Population regulation of African buffalo in the Mara–Serengeti ecosystem
Holly T. Dublin A and Joseph O. Ogutu B C DA ℅ IUCN ESARO, Wasaa Conservation Centre, PO Box 68200, Nairobi 00200, Kenya.
B University of Hohenheim, Institute of Crop Science, Biostatistics Unit, Fruwirthstrasse 23, 70599 Stuttgart, Germany.
C International Livestock Research Institute (ILRI), Old Naivasha Road, Box 30709, Nairobi 00100, Kenya.
D Corresponding author. Email: jogutu2007@gmail.com
Wildlife Research 42(5) 382-393 https://doi.org/10.1071/WR14205
Submitted: 5 October 2014 Accepted: 16 July 2015 Published: 31 August 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Context: The processes regulating ungulate populations have been the focus of numerous studies. For the African buffalo (Syncerus caffer Sparrman) population inhabiting the Mara–Serengeti ecosystem, rinderpest was the primary regulatory factor up to the mid-1960s. Following reduction of rinderpest and buffalo population increase, interspecific competition for food, notably with cattle and wildebeest (Connochaetes taurinus Burchell), was thought to be the primary regulatory factor in the ecosystem.
Aims: We analysed buffalo population trends and the relationship between buffalo population growth and rainfall and density dependence in the Mara–Serengeti ecosystem and discuss the findings in the context of the key ecosystem processes governing buffalo population dynamics in African savannas, namely, food limitation, competition, predation, disease and land use changes.
Methods: We analysed buffalo population dynamics in the Mara–Serengeti ecosystem in relation to rainfall and density dependence feedback between 1984 and 2010.
Key results: Buffalo population growth was both significantly density-dependent and positively correlated with the dry season rainfall after, but not before, a severe drought in 1993. Buffalo numbers crashed by 48.6% in 1984–85 and by 76.1% in 1993–94 during severe droughts when food availability was lowest and competition with the more numerous cattle and wildebeest was highest.
Conclusions: Recovery of buffalo numbers to pre-drought levels took 8–9 years after the 1984–85 drought but was much slower, with buffaloes numbering merely 36% of their 1993 population (12 895 animals) 18 years after the 1993–94 drought despite intermittent periods of high rainfall, probably due to demographic and/or reproductive factors, heightened competition with livestock, land use changes in the adjoining pastoral ranches, lion predation and recurrent severe droughts.
Implications: Our findings demonstrate how food limitation caused by droughts associated with the hemispheric El Niño–Southern Oscillation can cause severe declines in and threaten the persistence of large ungulate populations. The findings also portray how density-dependent food limitation, competition, predation, land use changes and other factors can accentuate the effect of droughts and greatly prolong population recovery.
Additional keywords: climate change, competition, disease, food limitation, land use change, predation.
Introduction
The ecological processes that structure the ungulate community in the Mara–Serengeti ecosystem have been the focus of many studies since the 1950s. The early studies primarily focussed on the processes that facilitate coexistence of its diverse ungulate community (e.g. Sinclair and Norton-Griffiths 1979; Sinclair and Arcese 1995). Niche differentiation was described for the ungulates at various levels: habitat (Lamprey 1963; Bell 1970; Jarman 1972; Ferrar and Walker 1974; Sinclair 1977), plant species eaten (Field 1968, 1972; Jarman 1971) and plant parts eaten (Gwynne and Bell 1968; Bell 1970; Sinclair 1977). These observations led Sinclair (1977, 1979) to conclude that interspecific competition was the dominant structuring process in the Serengeti ungulate community. However, these studies were largely conjectural as they were not based on firm empirical evidence. The link between the described overlaps or separations and the dynamic effects were not always substantiated; it may not have been limited resources that caused the described effects on population growth, densities and/or distribution (Bender et al. 1984). There may have been factors mimicking the effects of competition (Connor and Simberloff 1979; Diamond and Gilpin 1982; Gilpin and Diamond 1982; Simberloff 1983, 1984). Subsequently, re-analyses of the data on the Serengeti ungulate community supported the conclusion that predation plays as much of a role in regulating populations as interspecific competition (Sinclair 1985), thus explaining why the plains zebra (Equus quagga burchellii Gray) population (of 200 000 individuals) remained relatively stable while the wildebeest population expanded from 250 000 to 1.5 million between 1961 and 1985 (Sinclair and Norton-Griffiths 1982; Sinclair et al. 1985).
Despite these studies, the factors underpinning population dynamics of African buffaloes in the Mara–Serengeti ecosystem remain unknown, although natural catastrophes, predation (including poaching by humans), disease, poor recruitment and/or emigration are suggested. Thus, the conclusion that competition alone was of overriding importance in the regulation of the buffalo (Sinclair 1974b, 1977; Dublin et al. 1990a) seems insufficient to explain dramatic changes in the buffalo population in the Mara–Serengeti between 1984 and 2010.
The African buffalo respond strongly to variation in rainfall (Sinclair 1977; Walker et al. 1987; Mills et al. 1995; Ogutu and Owen-Smith 2003; Estes et al 2006), through the effect of rainfall on forage availability and quality (Coe et al. 1976; Boutton et al. 1988; Fritz and Duncan 1994). Extreme food scarcity during severe droughts are often associated with massive die-off of large numbers of grazing ungulates in African savannas. For example, the severe drought of 1993–94 killed ~14 448 (40%) of 36 119 buffalo (Metzger et al. 2010) and a quarter of a million of 1.5 million wildebeest (Mduma et al. 1999) in the Serengeti National Park. Likewise, the 1999–2000 drought, which was also extreme and widespread, killed 1500 buffaloes plus virtually all buffalo calves under 9 months old in the Ngorongoro Crater (Estes et al. 2006). Hence, recurrent severe droughts in the Mara–Serengeti (Ogutu et al. 2008), competition between buffalo and other herbivores, including livestock, plus major land use changes in the pastoral ranches of the Mara (Ogutu et al. 2009) may exacerbate these effects, increasing the susceptibility of buffalo to food scarcity and delaying their population recovery, even after forage supplies have improved. The effect of droughts on buffalo can also be interactive with predation, including poaching. For example, in southern Africa, lions (Panthera leo Linnaeus) apparently switch their prey selection in favour of buffalo after droughts; a selection that is negatively correlated with rainfall, reflecting an increase in the susceptibility of buffalo to predation due to drought conditions, lowered forage availability and body condition (Owen-Smith and Mills 2008).
Limited food and predation are not the only factors that have been offered as explanations for the regulation of buffalo in the Mara–Serengeti ecosystem. Indeed, it is widely recognised that disease, specifically rinderpest, played the primary role in limiting buffalo populations up to the mid-1960s (Sinclair 1973a, 1973b, 1977, 1979; Dobson 1995; Sinclair and Arcese1995). However, rinderpest disappeared from the Mara–Serengeti ecosystem by 1964 through widespread inoculation of cattle, which acted as a reservoir for the virus (Plowright 1982), in the surrounding area. The apparent disappearance of the disease was verified both by the reduction to zero of the percentage of buffalo with rinderpest antibodies (Sinclair 1977) and by the rise in both buffalo and wildebeest populations following this date (Sinclair 1973a, 1974a, 1977). Since then there has been only one documented outbreak of rinderpest in the Mara–Serengeti ecosystem, in 1982 (P. B. Rossiter, unpubl. data; Anderson et al. 1990; Kock et al. 1999). The actual mortality attributed to this outbreak was low, probably because of the restricted area affected by the virus (Anderson et al. 1990).
We tested predictions of hypotheses concerning expected influences of food limitation, density dependence, predation, competition and outbreaks of infectious diseases on the population dynamics of the Mara–Serengeti buffalo. H1: Food limitation hypothesis. We expected to find a positive relationship between the annual buffalo population growth rate and rainfall in the current or preceding year, indexing food availability. Rainfall is the key climatic component governing the production and quality of forage for ungulates in African savannas (Coe et al. 1976; East 1984; Boutton et al. 1988). The wet season rainfall thus determines the amount of grass produced in the wet season and carried over through the dry season as well as the amount of green grass produced in the dry season. H2: Density dependence hypothesis. Buffalo population growth rate is expected to be negatively dependent on prior buffalo population density. We therefore expected the effect of density-dependent food limitation on population growth rate to be negative and stronger before than after the buffalo population crash during the 1993–94 drought. H3: Food competition hypothesis. We expected buffalo to compete with livestock and migratory wildebeest in the dry season and the intensity of the competition to increase over time because of increasing livestock numbers, expansion of their spatial distribution in the Mara pastoral ranches and increasing illegal incursions into the Masai Mara National Reserve. Moreover, gradual exclusion of buffalo from the pastoral ranches by rapid expansion of settlements and intensification of livestock grazing linked to sedentarisation of the formerly semi-nomadic Masai pastoralists (Bhola et al. 2012a, 2012b) should further aggravate the competition. H4: Predation hypothesis. During droughts, when buffalo are already weakened by food scarcity, predation by lions and spotted hyenas (Crocuta crocuta) and by poachers can be expected to accentuate buffalo population crash. H5: Disease hypothesis. Outbreaks of infectious diseases such as rinderpest and anthrax can also exacerbate ungulate population crash during severe droughts.
We analyse fluctuations in buffalo numbers and the relationship between buffalo population growth and rainfall and prior density, focusing on the buffalo population in the northern Serengeti, the Masai Mara National Reserve (MMNR) and its adjoining pastoral ranches. We discuss our findings in the context of the key processes limiting buffalo population dynamics in African savannas, specifically food limitation, competition, predation, disease and land use changes.
Materials and methods
Study area
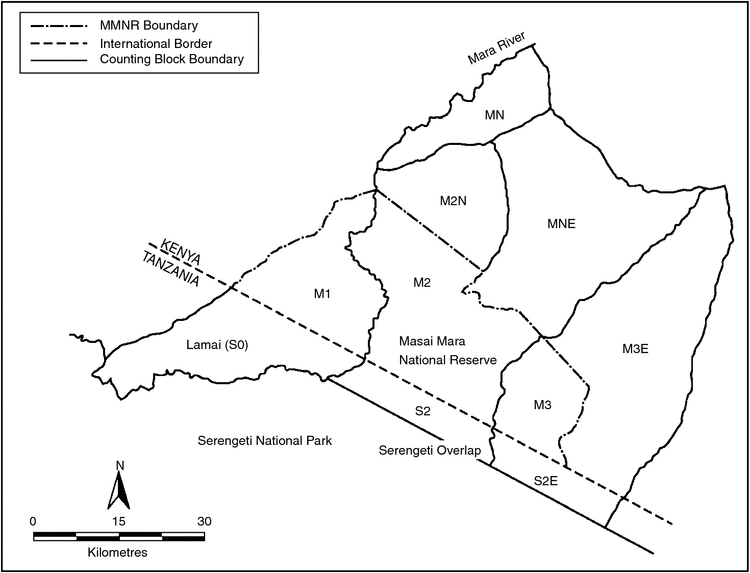
The 25 000 km2 Mara–Serengeti ecosystem lies in the Acacia savanna zone of east Africa and straddles the international boundary between Kenya and Tanzania. The ecosystem has been described in detail by Sinclair (1977), Sinclair and Norton-Griffiths (1979) and Sinclair and Arcese (1995). The location for this study, the northernmost portion of the Mara–Serengeti ecosystem, covered an area of 4238 km2. This included the MMNR (1530 km2), the pastoral ranches surrounding the reserve in Kenya, the Lamai wedge and a small (5-km strip) adjacent area across the northern Serengeti (Fig. 1).
The pastoral ranches of the Mara support large numbers of livestock. The number of livestock in the ranches has been expanding in recent decades. Livestock from the ranches are grazed illegally in the MMNR, especially during droughts, a phenomenon that has become more frequent and acute since 2006, when conversion of large parts of the pastoral ranches adjoining the MMNR into communal wildlife conservancies with controlled livestock access began (Butt et al. 2009; Ogutu et al. 2009, 2011). Apart from livestock numbers, livestock distribution, human population, settlements, fences and cultivation have also expanded in the Mara ranches, resulting in progressive habitat degradation and fragmentation and intensification of land use (Serneels et al. 2001; Sitati 2003; Lamprey and Reid 2004; Ogutu et al. 2009; Bhola et al. 2012a, 2012b).
The vegetation of the Mara has also changed substantially in recent decades (Dublin et al. 1990b; Dublin 1991; Dublin 1995; Obara 1999; Ogutu et al. 2009), a change evidenced by trends in the normalised difference vegetation index during the 1990s and early 2000s and accompanied by progressive desiccation of vegetation (Ogutu et al. 2008). Notably, outside the MMNR, the standing crop of grass was removed primarily by cattle, whereas within the MMNR, the migratory wildebeest in the dry season and frequent illegal cattle grazing throughout the year significantly reduced the standing crop of grass (Dublin 1991; Dublin 1995; Obara 1999). The long-term effects of sustained and heavy grazing pressure, both inside and outside the MMNR, are similar to those reported by Bronner (1990), changing the composition of the existing grassland communities in favour of unpalatable species, with negative repercussions for ungulate populations.
The Mara supports many lions and spotted hyenas (Ogutu et al. 2005). The lions largely depend on buffaloes in the wet season when the migratory ungulates are away in the Serengeti Park. Besides large predators, poachers also kill large ungulates in the Mara, especially along the south-western boundary of the MMNR. The extent and impact of poachers on buffaloes in the Mara is likely higher than is widely recognised because between August 2001 and February 2010 alone, for example, 1500 poachers were arrested and 16 045 snares removed in the Mara Triangle covering some 482 km2 of the western part of the MMNR (http://www.maratriangle.org/monthly-reports/).
Data collection
Rainfall
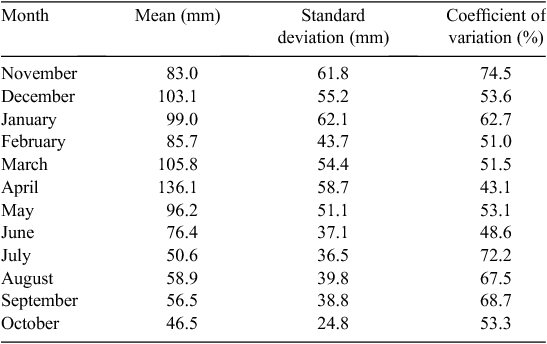
Rainfall is generally bimodal in the Mara, with one long dry season from July to October and a short dry season during January and February. The remainder of the year is generally wet, although the dry season may start in June (Table 1; Norton-Griffiths et al. 1975; Ogutu et al. 2008). Because the calendar year ends in the middle of the wet season, the ‘rainfall year’ runs from November of the previous year to October of the current year and, therefore, begins with the wet season and finishes with the dry season (Norton-Griffiths et al. 1975). Long-term daily rainfall records for the Masai Mara (1965 to 1997) were kept at Keekorok game lodge by the Kenya meteorological department. These were complemented with rainfall data collected by the Masai Mara ecological monitoring program (MMEMP) using a network of 14 gauges between 1989 and 2003, and by the Hyena research camp between 1988 and 2003.
|
Buffalo censuses
Buffalo numbers in the study area were estimated using total aerial photographic counts following methods described by Norton-Griffiths (1978), and were identical to earlier aerial censuses (Sinclair 1973a, 1977; Dublin and Douglas-Hamilton 1987). The accuracy of the method has been verified by Thouless (1992). Between 1984 and 2010, 31 such aerial counts were undertaken at the end of the wet (n = 18 counts) and dry (n = 13) seasons.
The study area was subdivided into blocks of a size suitable for census by one aircraft in a single day (Fig. 1; Dublin et al. 1990a). The blocks were systematically searched from an aircraft flying ~100–150 m above the ground. Flight lines were spaced such that the entire area was covered with more intensive surveying of areas within thick vegetation. To avoid double counting, an overlap zone in adjacent blocks was counted on consecutive days to account for herds moving between blocks. When groups of animals were encountered, their numbers were visually assessed. Those less than 25 were counted immediately, while those over 25 were photographed and counted later from photographic prints. The positions of all groups were plotted on a 1 : 250 000 scale map of the area, making adjustments for the herds that were double counted in adjacent blocks. It was not always possible to distinguish breeding and non-breeding groups of buffalo from the air and hence the analyses included all groups of buffalo.
Over the course of the study there were some slight variations in the areas sampled: between 1984 and 1995 the counts were carried out and analysed by H. T. Dublin. Thereafter, the counts were carried out and analysed by the Kenya wildlife service (KWS). The KWS flights covered slightly different areas. Hence, only data from KWS flights consistent with the areas flown in the previous counts were used in this study. The estimated total population size of buffalo in the Mara Region from 1958 to 2010 based on aerial counts for the dry season (July–October) and the wet season (November–June) are provided in Table S1 in the supplementary materials. The estimated total number of buffalo inside and outside the Masai Mara National Reserve and in a small strip in northern Serengeti in Tanzania, including the Lamai Wedge, from 1984 to 2010 are provided in Table S2 in the supplementary materials.
Data analyses
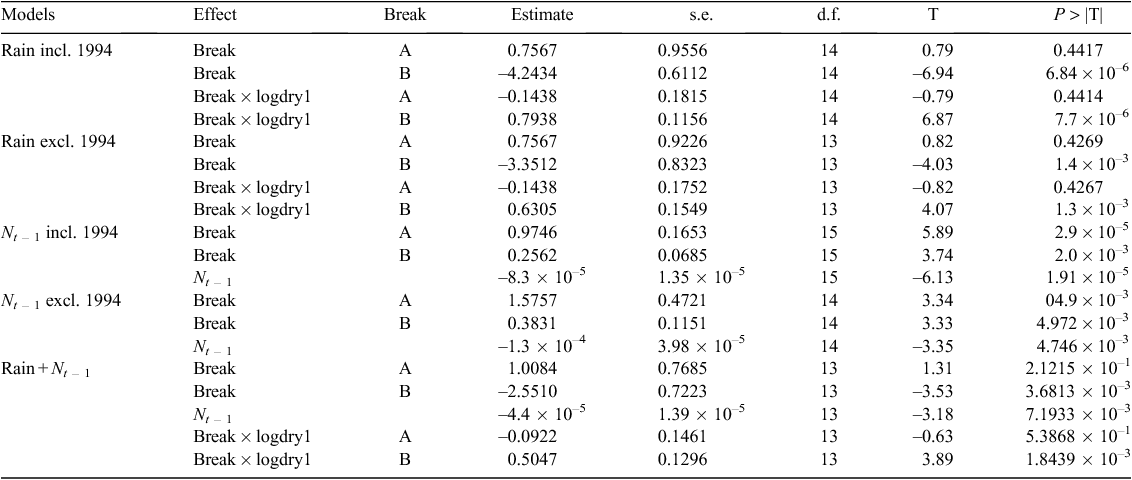
To reduce potential bias due to seasonal difference in buffalo group sizes, only the wet season counts were used for all the analyses. The temporal trend for the Mara buffalo population was modelled using a semiparametric generalised linear mixed model assuming a negative binomial error distribution, a log link function and allowing for an abrupt transition in mean density in 1994 due to the 1993–94 drought. The model incorporated a radial basis smoothing spline covariance structure (Ruppert et al. 2003) and enabled testing for significance of the transition in mean density in 1994 and separate spline smoothing of the time series of buffalo counts for the periods before and after 1994. The radial smoother covariance structure is equivalent to an approximate low-rank thin-plate spline based on the automatic smoother in Ruppert et al. (2003: ch. 13.4–13.5). The denominator degrees of freedom for testing for the significance of the transition in mean population size in 1994 was synthesised using the method of Kenward and Roger (1997). Allowing for potential serial autocorrelation of residuals according to the spatial generalisation of the first-order autoregressive errors model using the spatial exponential model did not improve model fit. The trend model was fitted using the SAS procedure GLIMMIX (SAS Institute Inc. 2014).
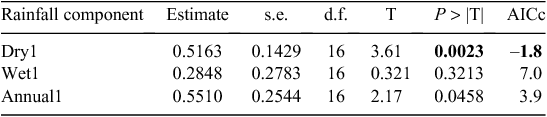
We evaluated evidence for density dependence in buffalo population growth and decomposed the total error variance in the buffalo count totals into contributions due to process noise (demographic error) and observation (counting) error using the Gompertz state-space method of Dennis et al. (2006) fitted by the restricted maximum likelihood method in the MIXED procedure of SAS. We used model selection based on the Akaike information criterion corrected for small sample size (AICc; Burnham and Anderson 2002) and computed by the maximum likelihood method to select the order of density dependence, the rainfall component and the lag most strongly correlated with the buffalo population growth. We considered log-transformed annual, dry and wet season rainfall components singly and jointly (wet and dry) and lagged over one or two years before the count year. Finally, we fitted a model incorporating density dependence, dry season rainfall and the period before and after the 1993 drought. We fitted models allowing for common as well as different slopes for the regression of population growth rate on rainfall and density dependence for the period before and after the crash in the buffalo population size during the 1993–94 drought. We compared the support for the two models using AICc.
Results
Rainfall
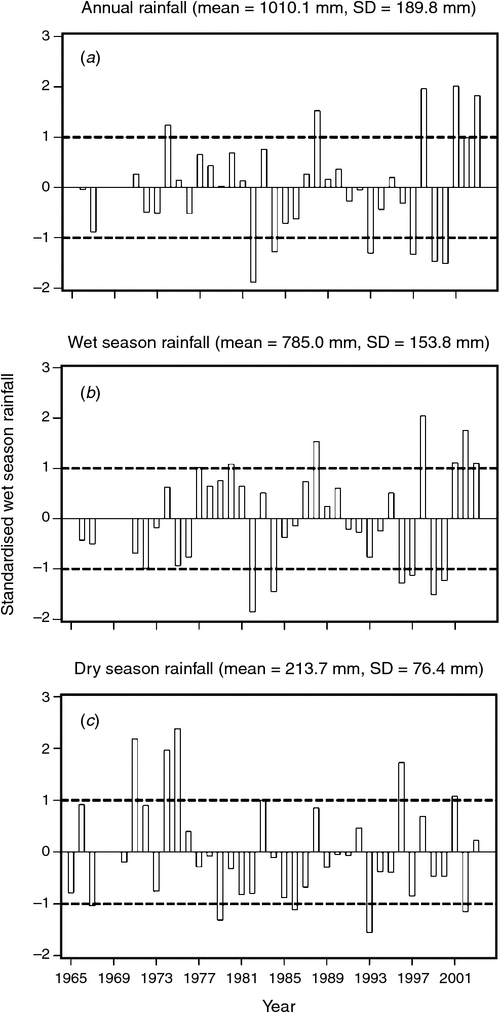
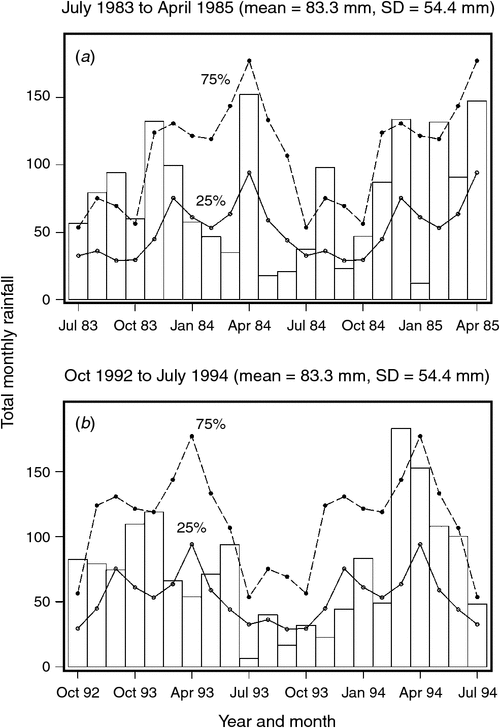
The mean ± 1 sd annual rainfall in the Mara between 1965 and 2003 was 1010.1 ± 187.3 mm (range = 653.4–1391.0 mm; Fig. 2a). Corresponding means for the wet (Fig. 2b) and dry (Fig. 2c) seasons were 785 ± 153.8 mm (range 500.7–1098.7 mm) and 213 ± 76.4 mm (95.4–395.8 mm), respectively. Based on the temporal pattern in annual rainfall between 1965 and 2003 (see also Ogutu et al. 2008) there were severe droughts in the Mara in 1984, 1993, 1997, 1999–2000, 2005–2006 and 2008–2009. The total annual rainfall during these droughts ranged from 723.0 to 767.3 mm or 72–76% of the long-term annual mean. Also, there was an extreme El Niño–Southern Oscillation flood in 1997–98 (Ogutu et al. 2008). However, the distribution of the monthly rainfall and, hence, intensity of the droughts varied markedly during the drought years. Rainfall was generally below the long-term monthly average (from the period 1965–2003) of 83.3 ± 54.4 mm between January and October 1984, and March 1993 and February 1994, when buffalo numbers declined most markedly in the Mara (Fig. 3a, b).
|
|
Buffalo numbers
Buffalo group size varied seasonally. During the dry season, the larger breeding herds split into smaller groups with declining forage availability (mean ± 1 s.e. group size for the wet season: 83.2 ± 4.3, n = 1515 groups; dry season: 49.1 ± 2.4, n = 1994, χ2 = 98.4, df = 1, P < 0.0005).
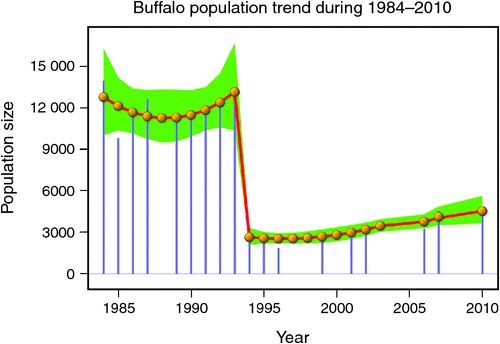
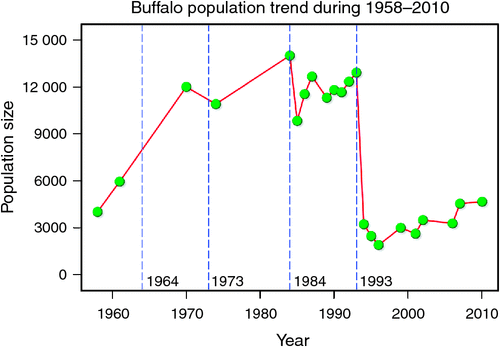
The number of buffaloes in the Mara fluctuated widely between 1984 and 2010 (range: 1885–13 988). There were two major episodes of decline in buffalo numbers: the first between 1984 and 1985, and the second between 1993 and 1994 (Figs 4 and 5). The decline in buffalo numbers between 1993 and 1994 was far more precipitous than that between 1984 and 1985 (Figs 4 and 5). Trend analysis showed that the mean buffalo population size dropped significantly in 1993–94 (F2,4.704 = 10.01, P = 0.0202). Following the decline in numbers in 1984–85, there was a recovery in the buffalo population almost to pre-drought levels within 8–9 years. In contrast, from 1993–94, recovery was delayed and buffalo population size was still merely 36% of the pre-drought population 18 years later in 2010, despite higher than average dry season rainfall levels recorded by the MMEMP in several subsequent years.
|
|
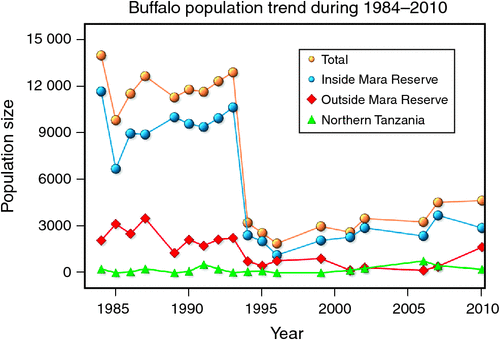
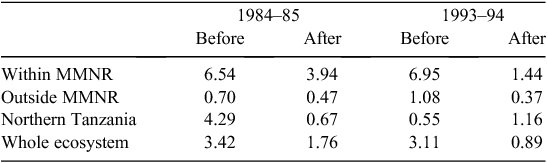
When the data were analysed at finer spatial scales, the decline in 1993–94 was proportionally greater within the MMNR than in the group ranches or in northern Tanzania. Ninety per cent of the net change in buffalo numbers occurred within the MMNR relative to 15% in the group ranches and a 5% increase in northern Tanzania (Figs 4 and 5). These patterns generally compare with those recorded for 1984–85, when the proportional declines in the total buffalo numbers were 56% within the MMNR, 6% in the group ranches and 37% in northern Tanzania. Even after the losses in the 1993–94 drought, buffalo densities remained greater within the MMNR than in the adjoining areas (Table 2).
|
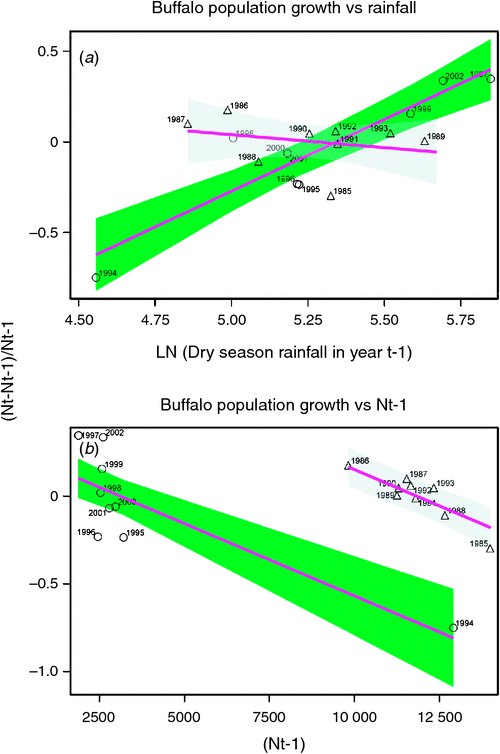
Buffalo population growth was most strongly correlated with rainfall in the dry season (July–October) in the year immediately preceding the count year between 1984 and 2010 (Fig. 6a; Tables 3, 4). However, the dry season rainfall effect was significantly positively correlated with buffalo population growth only after the 1993 drought (Fig. 6; Table 4). In addition, the proportional declines in buffalo numbers (48.6% in 1984–85 and 76.1% in 1993–94) or declines in buffalo density (1.7 animals km–2 in 1984–85 and 2.2 animals km–2 in 1993–94) suggest that the extent of the declines was positively related to the duration of the drought (10 months in 1984–85 and 17 months in 1993–94; Fig. 3a, b).
|
|
|
Following the 1984 decline and up to the 1993 decline, there was an average annual increase in the buffalo population of 10.6% (Figs 4 and 5). In contrast, following the 1993 decline in the buffalo population, the population continued to drop until after 1996 and thereafter the recovery has been slight, despite the higher than average dry season rainfall in 1998, 2001–2003 (Fig. 2a), 2004 and 2007. This apparent delayed or failed recovery was even more pronounced in the areas outside the MMNR and in the northern Serengeti overlap zone. The severe droughts of 1999–2000, 2005–2006 and 2008–2009 all contributed to delaying the recovery of the Mara buffalo. For example, the 1999–2000 drought killed ~405 or 13.2% of the Mara buffalo population.
The decline in buffalo numbers in 1993 resulted in a significant decline in the mean population size (F1, 3.33 = 341.50, P = 0.0002) (Figs 4 and 5). The estimated process (demographic) noise was 1.4108 × 10–2 and the observation (counting) error was 8.3370 × 10–3, showing that process noise constituted 62.9% of the total error in the count totals. Also, there was strong direct density-dependent feedback in the buffalo population growth for both the periods before and after the 1993 drought (Fig. 6b; Table 4). Note that exclusion of the 1994 observation did not materially affect the slope of the regression of population growth rate on density-dependence feedback (Fig. 6b; Table 4). The slope of density dependence was negative and similar for the two periods; only the intercept (or the average population growth rate) differed between the two periods (Fig. 6a; Table 4). Addition of density dependence to the model with the dry season rainfall effect improved the fit (AICc = –11.7) relative to the model with either the dry season rainfall (AICc = –8.3) or density dependence (AICc = –9.2) effect only.
Discussion
We analysed changes in buffalo population growth in relation to rainfall and prior density and inferred the additional contributions of competition, predation, diseases and land use changes to population growth of buffalo inhabiting the northern section of the Mara–Serengeti ecosystem. The Mara buffalo first increased from ~4000 animals in Octover–November 1958 to 5934 animals in May 1961, 11 981 in 1970 to over 10 882 by 1974, before decreasing to fewer than 3000 animals in 1993 (Darling 1960; Talbot and Stewart 1964; Dublin et al. 1990a; Ottichilo et al. 2000; Ogutu et al. 2011, 2015). Similarly, the Serengeti buffalo population declined from 65 000 in 1965 to 19 000 in 1990 (Estes et al. 2006).
The buffalo population counts between 1984 and 2010 indicate that the buffalo population growth rate in the Mara was significantly related to prior population density and the amount of dry season rainfall in the preceding year as predicted by H1 and H2. However, the changes in buffalo numbers were not significantly correlated with prior density before 1994 even though the droughts of 1984–85 occurred in the Mara during a period of high buffalo density, contrary to expectation (H2). The significant negative influence of prior density on population growth after but not before the 1993–94 drought is therefore surprising as we expected density-dependent food limitation to be more severe at high than low buffalo density.
African buffalo typically experience considerable drought-related mortality (Walker et al. 1987; Ogutu and Owen-Smith 2003; Ryan et al. 2007; Ogutu et al. 2014). The effects of drought are most strongly felt when animal populations experience extreme food limitation and either engage in limited movements or occupy habitats that experience drought-related food limitation at some stage on their migration – like the Mara–Serengeti wildebeest. Our finding that the Mara–Serengeti buffalo population is limited by food supply not only supports the food limitation hypothesis (H1) but is also supported by several previous studies in the region (Sinclair 1973b, 1974b, 1977, 1983, 1985; Dublin et al. 1990a). Indeed, following his long-term study on the African buffalo in the Serengeti, Sinclair (1977), argued that the importance of food limitation to buffalo has led to the evolution of specific behavioural strategies and physiological mechanisms to detect and avoid food shortages.
These studies generally assumed that food limitation is mediated through intra and interspecific competition. Although intraspecific competition is not discounted (e.g. Dublin et al. 1990a), interspecific competition is also important in the regulation of buffalo (Sinclair 1977). This is primarily because of the huge disparity in numbers between the competing wildebeest and buffalo populations. By extension, this should also include the cattle populations in the areas surrounding, and frequently grazed illegally in the MMNR, especially during droughts (Butt et al. 2009; Ogutu et al. 2009, 2011). It is noteworthy that buffalo occurred in sizable numbers in the pastoral ranches adjoining the MMNR through the 1970s and 1980s. However, by 1990s and 2000s they were almost completely excluded from the ranches and became largely confined to the MMNR by competition with livestock for forage, human population growth, settlements, cultivation and other land use changes (Serneels et al. 2001; Sitati 2003; Lamprey and Reid 2004; Ogutu et al. 2009; Bhola et al. 2012a, 2012b). This exclusion likely exacerbated the severity of food limitation for buffaloes during the droughts. It is therefore not surprising that, based on 49 aerial counts conducted in the Mara by the Kenya Department of Resource Surveys and Remote Sensing between 1977 and 2009 (Ogutu et al. 2011), declines in buffalo numbers in the MMNR were negatively associated with an increase in cattle numbers in both the MMNR (rs = –0.3248, P = 0.0222, n = 49 surveys) and the ranches (rs = –0.5531, P = 2.4 × 10–5, n = 49). The recent significant temporal changes in the vegetation of the Mara, including in the grassland communities (Dublin et al. 1990b; Dublin 1991, 1995; Obara 1999; Ogutu et al. 2009) and contemporaneous progressive desiccation of vegetation (Ogutu et al. 2008), likely increased the scale of the buffalo declines and amplified competition for forage between buffalo, cattle and migratory wildebeest.
The food scarcity experienced during droughts likely amplified competition between buffalo and other large herbivore species. The outcome of such competitive interactions is often highly asymmetrical when there is a large disparity in numbers of competitors. As a result, we expected the effect of competition to be much more pronounced on the less abundant buffalo than on the more abundant wildebeest and cattle. For buffalo, the effect was probably also exacerbated because buffalo are highly philopatric and remain within their relatively restricted home range even during disturbances (Sinclair 1977). Unlike buffalo, wildebeest and cattle are more mobile, and move widely in response to seasonal fluctuations in rainfall. Populations of the more abundant migratory wildebeest may, however, also be regulated by food limitation operating through intraspecific competition (Sinclair and Norton-Griffiths 1982; Sinclair 1985; Sinclair et al. 1985; Dublin et al. 1990a; Mduma et al. 1999). Competition with yet other herbivore species, most notably hippos (Hippopotamus amphibius), whose numbers have increased dramatically in recent years (Kanga et al. 2011), may have forced buffaloes to travel longer distances to and from water along the Mara river during and after the 1993–94 drought.
Competition can only regulate populations when food is limiting (Keddy 1989; Law and Watkinson 1989). The significant declines in buffalo numbers thus indicate that food limitation became more acute during droughts. It is, therefore, apparent that during these stochastic climatic events the Mara buffalo population was limited through a severe reduction in food availability exacerbated by both intraspecific and interspecific competition, as predicted by H3. Similar patterns of decline in buffalo numbers have also been reported for the Laikipia and Kajiado counties of Kenya, where buffalo numbers were also low in the pastoral lands supporting high densities of cattle (Georgiadis et al. 2007; Ogutu et al. 2013, 2014). In sharp contrast, buffalo numbers increased steadily between 1970 and 2011 in Lake Nakuru National Park, Kenya, which completely excludes livestock and experiences limited direct impacts of land use changes due to an electric perimeter fence (Ogutu et al. 2012).
Predation appears to have played only a minor role in regulating buffalo numbers in Mara–Serengeti since the late 1950s (Sinclair 1974a, 1977, 1985). Even though natural predators, primarily lions, do kill buffaloes (Pienaar 1969; Schaller 1972), predation is likely a secondary cause of the observed declines (Sinclair 1977; Metzger et al. 2010). This suggestion is supported by work carried out in the Kruger National Park, South Africa (Mills et al. 1995; Ogutu and Owen-Smith 2003; Owen-Smith and Mills 2006, 2008; Marshal et al. 2011) and in the Serengeti (Mduma et al. 1999) that showed that rainfall is the primary factor that underpins trends in ungulate populations, including buffalo, but that lion predation increased during droughts when buffaloes were weak and thus more vulnerable (H. T. Dublin, pers. obs.; Owen-Smith and Mills 2008). Hence, like other ungulates, the Mara buffalo became apparently more susceptible to other mortality agents, notably predation, during severe droughts when forage supplies were severely limited, as predicted by H4.
Therefore, although a significant proportion of buffaloes may be killed by predators, our results suggest that buffalo became vulnerable to predation because of severe food scarcity caused by the failure of dry season rainfall in 1993–94. In addition, predation was less likely to be an important factor in the regulation of the buffalo population because the lion population of the Mara–Serengeti ecosystem was reduced significantly by a canine distemper virus epidemic over the period of the 1993–94 drought (Haas et al. 1996; Roelke-Parker et al. 1996; Kock et al. 1998). Nonetheless, the numerous spotted hyenas likely heightened predation pressure on the weakened buffaloes. It is thus likely that many buffalo deaths resulted from predation on weakened animals, and the two episodic buffalo population declines were primarily caused by a severe scarcity of food exacerbated by the large numbers of interspecific competitors within the ecosystem (Braun 1973; Field 1976; Sinclair 1977, 1979; McNaughton and Tarrants 1983), implying an interaction between food scarcity, competition and predation.
In contrast to natural predation, poaching has had significant effects on buffalo populations in certain areas. These include parts of the northern and far western Serengeti that lack effective buffers between the park and neighbouring settlement areas. Here, the 80% and 45% declines in buffalo numbers between 1976 and 1984 and 1986 and 1992, respectively, and more recent declines between 1992 and 2008, were directly attributed to the proximity of the population to human habitation and to killing of animals for meat (Dublin et al. 1990a; TWCM 1992, 1999; Arcese et al. 1995; Hilborn et al. 2006; Metzger et al. 2010). It is conspicuous that these declines in the northern and far western Serengeti occurred at a time when the buffalo population in the Mara ecosystem was increasing (Figs 4 and 5), and far less severe declines were observed in the central and southern Serengeti (Dublin et al. 1990a; TWCM 1992, 1999; Metzger et al. 2010), which are not adjacent to dense human populations and did not show evidence of large-scale poaching during that period.
Disease has been eliminated as a primary cause of the Mara buffalo declines (Kock et al. 1999). Specifically, rinderpest and other diseases, particularly anthrax and bovine tuberculosis, were excluded from causing the 1984–85 or 1993–94 declines in buffalo numbers in the Mara or in the adjacent Serengeti Park (Metzger et al. 2010). Examined animals had high ectoparasite loads, but this was primarily attributed to the poor condition of the animals (P. B. Rossiter and R. Kock, pers. comm.). Accordingly, H5 is not supported.
In conclusion, the factors regulating buffalo numbers in the Mara–Serengeti have apparently changed over time, thus emphasising the dynamic nature of this ecosystem. No single factor explanation seems sufficient; each is contextual, probably relevant only to the specific period under consideration. In particular, the delayed recovery in the Mara buffalo population between 1994 and 2010, despite several years with higher than average rainfall during this time, suggests that factors other than, or in addition to, food limitation may prevent or slow population increase. This contrasts directly with the more immediate recovery of the buffalo population after the 1984–85 drought. A similar situation was recorded in the Kruger National Park, where buffalo numbers declined by 60% during a drought in 1992 (Mills et al. 1995). The resulting buffalo densities in Kruger were similar to those recorded after the 1993–94 drought in the Mara. In both areas there has been little recovery of buffalo numbers to previous levels. This suggests that some factor other than rainfall may be having an important regulatory effect. Though there are concerns over the impacts of bovine tuberculosis on Kruger’s buffalo population (Caron et al. 2003; Cross et al. 2009), both disease and predation are considered density-dependent means of population regulation (e.g. Begon et al. 1990). So these causes can probably be discounted in the current circumstance of low densities.
It may be that at the current low densities – far below those predicted from their respective annual rainfalls (Sinclair 1977: fig. 67, p. 199) – the population is limited by other demographic and/or reproductive constraints such as the repeated failure of annual recruitment into the population and the subsequent effects of greatly reduced numbers in the reproductive age class (e.g. Lande 1987, 1993; Saltz and Rubenstein 1995). Hence, investigation into the population dynamics, including sex and age structures, warrants serious consideration.
Overall, the dynamic nature of the Mara–Serengeti ecosystem and the manifold and shifting processes that drive these dynamics make prediction of future population trends difficult. The consequence of such unpredictability is that management strategies will be dependent on constant and continued monitoring of this population. Effective law enforcement is more critical than ever with the populations at such low levels (Hilborn et al. 2006), particularly if recovery is uncertain. To enhance the chances of a full recovery, interspecific competition, especially during and following drought periods should be reduced as much as possible. Therefore, illegal cattle grazing in the MMNR, heightened in recent times by constriction of grazing areas due to creation of wildlife conservancies in the pastoral ranches adjoining the MMNR should be prevented.
When the extent of the declines we found are considered in the light of other declines in African buffalo populations (e.g. Mills et al. 1995; Ogutu and Owen-Smith 2003; Estes et al. 2006; Owen-Smith and Mills 2006), there appears to be a need for greater vigilance and continued monitoring to avoid the possibility that a species that is perceived to be abundant and widespread falls to levels where its very persistence is threatened.
Acknowledgements
We would like to thank the Office of the President of Kenya, the Narok County Government (and its predecessor Narok County Council), the Kenya Wildlife Service and the Tanzania National Parks for their permission and collaboration in the monitoring of this important buffalo population for 27 years, a joint effort of unprecedented duration. Friends of Conservation (formerly Friends of Masai Mara) largely funded these counts from 1984 to 1995. Between 1996 and 2010 support was provided by the United States Fish and Wildlife Service, Worldwide Fund for Nature (WWF) and the Kenya Wildlife Service (KWS). Over the years, these counts have required the hard work of many flight and ground crews. We extend our appreciation to all the surveying colleagues for their support and friendship. We are proud that the commitment to long-term monitoring lives on. We thank Stuart Williams for assistance during the preparation of this paper. We also thank an anonymous reviewer and the associate editor for comments and suggestions that helped improve an earlier draft of this paper. Ogutu was supported by the German Research Foundation (DFG, Grant # OG 83/1-1). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 641918.
References
Anderson, E. C., Jago, M., Mlengeya, T., Timms, S., Payne, A., and Hirji, K. (1990). A serological survey of rinderpest antibody in wildlife and sheep and goats in northern Tanzania. Epidemiology and Infection 105, 203–214.| A serological survey of rinderpest antibody in wildlife and sheep and goats in northern Tanzania.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3czks1SrtA%3D%3D&md5=1c257e3f7c97d21c909852f688922c11CAS | 2384143PubMed |
Arcese, P., Hando, J., and Campbell, K. (1995). Historical and present-day anti-poaching efforts in Serengeti. In ‘Serengeti II: Dynamics, Management, and Conservation of an Ecosystem’. (Eds A. R. E. Sinclair and P. Arcese.) pp. 506–533. (University of Chicago Press: Chicago, IL.)
Begon, M., Harper, J. L., and Townsend, C. R. (1990). ‘Ecology: Individuals, Populations and Communities.’ (Blackwell Science: Oxford, UK.)
Bell, R. (1970). The use of the herb layer by grazing ungulates in the Serengeti. In ‘Animal Populations in Relation to Their Food Resources’. (Ed. A. Watson.) pp. 111–124. (Blackwell: Oxford, UK.)
Bender, E. A., Case, T. J., and Gilpin, M. E. (1984). Perturbation experiments in community ecology: theory and practice. Ecology 65, 1–13.
| Perturbation experiments in community ecology: theory and practice.Crossref | GoogleScholarGoogle Scholar |
Bhola, N., Ogutu, J. O., Piepho, H.-P., Said, M. Y., Reid, R. S., Hobbs, N. T., and Olff, H. (2012a). Comparative changes in density and demography of large herbivores in the Maasai Mara Reserve and its surrounding human-dominated pastoral ranches in Kenya. Biodiversity and Conservation 21, 1509–1530.
| Comparative changes in density and demography of large herbivores in the Maasai Mara Reserve and its surrounding human-dominated pastoral ranches in Kenya.Crossref | GoogleScholarGoogle Scholar |
Bhola, N., Ogutu, J. O., Said, M. Y., Piepho, H.-P., and Olff, H. (2012b). The distribution of large herbivore hotspots in relation to environmental and anthropogenic correlates in the Mara region of Kenya. Journal of Animal Ecology 81, 1268–1287.
| The distribution of large herbivore hotspots in relation to environmental and anthropogenic correlates in the Mara region of Kenya.Crossref | GoogleScholarGoogle Scholar | 22640527PubMed |
Boutton, T. W., Tieszen, L. L., and Imbamba, S. K. (1988). Seasonal changes in the nutrient of east African grassland vegetation. African Journal of Ecology 26, 103–115.
| Seasonal changes in the nutrient of east African grassland vegetation.Crossref | GoogleScholarGoogle Scholar |
Braun, H. M. H. (1973). Primary production in the Serengeti: purpose, methods and some results of research. Annals of the University of Abidjan 6, 171–188.
Bronner, G. (1990). ‘Vegetation and Land Use in the Matthews Range Area, Samburu District.’ (Gebrüder Borntraeger Verlag: Berlin.)
Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multi-Model Inference.’ 2nd edn. (Springer Verlag: New York, NY.)
Butt, B., Shortridge, A., and WinklerPrins, A. M. G. A. (2009). Pastoral herd management, drought coping strategies and cattle mobility in southern Kenya. Annals of the Association of American Geographers 99, 309–334.
| Pastoral herd management, drought coping strategies and cattle mobility in southern Kenya.Crossref | GoogleScholarGoogle Scholar |
Caron, A., Cross, P. C., and du Toi, J. T. (2003). Ecological implications of bovine tuberculosis in African buffalo herds. Ecological Applications 13, 1338–1345.
| Ecological implications of bovine tuberculosis in African buffalo herds.Crossref | GoogleScholarGoogle Scholar |
Coe, M. J., Cumming, D. H., and Phillipson, J. (1976). Biomass and production of large African herbivores in relation to rainfall and primary production. Oecologia 22, 341–354.
Connor, E., and Simberloff, D. (1979). The assembly of species: chance or competition. Ecology 60, 1132–1140.
| The assembly of species: chance or competition.Crossref | GoogleScholarGoogle Scholar |
Cross, P. C., Heisey, D. M., Bowers, J. A., Hay, C. T., Wolhuter, J., Buss, P., Hofmeyr, M., Miche, A. L., Bengis, R. G., Bird, T. L. F., du Toit, J. T., and Getz, M. W. (2009). Disease, predation and demography: assessing the impacts of bovine tuberculosis on African buffalo by monitoring at individual and population levels. Journal of Applied Ecology 46, 467–475.
| Disease, predation and demography: assessing the impacts of bovine tuberculosis on African buffalo by monitoring at individual and population levels.Crossref | GoogleScholarGoogle Scholar |
Darling, F. F. (1960). ‘An Ecological Reconnaissance of the Mara Plains in Kenya Colony.’ Wildlife Monograph no. 5, 41 pp. (The Wildlife Society: Washington, DC.)
Dennis, B., Ponciano, J. M., Subhash, R. L., Taper, M. L., and Staples, D. F. (2006). Estimating density dependence, process noise, and observation error. Ecological Monographs 76, 323–341.
| Estimating density dependence, process noise, and observation error.Crossref | GoogleScholarGoogle Scholar |
Diamond, J. M., and Gilpin, M. E. (1982). Examination of the null model of Connor and Simberloff for species co-occurrences on islands. Oecologia 52, 64–74.
| Examination of the null model of Connor and Simberloff for species co-occurrences on islands.Crossref | GoogleScholarGoogle Scholar |
Dobson, A. (1995). The ecology and epidemiology of rinderpest virus in the Serengeti and Ngorongoro conservation area. In ‘Serengeti II: Dynamics, Management, and Conservation of an Ecosystem’. (Eds A. R. E. Sinclair and P. Arcese.) pp. 485–505. (University of Chicago Press: Chicago, IL.)
Dublin, H. T. (1991). Dynamics of the Mara–Serengeti woodlands: an historical perspective. Forest Conservation History 35, 169–178.
Dublin, H. T. (1995). Vegetation dynamics in the Mara–Serengeti ecosystem: the role of elephants, fire, and other factors. In ‘Serengeti II: Dynamics, Management, and Conservation of an Ecosystem’. (Eds A. R. E. Sinclair and P. Arcese.) pp. 71–90. (University of Chicago Press, Chicago, IL.)
Dublin, H. T., and Douglas-Hamilton, I. (1987). Status and trends of elephants in the Serengeti-Mara ecosystem. African Journal of Ecology 25, 19–33.
| Status and trends of elephants in the Serengeti-Mara ecosystem.Crossref | GoogleScholarGoogle Scholar |
Dublin, H. T., Sinclair, A. R. E., Boutin, S., Anderson, E., Jago, M., and Arcese, P. (1990a). Does competition regulate ungulate populations: further evidence from Serengeti, Tanzania. Oecologia 82, 283–288.
| Does competition regulate ungulate populations: further evidence from Serengeti, Tanzania.Crossref | GoogleScholarGoogle Scholar |
Dublin, H. T., Sinclair, A. R. E., and McGlade, J. (1990b). Elephants and fire as causes of multiple stable states in the Mara–Serengeti woodlands. Journal of Animal Ecology 59, 1147–1164.
| Elephants and fire as causes of multiple stable states in the Mara–Serengeti woodlands.Crossref | GoogleScholarGoogle Scholar |
East, R. (1984). Rainfall, soil nutrient status and biomass of large African savanna mammals. African journal of Ecology 22, 245–270.
Estes, R. D., Atwood, J. L., and Estes, A. B. (2006). Downward trends in Ngorongoro Crater ungulate populations 1986–2005: conservation concerns and the need for ecological research. Biological Conservation 131, 106–120.
| Downward trends in Ngorongoro Crater ungulate populations 1986–2005: conservation concerns and the need for ecological research.Crossref | GoogleScholarGoogle Scholar |
Ferrar, A. A., and Walker, B. H. (1974). An analysis of herbivore/habitat relationships in Kyle National Park, Rhodesia. Journal of South African Wildlife Management Association 4, 137–147.
Field, C. R. (1968). The food habits of some wild ungulates in Uganda. PhD Thesis, University of Cambridge, UK.
Field, C. R. (1972). Food habits of some wild ungulates in Uganda by analysis of stomach contents. East African Wildlife Journal 10, 17.
| Food habits of some wild ungulates in Uganda by analysis of stomach contents.Crossref | GoogleScholarGoogle Scholar |
Field, C. R. (1976). Palatability factors and nutritive values of the food of buffaloes (Syncerus caffer) in Uganda. East African Wildlife Journal 14, 181–201.
| Palatability factors and nutritive values of the food of buffaloes (Syncerus caffer) in Uganda.Crossref | GoogleScholarGoogle Scholar |
Fritz, H., and Duncan, P. (1994). On the carrying capacity for large ungulates of African savanna ecosystems. Proceedings of the Royal Society of London B: Biological Sciences 256, 77–82.
| 1:STN:280:DyaK2c3nsl2qtQ%3D%3D&md5=e59927ebacf550eb0a7c5865a7ac99daCAS |
Georgiadis, N. J., Olwero, J. N., and Romañach, S. S. (2007). Savanna herbivore dynamics in a livestock-dominated landscape: I. Dependence on land use, rainfall, density, and time. Biological Conservation 137, 461–472.
Gilpin, M. E., and Diamond, J. M. (1982). Factors contributing to non randomness in species co-occurrences on islands. Oecologia 52, 75–84.
| Factors contributing to non randomness in species co-occurrences on islands.Crossref | GoogleScholarGoogle Scholar |
Gwynne, M. D., and Bell, R. H. V. (1968). Selection of vegetation components by grazing ungulates in the Serengeti National Park. Nature 220, 390–393.
| Selection of vegetation components by grazing ungulates in the Serengeti National Park.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1M%2FhsVKqtA%3D%3D&md5=bdf8ac4e29e7c2c243900d5980b783faCAS | 5684885PubMed |
Haas, L., Hofer, H., East, M., Wohlsein, P., Liess, B., and Barrett, T. (1996). Canine distemper virus infection in Serengeti spotted hyaenas. Veterinary Microbiology 49, 147–152.
| Canine distemper virus infection in Serengeti spotted hyaenas.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FhsVyiug%3D%3D&md5=2f7bbfadc1c23b203bdb3a276e0d5e19CAS | 8861651PubMed |
Hilborn, R., Arcese, P., Borner, M., Hando, J., Hopcraft, G., Loibooki, M., Mduma, S., and Sinclair, A. R. E. (2006). Effective enforcement in a conservation area. Science 314, 1266.
| Effective enforcement in a conservation area.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1agt7vF&md5=9b6a35a4107d05a4552c716476fe119fCAS | 17124316PubMed |
Jarman, P. J. (1971). Diets of large mammals in the woodlands around Lake Kariba, Rhodesia. Oecologia 8, 157–178.
| Diets of large mammals in the woodlands around Lake Kariba, Rhodesia.Crossref | GoogleScholarGoogle Scholar |
Jarman, P. J. (1972). Seasonal distribution of large mammal populations in the unflooded middle Zambezi valley. Journal of Applied Ecology 9, 283–299.
| Seasonal distribution of large mammal populations in the unflooded middle Zambezi valley.Crossref | GoogleScholarGoogle Scholar |
Kanga, E. M., Ogutu, J. O., Olff, H., and Santema, P. (2011). Population trend and distribution of the vulnerable common hippopotamus Hippopotamus amphibius in the Mara Region of Kenya. Oryx 45, 20–27.
| Population trend and distribution of the vulnerable common hippopotamus Hippopotamus amphibius in the Mara Region of Kenya.Crossref | GoogleScholarGoogle Scholar |
Keddy, P. A. (1989). ‘Competition.’ (Chapman: London.)
Kenward, M. G., and Roger, J. H. (1997). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53, 983–997.
| Small sample inference for fixed effects from restricted maximum likelihood.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2svntVGitw%3D%3D&md5=bfe664687c7909a6b15d97dee7b2847aCAS | 9333350PubMed |
Kock, R., Chalmers, W. S. K., Mwanzia, J., Chillingworth, C., Wambua, J., Coleman, P. G., and Baxendale, W. (1998). Canine distemper antibodies in lions of the Masai Mara. The Veterinary Record 142, 662–665.
| Canine distemper antibodies in lions of the Masai Mara.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1czjsFWjsw%3D%3D&md5=3111a0d73f6393faf517198cc85382ceCAS | 9670445PubMed |
Kock, R. A., Wambua, J. M., Mwanzia, J., Wamwayi, H., Ndungu, E. K., Barrett, T., Kock, N. D., and Rossiter, P. B. (1999). Rinderpest epidemic in wild ruminants in Kenya 1993–1997. The Veterinary Record 145, 275–283.
| Rinderpest epidemic in wild ruminants in Kenya 1993–1997.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FksVWgug%3D%3D&md5=038921094d38f23579bc7ee71880c93bCAS | 10579537PubMed |
Lamprey, R. H., and Reid, R. S. (2004). Expansion of human settlement in Kenya’s Maasai Mara: what future for pastoralism and wildlife? Journal of Biogeography 31, 997–1032.
Lamprey, H. K. (1963). Ecological separation of large mammal species in Tarangire Game Reserve, Tanzania. East African Wildlife Journal 1, 63–92.
| Ecological separation of large mammal species in Tarangire Game Reserve, Tanzania.Crossref | GoogleScholarGoogle Scholar |
Lande, R. (1987). Extinction thresholds in demographic models of territorial populations. American Naturalist 130, 624–635.
| Extinction thresholds in demographic models of territorial populations.Crossref | GoogleScholarGoogle Scholar |
Lande, R. (1993). Risks of population extinction from demographic and environmental stochasticity and random catastrophes. American Naturalist 142, 911–927.
| Risks of population extinction from demographic and environmental stochasticity and random catastrophes.Crossref | GoogleScholarGoogle Scholar |
Law, R., and Watkinson, A. R. (1989). Competition. In ‘Ecological Concepts’. (Eds J. M. Cherret and R. Law.) (Blackwell Scientific Publications: Oxford, UK.)
Marshal, J. P., Owen-Smith, N., Whyte, I. J., and Stenseth, N. C. (2011). The role of El Niño–Southern Oscillation in the dynamics of a savanna large herbivore population. Oikos 120, 1175–1182.
| The role of El Niño–Southern Oscillation in the dynamics of a savanna large herbivore population.Crossref | GoogleScholarGoogle Scholar |
McNaughton, S. J., and Tarrants, J. L. (1983). Grass leaf silicification: natural selection for and inducible defense against herbivores. Proceedings of the National Academy of Sciences of the United States of America 80, 790–791.
| Grass leaf silicification: natural selection for and inducible defense against herbivores.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cnivVehsQ%3D%3D&md5=d61e87358ecf98f6902fb71d6be93313CAS | 16578767PubMed |
Metzger, K. L., Sinclair, A. R. E., Hilborn, R., Hopcraft, J. G. C., and Mduma, S. A. R. (2010). Evaluating the protection of wildlife in parks: the case of African buffalo in Serengeti. Biodiversity and Conservation 19, 3431–3444.
| Evaluating the protection of wildlife in parks: the case of African buffalo in Serengeti.Crossref | GoogleScholarGoogle Scholar |
Mills, M. G. L., Biggs, H. C., and Whyte, I. J. (1995). The relationship between rainfall, lion predation and population trends in African herbivores. Wildlife Research 22, 75–88.
| The relationship between rainfall, lion predation and population trends in African herbivores.Crossref | GoogleScholarGoogle Scholar |
Mduma, S. A., Sinclair, A. R. E., and Hilborn, R. (1999). Food regulates the Serengeti wildebeest: a 40-year record. Journal of Animal Ecology 68, 1101–1122.
Norton-Griffiths, M. (1978). ‘Counting Animals.’ (African Wildlife Foundation: Nairobi, Kenya.)
Norton-Griffiths, M., Herlocker, D., and Pennycuick, L. (1975). Patterns of rainfall in the Serengeti ecosystem. East African Wildlife Journal 13, 347–374.
| Patterns of rainfall in the Serengeti ecosystem.Crossref | GoogleScholarGoogle Scholar |
Obara, G. A. O. (1999). The changes in the woody vegetation cover of Masai Mara National Reserve, Kenya. MSc Thesis, University of Kent, UK.
Ogutu, J. O., and Owen-Smith, N. (2003). ENSO, rainfall and temperature influences on extreme population declines among African savanna ungulates. Ecology Letters 6, 412–419.
| ENSO, rainfall and temperature influences on extreme population declines among African savanna ungulates.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Bhola, N., and Reid, R. S. (2005). The effects of pastoralism and protection on the density and distribution of carnivores and their prey in the Mara ecosystem of Kenya. Journal of Zoology 265, 281–293.
| The effects of pastoralism and protection on the density and distribution of carnivores and their prey in the Mara ecosystem of Kenya.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Piepho, H.-P., Dublin, H. T., Bhola, N., and Reid, R. S. (2008). El Niño–Southern Oscillation, rainfall, temperature and normalized difference vegetation index fluctuations in the Mara–Serengeti ecosystem. African Journal of Ecology 46, 132–143.
| El Niño–Southern Oscillation, rainfall, temperature and normalized difference vegetation index fluctuations in the Mara–Serengeti ecosystem.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Piepho, H.-P., Dublin, H. T., Bhola, N., and Reid, R. S. (2009). Dynamics of Mara–Serengeti ungulates in relation to land use changes. Journal of Zoology 278, 1–14.
| Dynamics of Mara–Serengeti ungulates in relation to land use changes.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Owen‐Smith, N., Piepho, H.-P., and Said, M. Y. (2011). Continuing wildlife population declines and range contraction in the Mara region of Kenya during 1977–2009. Journal of Zoology 285, 99–109.
| Continuing wildlife population declines and range contraction in the Mara region of Kenya during 1977–2009.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Owen-Smith, N., Piepho, H.-P., Kuloba, B., and Endebe, J. (2012). Dynamics of ungulates in relation to climatic and land use changes in an insularized African savanna ecosystem. Biodiversity and Conservation 21, 1033–1053.
| Dynamics of ungulates in relation to climatic and land use changes in an insularized African savanna ecosystem.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Owen-Smith, N., Piepho, H.-P., Said, M. Y., Kifugo, S., and Reid, R. S. (2013). Changing wildlife populations in Nairobi National Park and adjoining Athi-Kaputiei Plains: collapse of the migratory wildebeest. The Open Conservation Biology Journal 7, 11–26.
| Changing wildlife populations in Nairobi National Park and adjoining Athi-Kaputiei Plains: collapse of the migratory wildebeest.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Piepho, H.-P., Said, M. Y., and Kifugo, S. (2014). Herbivore dynamics and range contraction in Kajiado: climate and land use changes, population pressures, governance, policy and human–wildlife conflicts. Open Journal of Ecology 7, 9–31.
| Herbivore dynamics and range contraction in Kajiado: climate and land use changes, population pressures, governance, policy and human–wildlife conflicts.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J., Piepho, H.-P., Said, M., Ojwang, G., Kifugo, S. C., Wargute, P. W., and Njino, L. W. (2015). Extreme wildlife declines and concurrent increase in livestock numbers in Kenya rangelands. PLoS One, , .
Ottichilo, W. K., de Leeuw, J., Skidmore, A. K., Prins, H. H. T., and Said, M. Y. (2000). Population trends of large non-migratory wild herbivores and livestock in Masai Mara ecosystem, Kenya, between 1977 and 1997. African Journal of Ecology 38, 202–216.
| Population trends of large non-migratory wild herbivores and livestock in Masai Mara ecosystem, Kenya, between 1977 and 1997.Crossref | GoogleScholarGoogle Scholar |
Owen-Smith, N., and Mills, M. G. L. (2006). Manifold interactive influences on the population dynamics of a multispecies ungulate assemblage. Ecological Monographs 76, 73–92.
| Manifold interactive influences on the population dynamics of a multispecies ungulate assemblage.Crossref | GoogleScholarGoogle Scholar |
Owen-Smith, N., and Mills, M. G. L. (2008). Shifting prey selection generates contrasting herbivore dynamics within a large-mammal predator–prey web. Ecology 89, 1120–1133.
| Shifting prey selection generates contrasting herbivore dynamics within a large-mammal predator–prey web.Crossref | GoogleScholarGoogle Scholar | 18481536PubMed |
Pienaar, U. V. (1969). Observations on developmental biology, growth and some aspects of the population biology of African buffalo (Syncerus caffer Sparrman) in the Kruger National Park. Koedoe 12, 29–52.
| Observations on developmental biology, growth and some aspects of the population biology of African buffalo (Syncerus caffer Sparrman) in the Kruger National Park.Crossref | GoogleScholarGoogle Scholar |
Plowright, W. (1982). The effect of rinderpest and rinderpest control on wildlife in Africa. Symposium of the Zoological Society of London 50, 1–28.
Roelke-Parker, M. E., Munson, L., Packer, C., Kock, R., Cleaveland, S., Carpenter, M., Obrien, S. J., Pospischil, A., Hofmann-Lehmann, R., Lutz, H., Mwamengele, G. L. M., Mgasa, M. N., Machange, G. A., Summers, B. A., and Appel, M. J. G. (1996). A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379, 441–445.
| A canine distemper virus epidemic in Serengeti lions (Panthera leo).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XovVagsg%3D%3D&md5=d896645f378d61ccf74932db581fde43CAS | 8559247PubMed |
Ruppert, D., Wand, M. P., and Carroll, R. J. (2003). ‘Semiparametric Regression.’ (Cambridge University Press: Cambridge, UK.)
Ryan, S. J., Knechtel, C. V., and Getz, W. M. (2007). Ecological cues, gestation length, and birth timing in African buffalo (Syncerus caffer). Behavioural Ecology 18, 635–644.
| Ecological cues, gestation length, and birth timing in African buffalo (Syncerus caffer).Crossref | GoogleScholarGoogle Scholar |
Saltz, D., and Rubenstein, D. I. (1995). Population dynamics of a reintroduced Asiatic wild ass (Equus hemionus) herd. Ecological Applications 5, 327–335.
| Population dynamics of a reintroduced Asiatic wild ass (Equus hemionus) herd.Crossref | GoogleScholarGoogle Scholar |
SAS Institute Inc. (2014). ‘SAS System for Windows.’ Version 9.4. (SAS Institute Inc.: Carey, NC.)
Schaller, G. B. (1972). ‘The Serengeti Lion: a Study of Predator-Prey Relations.’ (Chicago University Press: Chicago, IL.)
Serneels, S., Said, M. Y., and Lambin, E. F. (2001). Land cover changes around a major east African wildlife reserve: the Mara ecosystem (Kenya). International Journal of Remote Sensing 22, 3397–3420.
| Land cover changes around a major east African wildlife reserve: the Mara ecosystem (Kenya).Crossref | GoogleScholarGoogle Scholar |
Simberloff, D. (1983). Competition theory, hypothesis testing, and other community ecological buzzwords. American Naturalist 122, 626–635.
| Competition theory, hypothesis testing, and other community ecological buzzwords.Crossref | GoogleScholarGoogle Scholar |
Simberloff, D. (1984). Properties of coexisting bird species in two archipelagos. In ‘Ecological Communities: Conceptual Issues and the Evidence’. (Eds D. R. Strong, D. Simberloff, L. G. Abele and A. B. Thistle.) pp. 234–253. (Princeton University Press: Princeton, NJ.)
Sinclair, A. R. E. (1973a). Population increases of buffalo and wildebeest in the Serengeti. East African Wildlife Journal 11, 93–107.
| Population increases of buffalo and wildebeest in the Serengeti.Crossref | GoogleScholarGoogle Scholar |
Sinclair, A. R. E. (1973b). Regulation and population models for a tropical ruminant. East African Wildlife Journal 11, 307–316.
| Regulation and population models for a tropical ruminant.Crossref | GoogleScholarGoogle Scholar |
Sinclair, A. R. E. (1974a). The natural regulation of buffalo populations in East Africa. III. Population trends and mortality. East African Wildlife Journal 12, 185–200.
| The natural regulation of buffalo populations in East Africa. III. Population trends and mortality.Crossref | GoogleScholarGoogle Scholar |
Sinclair, A. R. E. (1974b). The natural regulation of buffalo populations in East Africa. IV. The food supply as a regulating factor, and competition. East African Wildlife Journal 12, 291–311.
| The natural regulation of buffalo populations in East Africa. IV. The food supply as a regulating factor, and competition.Crossref | GoogleScholarGoogle Scholar |
Sinclair, A. R. E. (1977). ‘The African Buffalo.’ (University of Chicago Press: Chicago, IL.)
Sinclair, A. R. E. (1979). Dynamics of the Serengeti ecosystem: process and pattern. In ‘Serengeti: Dynamics of an Ecosystem’. (Eds A. R. E. Sinclair and M. Norton-Griffiths.) pp. 1–30. (University of Chicago Press: Chicago, IL.)
Sinclair, A. R. E. (1983). The adaptations of African ungulates and their effects on community function. In ‘Ecosystems of the World 13: Tropical Savannas.’ (Ed. F. Bourliere.) pp. 401–426. (Elsevier: Amsterdam.)
Sinclair, A. R. E. (1985). Does interspecific competition or predation shape the African ungulate community? Journal of Animal Ecology 54, 899–918.
| Does interspecific competition or predation shape the African ungulate community?Crossref | GoogleScholarGoogle Scholar |
Sinclair, A. R. E., and Arcese, P. (1995). ‘Serengeti II: Dynamics, Management, and Conservation of an Ecosystem.’ (The University of Chicago Press: Chicago, IL.)
Sinclair, A. R. E., and Norton-Griffiths, M. (1979). ‘Serengeti: Dynamics of an Ecosystem.’ (University of Chicago Press: Chicago, IL.)
Sinclair, A. R. E., and Norton-Griffiths, M. (1982). Does competition or facilitation regulate migrant ungulate populations in the Serengeti? A test of hypotheses. Oecologia 53, 364–369.
| Does competition or facilitation regulate migrant ungulate populations in the Serengeti? A test of hypotheses.Crossref | GoogleScholarGoogle Scholar |
Sinclair, A. R. E., Dublin, H., and Borner, M. (1985). Population regulation of Serengeti wildebeest – a test of the food hypothesis. Oecologia 65, 266–268.
| Population regulation of Serengeti wildebeest – a test of the food hypothesis.Crossref | GoogleScholarGoogle Scholar |
Sitati, N. W. (2003). Human–elephant conflicts in the Maasai Mara dispersal areas of Transmara district. PhD Thesis, University of Kent, UK.
Stewart, D. R. M., and Talbot, L. M. (1962). Census of wildlife on the Serengeti, Mara and Loita plains. East African Agriculture and Forestry Journal 28, 58–60.
Talbot, L. M., and Stewart, D. R. M. (1964). First wildlife census of the entire Mara–Serengeti region, East Africa. The Journal of Wildlife Management 28, 815–827.
| First wildlife census of the entire Mara–Serengeti region, East Africa.Crossref | GoogleScholarGoogle Scholar |
Thouless, C. R. (1992). ‘Samburu and Laikipia Elephant Count 1992.’ (Kenya Wildlife Service Laikipia Elephant Project: Nairobi, Kenya.)
TWCM (1992). ‘Census of Buffalo and Elephant in the Serengeti Ecosystem.’ (Tanzania Wildlife Conservation Monitoring: Arusha, Tanzania.)
TWCM (1999). ‘Total Count of Buffalo and Elephant in the Serengeti Ecosystem, February/March 1998.’ (TWCM/FZS/EU: Arusha, Tanzania.)
Walker, B. H., Emslie, R. H., Owen-Smith, R. N., and Scholes, R. J. (1987). To cull or not to cull: lessons from a southern African drought. Journal of Applied Ecology 24, 381–401.
| To cull or not to cull: lessons from a southern African drought.Crossref | GoogleScholarGoogle Scholar |