
Griffith, B., Scott, J. M., Carpenter, J. W., and Reed, C. (1989). Translocation as a species conservation tool: status and strategy. Science 245, 477–480.
| Translocation as a species conservation tool: status and strategy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvitlKqsA%3D%3D&md5=de6fb9f4f9b24852ce51ebaf19069fcaCAS | 17750257PubMed |
Hamede, R. K., McCallum, H., and Jones, M. (2013). Biting injuries and transmission of Tasmanian devil facial tumour disease. Journal of Animal Ecology 82, 182–190.
| Biting injuries and transmission of Tasmanian devil facial tumour disease.Crossref | GoogleScholarGoogle Scholar | 22943286PubMed |
Hawkins, C. E., Baars, C., Hesterman, H., Hocking, G. J., Jones, M. E., Lazenby, B., and Mann, D. (2006). Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation 131, 307–324.
| Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii.Crossref | GoogleScholarGoogle Scholar |
Hawkins, C. E., McCallum, H., Mooney, N., Jones, M., and Holdsworth, M. (2008). Sarcophilus harrisii. In ‘IUCN 2014. IUCN Red List of Threatened Species. Version 2014.1’. Available at http://www.iucnredlist.org/details/40540/0 [Accessed: 18 August 2015].
Hope J. H. 1972 Mammals of the Bass Strait Islands. Proceedings of the Royal Society of Victoria 85 163U195
Jones, M. E. (2001). Large marsupial carnivores. In ‘The New Encyclopedia of Mammals’. (Ed. D. Macdonald.) pp. 814–817. (Oxford University Press: Oxford, UK.)
Jones, K. E., Bielby, J., Cardillo, M., Fritz, S. A., O’Dell, J., Orme, C. D. L., Safi, K., Sechrest, W., Boakes, E. H., Carbone, C., and Connolly, C. (2009). PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648.
| PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals.Crossref | GoogleScholarGoogle Scholar |
Jule, K. R., Leaver, L. A., and Lea, S. E. G. (2008). The effects of captive experience on reintroduction survival in carnivores: a review and analysis. Biological Conservation 141, 355–363.
| The effects of captive experience on reintroduction survival in carnivores: a review and analysis.Crossref | GoogleScholarGoogle Scholar |
Maran, T., Põdra, M., Põlma, M., and Macdonald, D. W. (2009). The survival of captive-born animals in restoration programmes: case study of the endangered European mink Mustela lutreola. Biological Conservation 142, 1685–1692.
| The survival of captive-born animals in restoration programmes: case study of the endangered European mink Mustela lutreola.Crossref | GoogleScholarGoogle Scholar |
Martin, T. G., Nally, S., Burbidge, A. A., Arnall, S., Garnett, S. T., Hayward, M. W., Lumsden, L. F., Menkhorst, P., McDonald‐Madden, E., and Possingham, H. P. (2012). Acting fast helps avoid extinction. Conservation Letters 5, 274–280.
| Acting fast helps avoid extinction.Crossref | GoogleScholarGoogle Scholar |
Mathews, F., Orros, M., McLaren, G., Gelling, M., and Foster, R. (2005). Keeping fit on the ark: assessing the suitability of captive-bred animals for release. Biological Conservation 121, 569–577.
| Keeping fit on the ark: assessing the suitability of captive-bred animals for release.Crossref | GoogleScholarGoogle Scholar |
McCallum, H., Jones, M., Hawkins, C., Hamede, R., Lachish, S., Sinn, D. L., Beeton, L., and Lazenby, B. (2009). Transmission dynamics of Tasmanian devil facial tumour disease may lead to disease induced extinction. Ecology 90, 3379–3392.
| Transmission dynamics of Tasmanian devil facial tumour disease may lead to disease induced extinction.Crossref | GoogleScholarGoogle Scholar | 20120807PubMed |
Moehrenschlager, A., and Somers, M. (2004). Canid reintroductions and metapopulation management. In ‘Canids: Foxes, Wolves, Jackals, and Dogs: Status Survey and Conservation Action Plan’. (Ed. C. Sillero-Zubiri, M. Hoffman and D. W. Macdonald.) pp. 289–297. (IUCN/SSC Canid Specialist Group: Gland, Switzerland.)
Owen, D., and Pemberton, D. (2005). ‘The Tasmanian Devil: a Unique and Threatened Animal.’ (Allen and Unwin: Sydney.)
Pearse, A. M., and Swift, K. (2006). Transmission of devil facial-tumour disease. Nature 439, 549.
| Transmission of devil facial-tumour disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpsVCitA%3D%3D&md5=07b38d805b9cf3fbacf02c2ddd7d947dCAS | 16452970PubMed |
Pemberton, D., and Renouf, D. (1993). A field-study of communication and social-behavior of the Tasmanian devil at feeding sites. Australian Journal of Zoology 41, 507–526.
| A field-study of communication and social-behavior of the Tasmanian devil at feeding sites.Crossref | GoogleScholarGoogle Scholar |
Pemberton D. Gales S. Bauer B. Gales R. Lazenby B. Medlock K. 2008 The diet of the Tasmanian devil, Sarcophilus harrisii, as determined from analysis of scat and stomach contents. Papers and Proceedings of the Royal Society of Tasmania 142 13 –22
Rabin, L. A. (2003). Maintaining behavioural diversity in captivity for conservation: natural behaviour management. Animal Welfare 12, 85–94.
| 1:CAS:528:DC%2BD3sXnvV2rtQ%3D%3D&md5=75d01f117e94af78f2ca8a4d3089567dCAS |
Simón, M. A., Arenas-Rojas, R., Rodriguez-Siles, J., García-Tardío, M., Perez-Marin, J., Gil-Sánchez, J. M., and Lopez, G. (2013). Re-introduction of the Iberian lynx, Andalusia, Spain. In ‘Global Re-introduction Perspectives 2013. Further Case Studies from around the Globe’. (Ed. P. S. Soorae.) pp. 120–124. (IUCN/SSC Re-introduction Specialist Group Abu Dhabi and Environment Agency: Abu Dhabi.)
Sinn, D. L., Cawthen, L., Jones, S. M., Pukk, C., and Jones, M. E. (2014). Boldness towards novelty and translocation success in captive-raised, orphaned Tasmanian devils. Zoo Biology 33, 36–48.
| Boldness towards novelty and translocation success in captive-raised, orphaned Tasmanian devils.Crossref | GoogleScholarGoogle Scholar | 24375492PubMed |
Snyder, N. F., Derrickson, S. R., Beissinger, S. R., Wiley, J. W., Smith, T. B., Toone, W. D., and Miller, B. (1996). Limitations of captive breeding in endangered species recovery. Conservation Biology 10, 338–348.
| Limitations of captive breeding in endangered species recovery.Crossref | GoogleScholarGoogle Scholar |
Soorae, P. S. (Ed.) (2013). ‘Global Re-introduction Perspectives 2013. Further Case Studies from around the Globe.’ (SSC Re-introduction Specialist Group and Abu Dhabi, UAE Environment Agency: Abu Dhabi.)
Stoinski, T. S., Beck, B. B., Bloomsmith, M. A., and Maple, T. L. (2003). A behavioral comparison of captive-raised, reintroduced golden lion tamarins and their wild-born offspring. Behaviour 140, 137–160.
| A behavioral comparison of captive-raised, reintroduced golden lion tamarins and their wild-born offspring.Crossref | GoogleScholarGoogle Scholar |
Tarszisz, E., Dickman, C. R., and Munn, A. J. (2014). Physiology in conservation translocations. Conservation Physiology 2, .
| Physiology in conservation translocations.Crossref | GoogleScholarGoogle Scholar | 27293675PubMed |
Tucker, M. A., and Rogers, T. L. (2014a). Examining the prey mass of terrestrial and aquatic carnivorous mammals: minimum, maximum and range. PLoS One 9, e106402.
| Examining the prey mass of terrestrial and aquatic carnivorous mammals: minimum, maximum and range.Crossref | GoogleScholarGoogle Scholar | 25162695PubMed |
Tucker, M. A., and Rogers, T. L. (2014b). Examining predator-prey body size, trophic level and body mass across marine and terrestrial mammals. Proceedings of the Royal Society B: Biological Sciences 281, 2014–2103.
| Examining predator-prey body size, trophic level and body mass across marine and terrestrial mammals.Crossref | GoogleScholarGoogle Scholar |
van Heezik, Y., and Ostrowski, S. (2001). Conservation breeding for reintroductions: assessing survival in a captive flock of houbara bustards. Animal Conservation 4, 195–201.
| Conservation breeding for reintroductions: assessing survival in a captive flock of houbara bustards.Crossref | GoogleScholarGoogle Scholar |
Vickery, S. S., and Mason, G. J. (2003). Behavioural persistence and its implication for reintroduction success. Ursus 14, 35–46.
Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon 21, 213–251.
| Evolution and measurement of species diversity.Crossref | GoogleScholarGoogle Scholar |
Wolf, C. M., Griffith, B., Reed, C., and Temple, S. (1996). Avian and mammalian translocations: update and reanalysis of 1987 survey data. Conservation Biology 10, 1142–1154.
| Avian and mammalian translocations: update and reanalysis of 1987 survey data.Crossref | GoogleScholarGoogle Scholar |