Key properties governing sorption–desorption behaviour of poly- and perfluoroalkyl substances in saturated and unsaturated soils: a review
Rai S. Kookana A B * , Divina A. Navarro A B , Shervin Kabiri B and Mike J. McLaughlin B
A B * , Divina A. Navarro A B , Shervin Kabiri B and Mike J. McLaughlin B
A CSIRO Land and Water, Waite Campus, Locked Bag No. 2, Glen Osmond, SA 5064, Australia.
B School of Agriculture, Food and Wine, The University of Adelaide, Waite Campus, Locked Bag No. 1, Glen Osmond, SA 5064, Australia.
Soil Research 61(2) 107-125 https://doi.org/10.1071/SR22183
Submitted: 10 August 2022 Accepted: 3 November 2022 Published: 16 December 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Poly- and perfluoroalkyl substances (PFAS) have been widely used worldwide over the last seven decades in >200 diverse industrial applications. Thousands of different PFAS have been used in a wide range of products, such as food packaging, water-repellent and stain-resistant clothing and fire-fighting foams. Partially due to their extreme stability and high mobility, PFAS are now ubiquitous in the environment. Due to their prolonged persistence, some PFAS have been added to the list of persistent organic pollutants. Sorption is one of the fundamental processes that governs environmental fate and effects of organic chemicals. In recent years, a significant body of literature has been published on sorption of PFAS in soils. However, there are conflicting reports about the soil or sediment properties that may be used to predict the mobility of PFAS in the soil environment. This is not surprising because PFAS have complex chemical properties (anionic, cationic and zwitterionic charges together with surface active properties) that influence their sorption–desorption behaviour. Additionally, PFAS show a fluid–water interfacial adsorption phenomenon and such interfaces offer additional retention mechanisms in unsaturated or oil-contaminated soils. In this review, we analyse the literature on sorption and desorption of PFAS to evaluate the dominant soil and solution properties that govern their sorption–desorption behaviour in saturated and unsaturated soils. We also identify the knowledge gaps that need to be addressed in order to gain a sound understanding of their sorption–desorption behaviour in saturated as well as unsaturated soils.
Keywords: adsorption, air–water interfacial adsorption, desorption, perfluoroalkyl substances, PFAS, polyfluoroalkyl substances, sorption–desorption mechanism, vadose zone soils.
Introduction
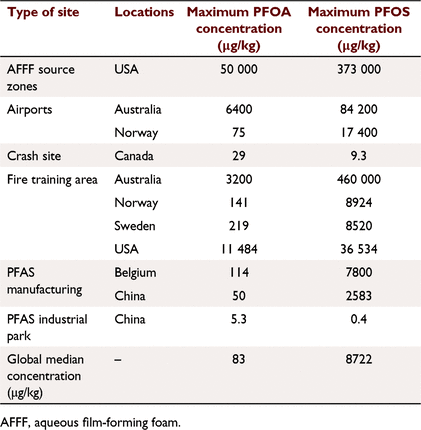
Manufactured over a period of seven decades, several thousand poly- and perfluoroalkyl substances (PFAS) have been introduced in the market globally (Organisation for Economic Co-operation and Development (OECD) 2018). Industrial usage of >1400 different PFAS (with >8000 unique known structures) have been noted to span across >200 diverse applications. Applications of PFAS range from food packaging, water-repellent and stain-resistant clothing to fire-fighting foams (Evich et al. 2022). The diverse range of applications reflect their unique chemical properties (e.g. amphiphilicity and thermal stability) which are, in part, responsible for the environmental concern they engender. Because of their extreme stability (C–F being a very strong bond) and high mobility, PFAS are now ubiquitous in the environment. Soils and sediments often serve as sinks for these chemicals (Brusseau et al. 2020; Sharifan et al. 2021; Wallis et al. 2022). Maximum reported concentrations of PFOS and perfluorooctanoic acid (PFOA) in soils from different countries have been presented in Table 1.
|
While bioaccumulation potential, endocrine disruption and other adverse outcomes for ecological and human health have been identified for PFAS, a full understanding of their toxicological properties is not yet clear (Fenton et al. 2021; Evich et al. 2022). However, due to their prolonged persistence, perfluorooctane sulfonic acid (PFOS) and its salts, perfluorooctane sulfonyl fluoride (PFOSF), PFOA and its salts and related compounds have been added to the list of Persistent Organic Pollutants (POPs) under the Stockholm Convention (United Nations Environment Program (UNEP) 2009). Some other PFAS are currently under consideration for listing.
Sorption–desorption, or partitioning to solid surfaces, is one of the fundamental processes that governs environmental fate and effects of organic chemicals. Partitioning not only determines transport and mobility of chemicals but also influences their bioavailability and uptake and thus moderates their effects on the environment. In recent years, numerous studies have been published on PFAS sorption in soils and sediments. However, a vast majority of these have focussed on perfluoroalkyl acids (PFAAs, e.g. PFOA and PFOS), perhaps because these were among the compounds that were regulated first, including their placement on the Stockholm Convention’s List of POPs.
There are conflicting reports in the literature on what soil properties may be used as predictors of PFAS partitioning in the soil environment. This is not surprising as PFAS have complex chemistries including various ionic states (anionic, cationic and zwitterionic charges) and surface active properties that influence their partitioning. Sorption or partitioning is often represented via a partitioning/distribution coefficient, or Kd value, where Kd represents the ratio of soil and solution concentrations. Initially, there were attempts to treat PFAS as conventional POPs and utilise organic carbon (OC)-based partition coefficients (Koc = Kd/foc, where foc is the fraction of OC in soil). This has been an attractive approach for conventional POPs, as it allowed extrapolation of data among soils based on foc in soil, which is a commonly measured soil property. However, as the database on PFAS grew it became clear that no single soil property could accurately predict the Kd of PFAS in soils (e.g. Li et al. 2018). In recent years, the database on sorption of PFAS in soils has grown markedly (e.g. Knight et al. 2019; Umeh et al. 2021) thus allowing a better understanding of the role of soil properties in governing their behaviour in saturated and unsaturated soils. It is noteworthy that the processes contributing to retention of PFAS in soils differ in soils depending on their degree of saturation (Brusseau 2019). Therefore, the partitioning behaviour of PFAS in saturated and unsaturated soils can be vastly different.
The principal aim of this study is to identify the soil and solution properties that are crucial in determining the partitioning behaviour of PFAS in saturated and unsaturated soils. Here, we analyse the literature to identify the most important properties controlling the retention of PFAS in saturated and unsaturated soils. The findings have a direct bearing on predictions of the mobility of PFAS from source zones in contaminated soils and their transport to ground and surface water. We believe this review will help facilitate the choice of relevant soil properties in transport models for prediction of the fate of PFAS in the soil environment. Recent studies on modelling of transport behaviour of PFAS in historically contaminated soils have highlighted the importance of sorption data under saturated as well as unsaturated conditions in predicting the mobility of PFAS in the real world (Wallis et al. 2022).
The chemistry of PFAS is markedly different from other POPs
The OECD (Organisation for Economic Co-operation and Development (OECD) 2018) recently released a revised definition of PFAS as ‘PFAS are fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom without any H/Cl/Br/I atom attached to it’. The PFAS generally have a hydrophobic ‘tail’ that contains a high proportion of fluorine (C–F chain), a hydrophilic head-group (e.g. carboxylate and sulfonate) and in many cases a ‘spacer’ organic group linking these two portions of the compound together (Buck et al. 2011). Replacing the hydrogen atoms (in a hydrocarbon chain in organic molecules) by fluorine atoms rendered a range of unique chemical properties that enabled this group of chemicals to be used for a diverse range of functions and applications mentioned above.
The PFAS, being a large group of substances (polymers and non-polymers) as solid, liquid and gases, have a broad range of physical, chemical and biological properties (Buck et al. 2021). The chemical properties of PFAS are very different from conventional organic compounds, especially the other POPs and nonpolar compounds. Unique properties of these chemicals include very high strength of the C–F bond, strong polarisation and greater hydrophobicity than alkyl chains of comparable lengths. The addition of functional head-groups increases their water solubility. Consequently, a functionalised fluorochemical has surfactant properties because of both hydrophobic and hydrophilic moieties (Ding and Peijnenburg 2013; Krafft and Riess 2015). The key features of fluorinated surfactants also include their surface activity in both aqueous solvent systems, reduced surface tension, which results in superior wetting, spreading and levelling properties for all types of surfaces, effective emulsification in specialty applications, and extreme stability both chemically and thermally (Buck et al. 2011).
Many of the PFAS compounds (e.g. carboxylates and sulfonates) are present in the anionic form (Table 2) over the environmentally relevant pH range (4–9). These PFAAs are highly water-soluble and consequently have greater mobility in the environment. The most common detections in wastewater treatment, fresh water and ground water systems have been of anionic PFAS such as PFOS, PFOA and perfluorohexanoic acid (PFHxA). Given that the C–F chain makes PFAS hydrophobic, their partitioning properties depend on the chain length. However, several PFAS containing amide and sulfonamido headgroups are cationic in nature (Table 2). Furthermore, some PFAS (e.g. fluorotelomer sulfonamido betaines, FtSaBs) are zwitterions. For example, perfluorooctane amidoalkyl betaine (PFOAB), in addition to a positive charge in its structure (–N+(CH3)2−), contains other ionisable functional groups which theoretically can result in four species with different charges. Clearly, PFAS have more complex chemistries than conventional POPs.

|
Key processes or mechanisms determining PFAS sorption in soils
Solid-phase adsorption is a well-recognised process and perhaps the dominant contributor to the sorption behaviour of PFAS in soils, in both saturated as well as unsaturated soils. Because of their complex chemistry (e.g. amphiphilic and surfactant properties as well as surface activity), the sorption mechanisms of PFAS in soils are more complex than many conventional organic chemicals, especially the non-ionic and highly hydrophobic organic chemicals such as POPs. A variety of mechanisms are responsible for their retention in soils, namely hydrophobic interactions (especially for long C–F chain compounds), electrostatic interactions (especially for ionic and ionisable compounds) and other mechanisms, including fluid–fluid interfacial adsorption as well as absorption and physical retention in inner pores of the solid-phase matrix (Fig. 1). These are discussed in more detail in the following sections. However, a direct evidence base for various mechanisms is still limited due to the relatively small number of mechanistic studies. Additionally, often low concentrations of many of these compounds in the environment makes spectroscopic investigations of the mechanisms difficult.
Electrostatic interactions
As mentioned earlier, PFAAs are anionic in nature, the PFAS containing amide and sulfonamido headgroups are cationic and the FtSaBs are zwitterionic. Therefore, electrostatic interactions of these PFAS with soil solid surfaces can play a dominant role in partitioning (Gao and Chorover 2012; Du et al. 2014; Barzen-Hanson et al. 2017; Mejia-Avendaño et al. 2020).
In soils, many aluminosilicate or phyllosilicate minerals carry permanent negative charges and thus offer significant adsorption sites for positively-charged PFAS (e.g. cationic or zwitterionic compounds) in soils. Organic matter, Fe and Al oxides and edges of phyllosilicate minerals such as kaolinite, have significant amounts of pH-dependent surface charge. The Fe and Al oxides can carry positive charge below their point of zero charge (pH ∼ 8). Therefore, soils rich in these sorbents, particularly Fe and Al oxides (such as in highly weathered soils in tropical and subtropical regions) can electrostatically adsorb PFAS from soil solution. In contrast, the negatively charged PFAS (e.g. PFAAs with a carboxylic acid or sulfonic acid head group) are repelled by the net negatively-charged adsorption sites on clay minerals and thus are generally adsorbed in smaller amounts than cationic or zwitterionic PFAS (depending on their hydrophobic chain lengths) in soils. The substantial body of literature on adsorption of dissolved humic acids on clay minerals may be relevant in understanding the nature of electrostatic interactions that anionic PFAS may undergo during the partitioning process in soils (Feng et al. 2005). Various mechanisms that drive the adsorption of humic acids on clay minerals (e.g. cation bridges, ligand exchange, anion exchange, van der Waals interactions and hydrophobic effects) may be involved in PFAA adsorption.
Du et al. (2014) in their review on sorption behaviour of PFAS concluded that, although a range of mechanisms are potentially relevant for partitioning of PFAS in soils, the electrostatic and hydrophobic mechanisms are expected to be dominant players. They argued that hydrogen bonding, van der Waals and π–π interactions of PFAS in soils and sediments are likely to be insignificant. Lu et al. (2016), while studying the adsorption behaviour of PFOS on nanosized inorganic oxides (Al2O3, Fe2O3, SiO2 and TiO2), noted that coexisting metal ions (Cu2+ and Pb2+) significantly enhanced the adsorption of PFOS on nano-oxides, through a cation bridging effect. The authors ascribed this to metallic cations forming inner-sphere complexes with nano-oxides and thus converting the neutral or negatively charged sites into positively charged sites that adsorbed PFOS anions. The PFOS molecule has been reported to replace the hydroxyl group on Al2O3 by ligand exchange (Wei et al. 2017).
Gao and Chorover (2012) reported that the sulfonate headgroup in PFOS forms outer-sphere complexes with haematite surfaces. This is one of the few spectroscopic studies on adsorption mechanisms, albeit the study had to employ PFAS concentrations in the range of 0.25–1 mM, which is several orders greater than those found in the environment. Campos-Pereira et al. (2020) in their adsorption study on 12 PFAS on ferrihydrite, using X-ray absorption near-edge structure (XANES), found no evidence of the formation of inner-sphere complexes. However, the strong pH-dependence of sorption of PFAS on ferrihydrite in their study was ascribed to the change in zeta potential of ferrihydrite surface with pH.
Recently, Loganathan and Wilson (2022) provided molecular-level insights into the adsorption of short- and long-chain PFAS molecules in mesopores of kaolinite clay through simulations and computations. They concluded that the PFAS molecules were exclusively adsorbed onto the hydroxyl surface of kaolinite. However, the interfacial adsorption and the coordination environment was strongly influenced by the nature of functional groups and hydrophobic chain length of the PFAS. These findings may apply to other minerals with basal hydroxyl groups (e.g. gibbsite).
Hydrophobic interactions
As mentioned earlier, the hydrophobicity of PFAS increases with the hydrophobic C–F chain length and consequently their partitioning on hydrophobic surfaces in soils, such as on soil organic matter, can become significant, especially for long-chain compounds (Fig. 2). Indeed, sorption of some long-chain anionic PFAAs (>C8) in soils may be primarily due to hydrophobic interactions with organic carbon, as the anionic headgroup would not favour adsorption to negatively charged mineral (or organic) surfaces. In contrast, the adsorption of short-chain compounds and ionic or ionisable compounds is primarily driven by the electrostatic interactions with charged surfaces in soil (Nguyen et al. 2020). Hydrophobic interactions between PFAS and the soil solid phase may be influenced by factors such as the ionic strength of the soil solution through salting out effects (Fig. 1) or polyvalent cations in soil solutions that may neutralise the negative charge on the headgroup, thus facilitating hydrophobic interactions of the PFAS tail with uncharged surfaces on soil solids.
|
|
Interfacial accumulation
The PFAS are surface active agents by design, i.e. they can readily form films at fluid–fluid interfaces (such as water–hydrocarbon and air–water). This process can impart additional opportunity for PFAS to accumulate at the air–water interface, which can potentially result in greater retention of PFAS in unsaturated soils. For a range of hydrocarbon surfactants, a significant adsorption on the air–water interface has been observed (e.g. Kim et al. 1998). Because PFAS are fluorinated surfactants they are more surface active than hydrocarbon surfactants and hence this retention process may be significant for PFAS in unsaturated soils (Brusseau 2019). In soils contaminated by PFAS, as well as other non-aqueous-phase liquids (NAPLs), water–hydrocarbon interfacial processes become important and the accumulation of PFAS at the NAPL–water interface may also contribute to the overall retention of PFAS by soils. The relative contribution of interfacial adsorption to overall retention of PFAS in unsaturated soils depends on their solid-phase sorption and degree of saturation in soils. As the solid phase sorption of PFAS increases (such as with increasing chain-length or favourable charge properties) the relative contribution of interfacial adsorption is expected to diminish (Brusseau 2019).
Other retention mechanisms
Other processes that could result in PFAS entrapment in soils include micelle and hemimicelle formation at soil/water surface (at high PFAS concentrations) as well as absorption and physical retention in inner pores of the solid-phase matrix. For example, PFOA can form hemimicelles on an adsorbent surface at 0.01–0.001 times its critical micelle concentration of 15 700 mg/L (Kissa 2001). In relation to physical entrapment, in a study on PFOS and its alternative 6:2 polyfluorinated ether sulfonate (F-53B) in a soil incubated over 240 days, Zhu et al. (2021) observed the formation of non-extractable residue of the two compounds that resisted solvent extraction and alkaline hydrolysis. However, they noted that the residues of both compounds underwent different stages of incorporation (decline in extractable fraction) and remobilisation (increase in extractable fraction). The authors ascribed the formation of nonextractable residue in the case of PFOS to the covalent bonding (via the head group) and strong adsorption (via the tail group), and in the case of F-53B to physical entrapment (sequestration). The remobilisation was attributed to the decomposition of organic matter in soils. Although sequestration of residues of POPs has been commonly observed (Alexander 2000), definitive work on this phenomenon for PFAS is currently lacking.
In addition, accumulation and retention of PFAS can occur at the fluid–fluid interface under both saturated (such as NAPL–water) as well as unsaturated conditions (air–water interfaces), as shown in Fig. 1. Although the partitioning into the air phase (soil atmosphere) has been found to be a significant contributor towards the retention of volatile aromatic compounds in soils (Kim et al. 1998), this phenomenon is expected to be insignificant for most PFAAs, which have low volatility (i.e. the Henry’s constant H in the range of 10−3 even in protonated form and lower in anionic form). In contrast, the H values for some fluorotelomers are much higher (up to 10 000), and for these compounds partitioning into the soil atmosphere from the aqueous phase may be significant.
Factors affecting sorption of PFAS in soils under saturated conditions
The solid-phase sorption in saturated soils is one of most studied process in the literature on organic contaminants and several reviews have been published on sorption of PFAS in soils and sediments (e.g. Zareitalabad et al. 2013; Du et al. 2014; Li et al. 2018; Sharifan et al. 2021).
Sorbate chemistry
Chemistry of PFAS is the single most important property determining their sorption to soils. As mentioned earlier, PFAAs are expected to be anions at ambient pH in most soils with a hydrophobic tail of various C–F chain length (e.g. C4–C12). Their amphiphilic nature is a strong determinant of their partitioning behaviour. Being anionic, they are repelled from negatively-charged surfaces, especially clay minerals. In contrast, PFAAs are attracted by the net positive charge such as those on Al and Fe oxides commonly present in tropical soils, especially in subsoils. There is now a large body of literature on PFAS that demonstrates the dependence of sorption in soils and sediments on the chain length of PFAAs. In addition, the nature of the headgroup of PFAAs (such as carboxylic acid or sulfonic acid) also influences their sorption behaviour, with sulfonates showing greater sorption than corresponding carboxylates (e.g. Higgins and Luthy 2006; Campos Pereira et al. 2018; Nguyen et al. 2020). Higgins and Luthy (2006) noted 0.5–0.6 log unit increase in PFAS partition coefficient on sediments with each increase in CF2 moiety. Similarly, Campos Pereira et al. (2018) reported an increase in PFAS sorption of the order of 0.60 and 0.83 log Koc units per CF2 moiety for perfluorocarboxylates and perfluorosufonates, respectively.
Recently, Nguyen et al. (2020) examined the sorption behaviour of a range of PFAS with diverse chemistry (Fig. 2). Their work showed a strong dependence of sorption of PFAAs on their chain length as well as headgroup. Similarly, while the zwitterions showed greater sorption than their corresponding PFAAs analogues, their sorption was also affected by the nature of functional group. It is noteworthy that new (replacement) PFAS (such as GenX and ADONA) also showed low sorption to soils, generally in the range shown by C4–C5 PFAAs. Sometimes, the partitioning process of polyfluorinated PFAS could be so complex that their sorption behaviour may not be predicted by soil properties alone (Barzen-Hanson et al. 2017).
Soil organic matter and its chemistry
The Koc value has been used extensively in modelling behaviour of organic contaminants in soil due to ease of extrapolation of data between soils based on a single soil property (OC content) and its relationship with chemical parameters such as octanol–water partition coefficient (Kow). However, many studies have since established that the Koc approach is a highly simplified representation of sorption of even non-ionic organic compounds in soils and sediments (Ahmad et al. 2001). However, as mentioned earlier, PFAS are atypical of organic compounds and encompass a range of complex hydrophobic, hydrophilic, surface activity and surfactant-type behaviour which makes it difficult to even measure their hydrophobicity via Kow.
Results from studies on the role of OC in PFAS sorption in soils are not consistent. While some studies have reported significant correlations with soil OC contents (e.g. Milinovic et al. 2015; Oliver et al. 2020a), others have observed that a simple correlation with soil OC does not hold for all systems (e.g. Li et al. 2018; Knight et al. 2019) and it may depend on PFAS chemistry (long-chain versus short-chain) and other factors. In the study by Milinovic et al. (2015), four of the six soils studied contained OC in the range of 3.9–39.0% and the carbon-rich soil perhaps had a stronger leverage on the correlation. The study by Oliver et al. (2020a) employed 19 estaurine coastal sediment samples containing OC in the range of 0.1–11.0%, with a narrow range of pH (6.2–7.7) and high salinity (electrical conductivity of 25.6–46.7 mS/cm). Li et al. (2018) evaluated the relative role of solid phase properties on sorption of PFAS in soils as well as sediments, based on a review of published data. The largest datasets available were for PFOA (n = 147) and PFOS (n = 178), and these analyses showed that OC content of soils and sediments only explained ≤10% variance in Kd of these two compounds. However, this dataset was drawn from various studies on sediments as well as soils, and the methods employed in soil or sediment characterisation were not consistent. The diversity of the sources may have been one reason for such poor correlation with OC in the dataset examined by Li et al. (2018). Since then, two major studies on sorption of PFAS employing a large number of soils have been reported, wherein the soils were characterised in a single laboratory. Knight et al. (2019) studied sorption of radiolabelled PFOA (14C) on 100 Australian soils with diverse physico-chemical properties (e.g. OC range 1–3.5% and silt + clay content range 5–88%) and found that the Kd values did not correlate well with any single soil property. Although OC had the highest R2 value (R2 = 0.36), it only explained about one-third of the variation in PFOA sorption in these soils. Similarly, in a subsequent study on sorption of PFOS on a set of 114 soils from Australia and Fiji, Umeh et al. (2021) found that while total OC showed a significant positive relationship with Kd, it accounted for approximately 35% of variance in PFOS sorption in soils. In both studies, while OC could not explain some two-thirds of variance in sorption of PFOA and PFOS, it still had the dominant effect on sorption of these two PFAAs. The importance of soil OC may increase with increasing size and hydrophobicity of PFAS (Nguyen et al. 2020). Generally, OC contents decline rapidly with depth in soil profiles. The Kd of PFOA also showed a decline with depth in Australian soils (Fig. 3). This shows that OC content, although not the sole predictor, is an important factor affecting sorption of PFAS in soils.
|
|
Carbon chemistry is also expected to play a role in the sorption of PFAS. For example, activated carbon is a very effective sorbent of most PFAS and hence routinely employed in removing PFAS from groundwaters using ‘pump and treat’ method and also for stabilisation of soils (Kabiri et al. 2021; Kah et al. 2021). In a study on 14 PFAS sorption to an organic soil, Campos Pereira et al. (2018) concluded that most long-chain PFAS are preferentially sorbed on highly condensed domains of the humin fraction and sorption is affected by the net charge, and hence pH. In another study, Campos-Pereira et al. (2022), while investigating the role of carbon chemistry (using 13C nuclear magnetic resonance spectroscopy) in sorption of a range of PFAS in three organic soil samples, observed a positive relationship between sorption and the contents of carbohydrates (i.e. O-alkyl carbon) and a negative relationship with pH. Zhao et al. (2014), in a study on sequentially extracted humic substances from a peat soil, found an inverse relationship between PFAS sorption and the contents of aromatic OC as well as phenolic and carboxylic moieties. Oliver et al. (2020a) found no correlation between sorption of PFOS and PFHxS with any of the carbon structures in 19 estuarine sediments.
Umeh et al. (2021) compared the sorption of PFOS by total OC and nonoxidisable organic matter in some soils by treating the soils with persulfate. They found that the Kd of PFOS in treated soils was 2–6 times greater than in untreated soils and concluded that soil organic matter quality also plays an important role in PFAS sorption. This is consistent with previous studies by Zhang et al. (2015) and Campos Pereira et al. (2018), wherein the humin fraction accounted for most of the PFOS sorption in contrast to humic and fulvic acids. Proteinaceous carbon has also been found to favour the sorption of some long-chain PFAAs in soils (Li et al. 2019). Overall, organic carbon in soils remains one of the most important soil properties affecting PFAS sorption. However, the soil mineral matter may also contribute significantly to the sorption of PFAS, especially in soils with low OC or for cationic and zwitterionic PFAS.
Soil minerals (including clays) and their charge characteristics
In a study on 29 PFAS (representing a range of chemistries and charge characteristics) in 10 soils, Nguyen et al. (2020) found that sorption of PFAS in soils decreased in the order zwitterions > sulfonamides > telomers > PFSAs > PFCAs > ethers (Fig. 2). Mejia-Avendaño et al. (2020) carried out a batch sorption study on five anionic, three zwitterionic and one cationic PFAS in five soils. They observed that cationic PFAS (a perfluorooctaneamide ammonium compound) showed an order of magnitude greater sorption than PFOA (an anion). Furthermore, sorption behaviour of some PFAS (e.g. zwitterionic perfluorooctane sulfonamidoalkyl betaine due to its marked change in speciation at around neutral pH) was pH dependent in this study. Adsorption of PFAAs on soils in this study and that by others has been found to be insensitive to pH as they remain anionic in the pH range of most soils (e.g. Oliver et al. 2020b).
Barzen-Hanson et al. (2017) examined the sorption behaviour of aqueous film-forming foams (AFFF) containing anionic FtSs, zwitterionic FtSaBs and cationic 6:2 fluorotelomer sulfonamido amine (FtSaAm) in six soils with varying physico-chemical properties. They observed the greatest sorption of 8:2 FtSaB followed by 8:2 FtS, 6:2 FtSaB and 6:2 FtS. Within each class of compounds, sorption increased with increasing fluorinated chain-length. The increase in log Kd with increasing C–F chain length showed that hydrophobic binding was also contributing to the sorption of these PFAS. For compounds with comparable chain lengths, sorption was dependent on the molecular size, molecular weight and charge properties of compounds. The cationic 6:2 FtSaAm was completely sorbed in all but one soil. A comparison of sorption among various compounds showed that not only the number of positive charges, but also the position of the charge and the net charge of PFAS played role in determining the extent to which the compound was sorbed by the soils.
In another study on sorption of PFOS (an anionic compound) in 114 soils (mostly from the tropical regions of Australia and Fiji), Umeh et al. (2021) observed that variable charge soils carrying net positive charge showed higher sorption than the other soils. Many Fijian soils (63%) showed PFOS Kd values greater than the median value (28 L/kg), whereas only 35% of Australian soils exceeded this value. The sorption behaviour of various PFAS in these studies and in others (reviewed by Li et al. (2018)) is consistent with the electrostatic interactions driven by the permanent negative charge on aluminosilicates and pH-dependent charges on organic matter and Al and Fe oxides/hydroxides in soils.
Soil solution chemistry (pH, ionic strength, cations and DOC)
Soil solution chemistry can have a major effect on the sorption behaviour of PFAS in soils. For example, pH, ionic strength and the nature of polyvalent cations as well as dissolved organic carbon (DOC) can significantly influence the overall net charge of the soil solid phase (e.g. reduced negative electrostatic potential on organic matter). Dissolved organic matter has been found to interact with PFAS molecules or sorption surfaces (e.g. soil and activated carbon) and affect their partitioning onto the solid phase (e.g. Jeon et al. 2011; Qi et al. 2022). The work by Higgins and Luthy (2006) on sorption of PFAS on sediments noted a strong relationship with electrostatic potential for sediment organic matter. Polyvalent cations in soil solution (and on the soil exchange complex) have been found to affect the sorption behaviour of PFAS, through their influence on the electrostatic interactions with the soil solid phase (e.g. negative charge on soil OC and aluminosilicates). Polyvalent ions can enhance PFAS sorption through cation-bridging (e.g. of anionic PFAAs) and through complexation with soil minerals. While monovalent cations such as Na+ and K+ in solution have been found to have little effect on PFAS sorption, polyvalent ions such as Ca2+, Mg2+, Fe2+ and Al3+ have been found to enhance the sorption of PFAS in soils (Higgins and Luthy 2006; Campos Pereira et al. 2018; Cai et al. 2022). Cai et al. (2022) studied the sorption of 18 anionic PFAS (with varying chain lengths and headgroups) in two soils in the presence of a range of ionic strengths of three cations (Na+, Ca2+ and Mg2+). They observed that, at equimolar concentrations, Ca2+ and Mg2+ were more effective than Na+ in enhancing the sorption of several PFAS. The effect of cations was insignificant for some short-chain compounds (e.g. PFPeA, GenX and ADONA). This indicates that the enhancement of sorption involved hydrophobic interactions which were more prominent in the case of long-chain compounds. The Kd of PFAS increased linearly with increasing cation concentration. The authors ascribed this effect to the interplay of electrostatic and hydrophobic interactions, both favouring sorption at higher ionic strengths of divalent cations through reduction of the intramolecular repulsion of anionic PFAS and neutralising the negative surface charge on soil solids with increasing concentration of cations.
The effect of cations may become even more complex in soils with pH-dependent charge (Oliver et al. 2020b) and for cationic PFAS. For example, sorption of cationic PFAS (perfluorooctane-amido quaternary ammonium salt (PFOAAmS)) decreased with increasing concentration of Ca2+ (Mejia-Avendaño et al. 2020). Contaminants co-existing with PFAS in soil may affect PFAS sorption in both saturated and unsaturated soils. Co-contaminants may include DOC, hydrocarbon surfactants, NAPLs and other sorbates (such as mixture of PFAS, DOC and metals). Column studies on activated carbons and anion exchange resins have demonstrated that DOC competes with PFAS for sorption sites on sorbents employed to remove PFAS from water (McCLeaf et al. 2017). The presence of humic acid on clay mineral surfaces has been found to inhibit adsorption of PFOS (Zhang et al. 2014). The addition of P in soil solution has also been found to reduce the sorption of PFOS in a soil in the presence as well as absence of organic matter and ferric oxides (Qian et al. 2017).
Effect of co-contaminants in PFAS products on sorption
Firefighting activities over decades have employed AFFF with a complex composition of fluorinated surfactants, hydrocarbon surfactants, organic solvents, polymers and other additives (Interstate Technology and Regulatory Council (ITRC) 2020). Indeed, hydrocarbon surfactants in AFFFs are more abundant (5–10%) than PFAS (0.9–1.5%) and these can co-exist with PFAS in soil as well as in contaminated groundwaters (García et al. 2019). The presence of hydrocarbon surfactants at higher concentrations than PFAS in AFFF and their greater surface activity means that co-contaminants can make a major difference in the retention of PFAS in saturated and unsaturated soils. However, most PFAS sorption studies in soils have been conducted using very simple systems. Often a single PFAS compound has been studied in the absence of co-contaminants, such as hydrocarbon surfactants. Additionally, during the electrochemical fluorination process both linear and branched isomers of PFASs are produced and their reactions with sorbing surfaces are different (McCleaf et al. 2017). Therefore, their field transport behaviour in both saturated and unsaturated soils may be quite different (Nickerson et al. 2021; Sharifan et al. 2021).
Based on a leaching study of historically contaminated soils, Høisæter et al. (2019) hypothesised that complex mixtures of AFFF affect the retention behaviour of PFAS in field-contaminated soils. Similarly, leaching experiments showed that longer chain PFAS can effectively displace the shorter chain PFAS from sorption sites in soil columns (e.g. Gellrich et al. 2012). However, the effect of co-contaminants has not been fully investigated so far.
Metals and PFAS can be released simultaneously into the surrounding environment, especially because some fluorinated surfactants (e.g. PFOS) are used as the chrome mist suppressant in the plating industry (Li et al. 2019). Although PFOS has been reported to form surface-bound complexes with haematite and copper (e.g. Gao and Chorover 2012), the effect of transition metals on sorption of PFAS in soils has not been investigated. Overall, a sound understanding of the effects of co-contaminants on PFAS sorption in soils is lacking.
Additional factors affecting PFAS retention in unsaturated soils
A range of processes could contribute to the retention of PFAS in unsaturated soils (Brusseau 2018). These may include sorption and partitioning occurring on the solid phase as discussed above, but also air-phase interactions and in NAPLs, as shown in Fig. 1. Thus a combination of several processes may determine the mobility of PFAS in unsaturated soils. The soil physical and chemical properties (mineralogy, particle size and organic matter content), PFAS properties (chain length and polarity) and NAPL physical and chemical properties (solubility, viscosity, density and solvency power) together will determine the relative importance of the interfacial processes in affecting PFAS mobility. Rarely do PFAAs and NAPLs exist as single compounds in the subsurface; more commonly consisting of multicomponent mixtures with perhaps a few dominant compounds dictating the behaviour or toxicity. However, the interaction between chemicals and the competition for interfacial or solid phase sorption sites is the subject of ongoing research.
Effect of PFAS chain length and functional groups on interfacial adsorption
Adsorption at the air–water interface (AWA) is directly dependent on the C–F chain length of the compound. This is because AWA depends on the surface activity of the compound which is strongly dependent on the C–F chain length and its concentration in solution (Costanza et al. 2019; Silva et al. 2019). Lyu et al. (2022) reported an excellent linear relationship between log Kai (air–water adsorption coefficient) and the number of fluorinated carbons for perfluoro carboxylates with chain lengths in the range of 3–9 (Fig. 4). With each additional fluorinated carbon, they observed an approximately 0.46 log unit increase in Kai. However, when Kai was incorporated into the retardation factor, the relationship showed that for short chain compounds the interfacial adsorption did not significantly contribute to the retardation of PFAS transport in unsaturated soils (Fig. 4). Simulations carried out by Guo et al. (2022), using a screening model on six different soils, showed that the retention in unsaturated soils was strongly dependent not only on the chain length of PFAS but also the type of functional group present (e.g. carboxylates versus sulfonates).
|
|
Effect of degree of saturation in soils
Several studies have compared the movement of PFOS and PFOA through soil and sand columns under saturated and unsaturated conditions (Lyu et al. 2018; Brusseau et al. 2019). They noted that movement of PFAS through these columns was universally slower under unsaturated conditions than when the soil or sand was saturated with water (Fig. 5). This retarded movement has been ascribed to the AWA phenomenon. Brusseau et al. (2019), for example, observed that PFOS movement, as represented by the retardation factor (which is a measure of delayed movement of chemical in comparison to water or a conservative, non-interacting chemical), was four times slower in unsaturated than in saturated sand. However, PFOA was only 1.4 times slower through the sand, but the conditions were not directly comparable as the water saturation was higher in the PFOA instance and therefore interfacial area was likely smaller. The AWA as well as the movement of PFAS in unsaturated soils may also be influenced by the immobile domains of water as the soils become unsaturated and the measurement of AWA by simple retardation factor may not be straightforward (Hasan et al. 2020; Stults et al. 2021).
|
|
Effect on soil texture and grain size
The relative contribution of air–water interface towards the retention of PFAS in unsaturated soils is likely to depend on soil properties determining the total interfacial area. Brusseau et al. (2019) observed that although their soil had a larger specific AWA than sand, the contribution of air–water interfacial interaction was larger in sand (due to its lower solid-phase sorption) than in the soil with greater sorption potential for PFAS. In other words, with increasing sorption capacity of the solid phase in a soil (such as through electrostatic or hydrophobic interactions), the relative role of interfacial adsorption is expected to diminish.
In another study, Lyu et al. (2018) noted that the effect of unsaturated conditions on the rate of movement of PFOA was affected by the size of sand grains. The authors noted that in a fine sand (average grain size of 0.35 mm) the retardation of PFOA was significantly higher under unsaturated than saturated conditions but this difference was very small in a coarser sand with average grain size of 1.2 mm. The lower retardation (faster movement) of PFOA in the coarser sand at the same level of water saturation of pores was ascribed to the smaller AWA and thus lower contribution of accumulation at the air–water interface. This was further supported by model simulations carried out by Guo et al. (2022) on transport of PFAS through six different soils, wherein greater retardation factors of PFAS were noted in sandy soils than fine-grained soils.
Effect of solution pH, ionic strength and type of cations on the air–water interfacial accumulation
In addition to soil texture, soil solution properties have been noted to influence the relative PFAS adsorption or accumulation at the air–water interface. These include water quality, i.e. pH and salinity of water (ionic strength), multiple species as well as the concentration of PFAS in the solution. For example, Costanza et al. (2019) and Lyu and Brusseau (2020) observed that the presence of salts in solution (ionic strength) as well as solution pH affected the interfacial interactions and movement of PFOA through soil columns. However, pH had a much smaller effect than ionic strength on PFOA movement through unsaturated columns (Lyu and Brusseau 2020). As the ionic strength of the solution increased, the movement of the PFOA became slower or more retarded under unsaturated conditions. This is because the surface tension of PFAS in solution is lower in saline water than in pure water. Similarly, Silva et al. (2019) compared the surface tension isotherms of several perfluoro carboxylates at different ionic strengths and noted that the surface tension of these carboxylates not only depends on the C–F chain length and PFAS concentration, but also on the ionic strength. In synthetic groundwaters, the surface tension decreased more markedly as the chain length and concentration of perfluoroalkyl carboxylic acid increased and consequently the Kai as well as adsorption energy increased with compound chain length. The effect of ionic composition on surface tension and on Kai was examined by Brusseau and Van Glubt (2019), Li et al. (2021) and Le et al. (2022). These studies showed that salt concentration and type have a strong influence on retention and mobility of PFAS in unsaturated soils. Based on transport studies in soil columns maintained at different degree of moisture saturation, Lyu and Brusseau (2020) reported that air–water retention of PFOA doubled as the ionic strength increased from 0.001 to 0.1 M. Similarly, the presence of CaCl2 in background solution increased PFOA retention by 67% in an unsaturated soil compared to that in the presence of NaCl. Most studies have been conducted in the presence of simple systems employing a monovalent salt such as NaCl. Modelling studies (e.g. Le et al. 2022) have highlighted, through an example, that the failure to account for the effect of divalent cations can significantly underestimate the retention of PFAS in unsaturated systems.
Effect of competing sorbates
As in the case of saturated soils, several co-contaminants can influence not only the solid-phase sorption (as measured in saturated soil) but also the interfacial adsorption in unsaturated soils. These may include other surfactants that enter the soils contaminated by AFFF, PFAS mixtures, DOC and other organic and inorganic contaminants. Several reports in the literature show that co-existing anionic hydrocarbons can perturb the surface activity of PFAS and thus can affect their AWA. Brusseau and Van Glubt (2019) reported that anionic hydrocarbons as co-contaminants can markedly increase the critical micelle concentration of PFOA and PFOS. Costanza et al. (2020) observed that AFFF showed greater interfacial activity than PFOA, PFOS or perfluorooctane sulfonamide (FOSA). Similarly, Lyu et al. (2022) observed that sodium dodecyl benzene sulfonate (a hydrocarbon surfactant) was preferentially adsorbed at the air–water interface and thus competed with the interfacial accumulation of PFOA in an unsaturated sand. In contrast, the co-existing hydrocarbons in AFFF can have a synergistic effect on enhancing the accumulation at the NAPL–water interface (Costanza et al. 2020).
The presence and size of alkali metal ions in solution can alter the surface activity of PFAS. At comparable concentrations, surface activity of NH4-PFOA was reported to be greater due to its smaller hydrated radius than Li-PFOA with larger hydrated radius (Brusseau and Van Glubt 2019). Consistent with this, PFOA showed greater retardation in column experiments in the presence of Ca2+ than Na+ and the retardation increased with increasing ionic strength (Li et al. 2021).
Relative importance of interfacial accumulation in relation to total retention in unsaturated soils
As a synthesis from several studies in the literature, Brusseau (2019) developed a generic nomograph to illustrate the relative role of interfacial processes (air–water and oil–water combined) to the total retention of PFAS. He demonstrated that the importance of interfacial adsorption will depend on the inherent ability of soil to retain PFAS through water–solid phase interactions. While, consistent with our previous work (Li et al. 2018), recognising the limitation of the Koc approach for PFAS (i.e. assuming only organic carbon controls the sorption of PFAS), for ease Brusseau used Koc as an indicator of the sorption on the solid phase. The other parameter needed was the specific fluid–fluid interfacial area (Anw in cm2/cm3), which represents the combined effect of degree of saturation of the geological media, such as soil and the PFAS molecule. Using these two parameters, Brusseau (2019) developed the nomograph for an estimation of the relative role of AWA in the total retention of PFAS (Fig. 6). This shows that the relative role of interfacial adsorption could be as small as <5% to as high as 85%, depending on the conditions related to the geological media, its degree of saturation and the PFAS chemical.
|
|
The foc is an easy-to-measure property and is usually part of basic characterisation of a soil and therefore is often a readily available parameter for most soils. However, there are different methods in the literature to measure Anw (Brusseau 2019), and these methods do not always give the same value for that parameter. Nevertheless, this level of uncertainty may or may not matter, depending on the geological conditions. Many surface soils, such as in Australia generally, have foc in the range 0.01–0.02. This often declines exponentially to about 1/10 at a depth of about 0.5–1 m for subsurface soil, and one can assume foc of about 0.001 down to the water table and perhaps lower than this in aquifer sediments. In terms of air–water interfacial area, Brusseau (2019) reported measured Anw values for the range of PFOA of 24–68 cm−1 for sand at 0.76 saturation and for PFOS of 120–140 cm−1 for a sand and a soil at 0.66 saturation. The nomograph (Fig. 6) shows that, in an unsaturated subsurface soil (with foc = 0.001), this combination may have a significant contribution of air–water interfacial adsorption to the total retention below and above the line of 0.35 for both PFOA and PFOS. It will be interesting to compare the predictions from this nomograph with the measured values in future studies.
Desorption and leaching behaviour of PFAS
Desorption to water from solid surfaces in soil
Most studies have focussed on sorption behaviour of PFAS in soils with only a limited number studying both the sorption and desorption aspects (e.g. Milinovic et al. 2015; Wei et al. 2017; Xiao et al. 2019). Despite the low sorption affinity of PFAAs in soils (especially the short-chain compounds), their sorption is not always reversible (Zhao et al. 2014; Milinovic et al. 2015; Wei et al. 2017). On sequentially extracted humic substances, Zhao et al. (2014) observed that sorption of PFHxS on humin was not as reversible as from humic acids. The PFOS sorption on sediments has been found to be hystereric and its irreversibility increased with increasing salinity (You et al. 2010). Indeed, a significant sorption–desorption hysteresis has been noted in studies on a range of PFAS ranging from PFAAs to cationic and zwitterionic compounds. For example, Xiao et al. (2019) in their sorption–desorption studies on cationic (PFOAAmS) and zwitterionic (PFOAB) PFAS in five soils, noted a more pronounced hysteresis in sorption–desorption isotherms of PFOAB than in the case of PFOAAmS. However, the degree of hysteresis was dependent on soil type. The authors could not rule out the possible transformation of PFOAB during their study, which may have contributed to sorption–desorption hysteresis (Xiao et al. 2019).
Leaching of PFAS through soils is obviously dependent on their desorption behaviour. Laboratory studies based on batch or column leaching procedures conducted under saturated conditions have shown that PFAS, particularly anionic PFAS, can easily leach from contaminated soils (Bräunig et al. 2019, 2021; Kabiri et al. 2022). Unlike sorption studies, leaching/desorption studies typically follow standard leaching methods that have been developed either to classify waste for disposal or to understand leaching mechanisms/behaviour, with method selection based on jurisdictional regulatory guidance. These methods vary based on procedural factors including leaching mode (batch or column), liquid-to-solid ratio, leaching solution conditions (pH, conductivity and solution chemistry), frequency and duration of leaching.
Desorption behaviour of PFAS is generally consistent with results from sorption studies (Nguyen et al. 2020), with desorption behaviour in soil influenced by PFAS chemistry, soil properties and solution conditions. This was evident in recent work on desorption and leaching of a broad range of PFAS (21 PFAS) from 12 AFFF-contaminated soils (Kabiri et al. 2022). In this study, short-chain PFAS (CF2-chain length ≤ 7) were readily desorbed from soil (Fig. 7). Desorption was not affected by the leaching conditions including liquid-to-solid ratio, leaching duration, frequency or batch versus column leaching procedures. In comparison, long-chain PFAS were more resistant to desorption, particularly under acidic conditions. Indeed, some of the long-chain PFAS including PFUnDA, PFDoDA, PFTrDA, PFTeDA and 10:2 FtS did not desorb from the soils under any circumstances. The PFAS functional head group also affected desorption from soil with greater desorption in following the order: carboxylic acids > sulfonic acids > sulfonamide head groups, given the same C–F chain length. A column desorption and leaching test showed that long-chain PFAS desorb and leach to a lesser extent than short-chain compounds. The desorption of long-chain PFAS varied markedly with soil properties. For example, there was greater PFAS desorption from sandy than clay-rich soils (Kabiri et al. 2022).
|
|
The above discussion suggests that, compared to short-chain PFAS, long-chain PFAS (including long-chain precursors) tend to accumulate in the soil and can serve as a significant pool and long-term source for PFAS leaching. Indeed, long-chain PFAS, especially PFOS, can resist complete leaching even with repetitive extraction (Kabiri et al. 2022). The less extractable PFAS could be entrapped or protected by soil components (e.g. minerals or organic carbon) in meso- or micro-pores in a process known as ageing or physical entrapment (Fig. 1), thus inhibiting their desorption. Clearly, these aspects need further investigations.
It is important to note that most desorption/leaching studies on PFAS have been performed under saturated conditions. Considering increased sorption of PFAS under unsaturated conditions, the role of the air–water interface in controlling PFAS desorption/leaching also needs to be considered.
Desorption to water from air–water and NAPL–water interfaces in soil
The PFAS accumulated at the air–water interface in unsaturated soils are released back when water saturation increases as a result of advancement of a wetting front, for example after a rain event. Through model simulations, Brusseau and Guo (2022) demonstrated that the PFAS adsorbed at the air–water interface were released to pore water and thus increased the pore-water concentrations of long-chain PFAS (such as PFOA and PFOS). For short-chain compounds this effect was minor. Clearly more work is needed on desorption behaviour of PFAS under unsaturated conditions. Pore water extracted from soils (e.g. through suction lysimeters) under field conditions may inform the real world desorption and leaching behaviour of PFAS in soils (Rayner et al. 2022). Studies on transport behaviour of PFAS at contaminated sites under field conditions indicate desorption of PFAS leading to both downward and upward movement in the soil profile, depending on the prevailing conditions (Wallis et al. 2022).
Integrated role of soil properties for sorption and desorption
It is evident from the literature that, unlike other organic compounds, the sorption processes are much more complex and soil OC content, or any other single soil property, may not be solely responsible for sorption of PFAS, except in the case of highly hydrophobic PFAS (Li et al. 2018). A study on sorption of anionic, cationic and zwitterionic fluorotelomers (precursors that can be metabolised into and degrade to PFAS) concluded that the sorption mechanisms are so complex that they cannot always be predicted easily (Barzen-Hanson et al. 2017). Studies have shown that often several properties need to be considered together to explain PFAS sorption behaviour in soils (e.g. Li et al. 2018; Knight et al. 2019; Umeh et al. 2021).
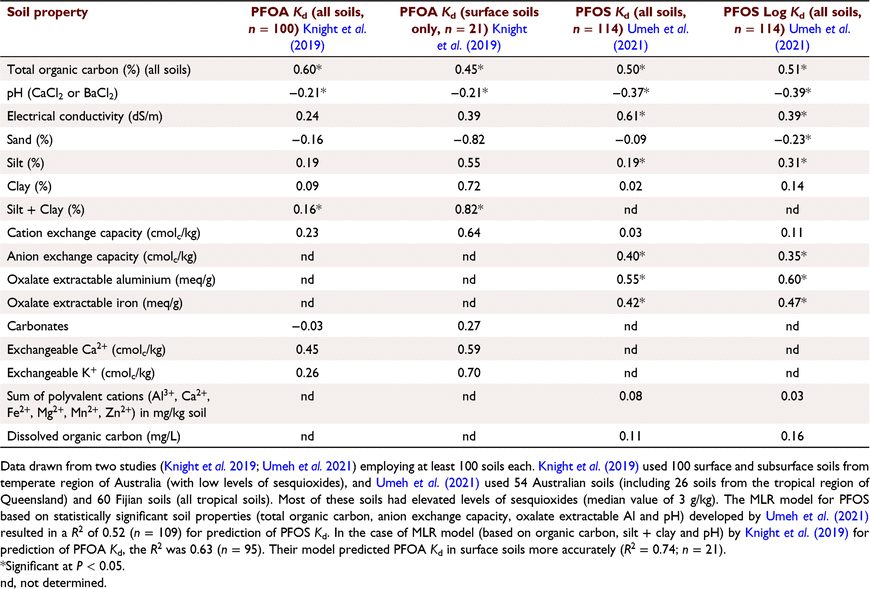
Based on a review of data, Li et al. (2018) found significant relationships between Kd values and OC plus clay contents for PFOS (R2 = 0.76, n = 15), PFOA (R2 = 0.43, n = 29), N-MeFOSAA (R2 = 0.96, n = 5), PFNA (R2 = 0.87, n = 9), PFDA (R2 = 0.84, n = 11), PFUnDA (R2 = 0.92, n = 7) and PFPeA (R2 = 0.91, n = 6). Although the limited number of observations available by 2018 indicated that OC content, clay content and pH needed to be considered together in explaining the sorption behaviour of PFAS in soils, more work on a larger set of well-characterised soils is necessary. Li et al. (2018) also noted that in many sorption studies on PFAS, the soils or sediments used in the studies were not fully characterised and sometimes even the basic properties (such as pH) were missing, thus limiting the value of sorption data obtained in such studies. Subsequently, Knight et al. (2019) investigated the role of various soil properties – OC, pH (CaCl2), particle size distribution, electrical conductivity, chloride, cation exchange capacity, exchangeable cations (Ca2+, Na+ and K+) and exchangeable sodium percentage – together to explain the variation of PFOA Kd measured in 100 surface and subsurface soils (Table 3). Their statistical analysis of the data showed that Kd was only significantly affected by OC content, silt+clay content (P < 0.05) and pH (P = 0.06) approaching the critical t-value (Table 3). Inclusion of pH only marginally improved the quality of their multiple linear regression model (R2 increased from 0.61 to 0.63). More recently, Umeh et al. (2021) conducted a major study on sorption of PFOS using a set of well-characterised 114 soils (Table 3), including for some soil properties that have rarely been measured, such as anion exchange capacities. Their multiple linear regression (MLR) analysis revealed that the combination of total OC, oxalate-extractable Al, anion exchange capacity, pH and silt (in the order of importance) accounted for approximately half of the total variation in PFOS Kd values across soils. Inclusion of these predictor properties in an artificial neural network model (trained on a subset of soils) provided satisfactory prediction of PFOS Kd in these soils.
|
Most of the above studies were conducted on anionic perfluoroalkyl acids (e.g. PFOA and PFOS). Limited studies on sorption of zwitterionic or cationic PFAS (e.g. FtSaB and FtSaAm) have shown that their sorption behaviour is principally driven by electrostatic interactions such as cation exchange. Barzen-Hanson et al. (2017) noted significant and very strong positive correlations of sorption coefficients with the cation exchange capacity of soil. For zwitterionic 6:2 FtSaB and 8:2 FtSaB, they noted R values of 0.94 and 0.99, respectively. However, such correlations were absent for anionic FtS, while the cationic FtSaAm was totally sorbed in five out of six soils. No correlation with OC or anion exchange capacity was noted for FtS or FtSaB.
Which soil properties matter most
The analysis above shows that the key soil properties that govern partitioning behaviour of PFAS depend strongly on the chemical characteristics of the PFAS molecule. Given the huge diversity in PFAS chemistry (surfactants to non-surfactants, from anions to zwitterions and cations and from non-polymers to polymers), it is presumptive to expect that the same set of properties will be applicable to all chemistries. Most existing literature is on sorption of PFAAs (e.g. PFOS and PFOA), and a handful of zwitterions and other PFAS, perhaps because these have received the most regulatory attention so far. Therefore, the following discussion is largely related to the PFAA group of PFAS. Additionally, the mechanisms governing partitioning, and therefore factors affecting it, differ in saturated and unsaturated soils.
In saturated soils, the current body of literature shows that firstly no single soil property can adequately explain the sorption behaviour of PFAS on the soil solid-phase. Total OC content of soil is one of major properties governing the sorption of PFAAs and its importance increases with increasing PFAS chain-length or molecular size. That is, the longer the chain length, the more hydrophobic is the tail of the PFAS molecule, leading to a greater role for OC in partitioning. However, several studies show that OC content could explain only about one-third of the total variation in sorption of PFAAs in soils. The other important predictors of Kd of PFAS include particle size distribution, oxalate-extractable Al and Fe contents (especially in tropical soils) and solution chemistry. The solution chemistry is important in terms of pH, ionic strength as well as the nature and concentrations of polyvalent cations such as Ca2+, Mg2+ and Al3+. The presence of co-contaminants such as AFFF constituents, DOC and other organic and inorganic contaminants (e.g. metals) can potentially affect PFAS sorption. It is noteworthy that the properties that govern the partitioning behaviour of PFAS in saturated soils do not directly extrapolate to sediments. For example, in the case of marine and estuarine sediments, due to their high salinity, buffered pH and narrower particle size distribution, the OC content alone may be a stronger predictor of PFAS partitioning (Oliver et al. 2020a).
In unsaturated soils, in addition to the solid-phase sorption (as in saturated soils), AWA can contribute significantly to the retention of PFAS in soils. For some short-chain PFAS, which do not show significant solid-phase sorption (e.g. PFBA and PFBS), the main mechanism of their retention is interfacial accumulation. The Kai of PFAAs is strongly dependent on the chemistry (surface activity) of the PFAS, the air–water interfacial area in the unsaturated soil (i.e. degree of saturation and texture of soil) and the solution chemistry (e.g. ionic strength and polyvalent cations).
Knowledge gaps
The work on sorption–desorption behaviour of PFAS remains limited, considering the diverse chemistry of compounds in this class and their myriad applications. Hence the following knowledge gaps are by no means exhaustive.
-
The importance of soil properties in explaining partitioning is very much dependent on PFAS chemistry. Most published work is on PFAAs and data for cationic, zwitterionic and precursor compounds (polyfluorinated compounds) are very limited.
-
Laboratory partitioning studies using defined compounds in isolation do not mimic field contamination because AFFF are complex mixtures of hydrocarbon surfactants and fluorinated surfactants. Furthermore, field soils may have other co-contaminants. Partitioning behaviour in the presence of co-contaminants is lacking under both saturated and unsaturated conditions.
-
Field-based measurements of partitioning should be attempted to obtain empirically driven sorption–desorption parameters.
-
Direct evidence for sorption mechanisms of PFAS on soil surfaces is lacking, partly due to the low abundance of these contaminants in most soils, which precludes the use of the many spectroscopic techniques able to provide mechanistic evidence.
-
Studies on reversibility of sorption reactions, either at solid surfaces or at air–NAPL–water interfaces are rare. The degree to which natural attenuation of PFAS and the appearance of non-extractable residues might reduce long-term risks to the environment is not known.
-
A wider range of soil types, with stronger solid-phase partitioning, should be used in studies of partitioning under unsaturated conditions, to determine the relative importance of air–water interfacial retention on overall partitioning. Most studies so far have deliberately employed sandy soils with little solid-phase sorption affinity for PFAS.
-
The measurement of air–water interfacial accumulation is difficult and requires complex laboratory techniques – simpler methods are needed to allow wider investigation of this phenomenon.
-
Sorption and desorption processes together determine the partitioning and mobility of contaminants in the soil environment. So far little work is available in the published literature on the desorption behaviour of PFAS in soils.
-
Unlike convention nonpolar organic compounds for which Kow- and Koc-based predictions of partitioning coefficients in soils provide a reasonable estimate of mobility, these parameters are clearly neither appropriate nor adequate for surface active chemicals such as PFAS. New predictive approaches (e.g. quantitative structure activity relationships) are needed where characterisation of PFAS structure and/or chemistry can be used to predict mobility in the environment.
Abbreviations
Data availability
Data used to generate the results in the paper are available in the public domain.
Conflicts of interest
The authors declare no known conflicts of interest.
Declaration of funding
The research work did not receive any specific funding. However, CSIRO and the University of Adelaide offered in-kind support to the project.
Acknowledgements
The authors are thankful to Professor Balwant Singh, the Editor-in-Chief of Soil Research, for inviting this contribution, and to the three anonymous reviewers for their constructive comments and suggestions for improving the quality of the manuscript. Authors are thankful to various publishers for permitting the use of copyright material.
References
Ahmad R, Kookana RS, Alston AM, Skjemstad JO (2001) The nature of soil organic matter affects sorption of pesticides. 1. Relationships with carbon chemistry as determined by 13C CPMAS NMR spectroscopy. Environmental Science & Technology 35, 878–884.| The nature of soil organic matter affects sorption of pesticides. 1. Relationships with carbon chemistry as determined by 13C CPMAS NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar |
Alexander M (2000) Aging, bioavailability, and overestimation of risk from environmental pollutants. Environmental Science & Technology 34, 4259–4265.
| Aging, bioavailability, and overestimation of risk from environmental pollutants.Crossref | GoogleScholarGoogle Scholar |
Barzen-Hanson KA, Davis SE, Kleber M, Field JA (2017) Sorption of fluorotelomer sulfonates, fluorotelomer sulfonamido betaines, and a fluorotelomer sulfonamido amine in National Foam aqueous film-forming foam to soil. Environmental Science & Technology 51, 12394–12404.
| Sorption of fluorotelomer sulfonates, fluorotelomer sulfonamido betaines, and a fluorotelomer sulfonamido amine in National Foam aqueous film-forming foam to soil.Crossref | GoogleScholarGoogle Scholar |
Bräunig J, Baduel C, Barnes CM, Mueller JF (2019) Leaching and bioavailability of selected perfluoroalkyl acids (PFAAs) from soil contaminated by firefighting activities. Science of the Total Environment 646, 471–479.
| Leaching and bioavailability of selected perfluoroalkyl acids (PFAAs) from soil contaminated by firefighting activities.Crossref | GoogleScholarGoogle Scholar |
Bräunig J, Baduel C, Barnes CM, Mueller JF (2021) Sorbent assisted immobilisation of perfluoroalkyl acids in soils – effect on leaching and bioavailability. Journal of Hazardous Materials 412, 125171
| Sorbent assisted immobilisation of perfluoroalkyl acids in soils – effect on leaching and bioavailability.Crossref | GoogleScholarGoogle Scholar |
Brusseau ML (2018) Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface. Science of the Total Environment 613–614, 176–185.
| Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface.Crossref | GoogleScholarGoogle Scholar |
Brusseau ML (2019) Estimating the relative magnitudes of adsorption to solid-water and air/oil-water interfaces for per- and poly-fluoroalkyl substances. Environmental Pollution 254B, 113102
| Estimating the relative magnitudes of adsorption to solid-water and air/oil-water interfaces for per- and poly-fluoroalkyl substances.Crossref | GoogleScholarGoogle Scholar |
Brusseau ML, Guo B (2022) PFAS concentrations in soil versus soil porewater: mass distributions and the impact of adsorption at air-water interfaces. Chemosphere 302, 134938
| PFAS concentrations in soil versus soil porewater: mass distributions and the impact of adsorption at air-water interfaces.Crossref | GoogleScholarGoogle Scholar |
Brusseau ML, Van Glubt S (2019) The influence of surfactant and solution composition on PFAS adsorption at fluid-fluid interfaces. Water Research 161, 17–26.
| The influence of surfactant and solution composition on PFAS adsorption at fluid-fluid interfaces.Crossref | GoogleScholarGoogle Scholar |
Brusseau ML, Yan N, Van Glubt S, Wang Y, Chen W, Lyu Y, Dungan B, Carroll KC, Holguin FO (2019) Comprehensive retention model for PFAS transport in subsurface systems. Water Research 148, 41–50.
| Comprehensive retention model for PFAS transport in subsurface systems.Crossref | GoogleScholarGoogle Scholar |
Brusseau ML, Anderson RH, Guo B (2020) PFAS concentrations in soils: background levels versus contaminated sites. Science of the Total Environment 740, 140017
| PFAS concentrations in soils: background levels versus contaminated sites.Crossref | GoogleScholarGoogle Scholar |
Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated Environmental Assessment and Management 7, 513–541.
| Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins.Crossref | GoogleScholarGoogle Scholar |
Buck RC, Korzeniowski SH, Laganis E, Adamsky F (2021) Identification and classification of commercially relevant per- and poly-fluoroalkyl Substances (PFAS). Integrated Environmental Assessment and Management 17, 1045–1055.
| Identification and classification of commercially relevant per- and poly-fluoroalkyl Substances (PFAS).Crossref | GoogleScholarGoogle Scholar |
Cai W, Navarro DA, Du J, Ying G, Yang B, McLaughlin MJ, Kookana RS (2022) Increasing ionic strength and valency of cations enhance sorption through hydrophobic interactions of PFAS with soil surfaces. Science of the Total Environment 817, 152975
| Increasing ionic strength and valency of cations enhance sorption through hydrophobic interactions of PFAS with soil surfaces.Crossref | GoogleScholarGoogle Scholar |
Campos Pereira H, Ullberg M, Kleja DB, Gustafsson JP, Ahrens L (2018) Sorption of perfluoroalkyl substances (PFASs) to an organic soil horizon – effect of cation composition and pH. Chemosphere 207, 183–191.
| Sorption of perfluoroalkyl substances (PFASs) to an organic soil horizon – effect of cation composition and pH.Crossref | GoogleScholarGoogle Scholar |
Campos-Pereira H, Kleja DB, Sjöstedt C, Ahrens L, Klysubun W, Gustafsson JP (2020) The adsorption of per- and polyfluoroalkyl substances (PFASs) onto ferrihydrite is governed by surface charge. Environmental Science & Technology 54, 15722–15730.
| The adsorption of per- and polyfluoroalkyl substances (PFASs) onto ferrihydrite is governed by surface charge.Crossref | GoogleScholarGoogle Scholar |
Campos-Pereira H, Makselon J, Kleja DB, Prater I, Kögel-Knabner I, Ahrens L, Gustafsson JP (2022) Binding of per- and polyfluoroalkyl substances (PFASs) by organic soil materials with different structural composition – charge- and concentration-dependent sorption behavior. Chemosphere 297, 134167
| Binding of per- and polyfluoroalkyl substances (PFASs) by organic soil materials with different structural composition – charge- and concentration-dependent sorption behavior.Crossref | GoogleScholarGoogle Scholar |
Costanza J, Arshadi M, Abriola LM, Pennell KD (2019) Accumulation of PFOA and PFOS at the air–water interface. Environmental Science & Technology Letters 6, 487–491.
| Accumulation of PFOA and PFOS at the air–water interface.Crossref | GoogleScholarGoogle Scholar |
Costanza J, Abriola LM, Pennell KD (2020) Aqueous film-forming foams exhibit greater interfacial activity than PFOA, PFOS, or FOSA. Environmental Science & Technology 54, 13590–13597.
| Aqueous film-forming foams exhibit greater interfacial activity than PFOA, PFOS, or FOSA.Crossref | GoogleScholarGoogle Scholar |
Ding G, Peijnenburg WJGM (2013) Physicochemical properties and aquatic toxicity of poly- and perfluorinated compounds. Critical Reviews in Environmental Science and Technology 43, 598–678.
| Physicochemical properties and aquatic toxicity of poly- and perfluorinated compounds.Crossref | GoogleScholarGoogle Scholar |
Du Z, Deng S, Bei Y, Huang Q, Wang B, Huang J, Yu G (2014) Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—a review. Journal of Hazardous Materials 274, 443–454.
| Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—a review.Crossref | GoogleScholarGoogle Scholar |
Evich MG, Davis MJB, McCord JP, Acrey B, Awkerman JA, Knappe DRU, Lindstrom AB, Speth TF, Tebes-Stevens C, Strynar MJ, Wang Z, Weber EJ, Henderson WM, Washington JW (2022) Per- and polyfluoroalkyl substances in the environment. Science 375, eabg9065
| Per- and polyfluoroalkyl substances in the environment.Crossref | GoogleScholarGoogle Scholar |
Feng X, Simpson AJ, Simpson MJ (2005) Chemical and mineralogical controls on humic acid sorption to clay mineral surfaces. Organic Geochemistry 36, 1553–1566.
| Chemical and mineralogical controls on humic acid sorption to clay mineral surfaces.Crossref | GoogleScholarGoogle Scholar |
Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, Smith JS, Roberts SM (2021) Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environmental Toxicology and Chemistry 40, 606–630.
| Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research.Crossref | GoogleScholarGoogle Scholar |
Gao X, Chorover J (2012) Adsorption of perfluorooctanoic acid and perfluorooctanesulfonic acid to iron oxide surfaces as studied by flow-through ATR-FTIR spectroscopy. Environmental Chemistry 9, 148–157.
| Adsorption of perfluorooctanoic acid and perfluorooctanesulfonic acid to iron oxide surfaces as studied by flow-through ATR-FTIR spectroscopy.Crossref | GoogleScholarGoogle Scholar |
García RA, Chiaia-Hernández AC, Lara-Martin PA, Loos M, Hollender J, Oetjen K, Higgins CP, Field JA (2019) Suspect screening of hydrocarbon surfactants in AFFFs and AFFF-contaminated groundwater by high-resolution mass spectrometry. Environmental Science & Technology 53, 8068–8077.
| Suspect screening of hydrocarbon surfactants in AFFFs and AFFF-contaminated groundwater by high-resolution mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
Gellrich V, Stahl T, Knepper TP (2012) Behavior of perfluorinated compounds in soils during leaching experiments. Chemosphere 87, 1052–1056.
| Behavior of perfluorinated compounds in soils during leaching experiments.Crossref | GoogleScholarGoogle Scholar |
Guo B, Zeng J, Brusseau ML, Zhang Y (2022) A screening model for quantifying PFAS leaching in the vadose zone and mass discharge to groundwater. Advances in Water Resources 160, 104102
| A screening model for quantifying PFAS leaching in the vadose zone and mass discharge to groundwater.Crossref | GoogleScholarGoogle Scholar |
Hasan S, Niasar V, Karadimitriou NK, Godinho JRA, Vo NT, An S, Rabbani A, Steeb H (2020) Direct characterization of solute transport in unsaturated porous media using fast X-ray synchrotron microtomography. Proceedings of the National Academy of Sciences of the United States of America 117, 23443–23449.
| Direct characterization of solute transport in unsaturated porous media using fast X-ray synchrotron microtomography.Crossref | GoogleScholarGoogle Scholar |
Higgins CP, Luthy RG (2006) Sorption of perfluorinated surfactants on sediments. Environmental Science & Technology 40, 7251–7256.
| Sorption of perfluorinated surfactants on sediments.Crossref | GoogleScholarGoogle Scholar |
Høisæter Å, Pfaff A, Breedveld GD (2019) Leaching and transport of PFAS from aqueous film-forming foam (AFFF) in the unsaturated soil at a firefighting training facility under cold climatic conditions. Journal of Contaminant Hydrology 222, 112–122.
| Leaching and transport of PFAS from aqueous film-forming foam (AFFF) in the unsaturated soil at a firefighting training facility under cold climatic conditions.Crossref | GoogleScholarGoogle Scholar |
Interstate Technology and Regulatory Council (ITRC) (2020) PFAS Technical and Regulatory guidance document and Factsheets PFAS-1. Interstate Technology and Regulatory Council, PFAS Team, Washington, DC. Available at https://pfas-1.itrcweb.org/
Jeon J, Kannan K, Lim BJ, An KG, Kim SD (2011) Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles. Journal of Environmental Monitoring 13, 1803–1810.
| Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles.Crossref | GoogleScholarGoogle Scholar |
Kabiri S, Centner M, McLaughlin MJ (2021) Durability of sorption of per- and polyfluorinated alkyl substances in soils immobilised using common adsorbents: 1. Effects of perturbations in pH. Science of the Total Environment 766, 144857
| Durability of sorption of per- and polyfluorinated alkyl substances in soils immobilised using common adsorbents: 1. Effects of perturbations in pH.Crossref | GoogleScholarGoogle Scholar |
Kabiri S, Tucker W, Navarro DA, Bräunig J, Thompson K, Knight ER, Nguyen TMH, Grimison C, Barnes CM, Higgins CP, Mueller JF, Kookana RS, McLaughlin MJ (2022) Comparing the leaching behavior of per- and polyfluoroalkyl substances from contaminated soils using static and column leaching tests. Environmental Science & Technology 56, 368–378.
| Comparing the leaching behavior of per- and polyfluoroalkyl substances from contaminated soils using static and column leaching tests.Crossref | GoogleScholarGoogle Scholar |
Kah M, Oliver D, Kookana R (2021) Sequestration and potential release of PFAS from spent engineered sorbents. Science of the Total Environment 765, 142770
| Sequestration and potential release of PFAS from spent engineered sorbents.Crossref | GoogleScholarGoogle Scholar |
Kim H, Annable MD, Rao PSC (1998) Influence of air–water interfacial adsorption and gas-phase partitioning on the transport of organic chemicals in unsaturated porous media. Environmental Science & Technology 32, 1253–1259.
| Influence of air–water interfacial adsorption and gas-phase partitioning on the transport of organic chemicals in unsaturated porous media.Crossref | GoogleScholarGoogle Scholar |
Kissa E (2001) ‘Fluorinated surfactants and repellents.’ (Marcel Dekker: New York)
Knight ER, Janik LJ, Navarro DA, Kookana RS, McLaughlin MJ (2019) Predicting partitioning of radiolabelled 14C-PFOA in a range of soils using diffuse reflectance infrared spectroscopy. Science of the Total Environment 686, 505–513.
| Predicting partitioning of radiolabelled 14C-PFOA in a range of soils using diffuse reflectance infrared spectroscopy.Crossref | GoogleScholarGoogle Scholar |
Krafft MP, Riess JG (2015) Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability—part one. Chemosphere 129, 4–19.
| Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability—part one.Crossref | GoogleScholarGoogle Scholar |
Le S-T, Gao Y, Kibbey TCG, Glamore WC, O’Carroll DM (2022) Predicting the impact of salt mixtures on the air-water interfacial behavior of PFAS. Science of the Total Environment 819, 151987
| Predicting the impact of salt mixtures on the air-water interfacial behavior of PFAS.Crossref | GoogleScholarGoogle Scholar |
Li Y, Oliver DP, Kookana RS (2018) A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs). Science of the Total Environment 628–629, 110–120.
| A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs).Crossref | GoogleScholarGoogle Scholar |
Li F, Fang X, Zhou Z, Liao X, Zou J, Yuan B, Sun W (2019) Adsorption of perfluorinated acids onto soils: kinetics, isotherms, and influences of soil properties. Science of the Total Environment 649, 504–514.
| Adsorption of perfluorinated acids onto soils: kinetics, isotherms, and influences of soil properties.Crossref | GoogleScholarGoogle Scholar |
Li Z, Lyu X, Gao B, Xu H, Wu J, Sun Y (2021) Effects of ionic strength and cation type on the transport of perfluorooctanoic acid (PFOA) in unsaturated sand porous media. Journal of Hazardous Materials 403, 123688
| Effects of ionic strength and cation type on the transport of perfluorooctanoic acid (PFOA) in unsaturated sand porous media.Crossref | GoogleScholarGoogle Scholar |
Loganathan N, Wilson AK (2022) Adsorption, structure, and dynamics of short- and long-chain PFAS molecules in kaolinite: molecular-level insights. Environmental Science & Technology 56, 8043–8052.
| Adsorption, structure, and dynamics of short- and long-chain PFAS molecules in kaolinite: molecular-level insights.Crossref | GoogleScholarGoogle Scholar |
Lu X, Deng S, Wang B, Huang J, Wang Y, Yu G (2016) Adsorption behavior and mechanism of perfluorooctane sulfonate on nanosized inorganic oxides. Journal of Colloid and Interface Science 474, 199–205.
| Adsorption behavior and mechanism of perfluorooctane sulfonate on nanosized inorganic oxides.Crossref | GoogleScholarGoogle Scholar |
Lyu Y, Brusseau ML (2020) The influence of solution chemistry on air-water interfacial adsorption and transport of PFOA in unsaturated porous media. Science of the Total Environment 713, 136744
| The influence of solution chemistry on air-water interfacial adsorption and transport of PFOA in unsaturated porous media.Crossref | GoogleScholarGoogle Scholar |
Lyu Y, Brusseau ML, Chen W, Yan N, Fu X, Lin X (2018) Adsorption of PFOA at the air–water interface during transport in unsaturated porous media. Environmental Science & Technology 52, 7745–7753.
| Adsorption of PFOA at the air–water interface during transport in unsaturated porous media.Crossref | GoogleScholarGoogle Scholar |
Lyu Y, Wang B, Du X, Guo B, Brusseau ML (2022) Air-water interfacial adsorption of C4-C10 perfluorocarboxylic acids during transport in unsaturated porous media. Science of the Total Environment 831, 154905
| Air-water interfacial adsorption of C4-C10 perfluorocarboxylic acids during transport in unsaturated porous media.Crossref | GoogleScholarGoogle Scholar |
McCleaf P, Englund S, Östlund A, Lindegren K, Wiberg K, Ahrens L (2017) Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Research 120, 77–87.
| Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests.Crossref | GoogleScholarGoogle Scholar |
Mejia-Avendaño S, Zhi Y, Yan B, Liu J (2020) Sorption of polyfluoroalkyl surfactants on surface soils: effect of molecular structures, soil properties, and solution chemistry. Environmental Science & Technology 54, 1513–1521.
| Sorption of polyfluoroalkyl surfactants on surface soils: effect of molecular structures, soil properties, and solution chemistry.Crossref | GoogleScholarGoogle Scholar |
Milinovic J, Lacorte S, Vidal M, Rigol A (2015) Sorption behaviour of perfluoroalkyl substances in soils. Science of the Total Environment 511, 63–71.
| Sorption behaviour of perfluoroalkyl substances in soils.Crossref | GoogleScholarGoogle Scholar |
Nguyen TMH, Bräunig J, Thompson K, Thompson J, Kabiri S, Navarro DA, Kookana RS, Grimison C, Barnes CM, Higgins CP, McLaughlin MJ, Mueller JF (2020) Influences of chemical properties, soil properties, and solution pH on soil–water partitioning coefficients of per- and polyfluoroalkyl substances (PFASs). Environmental Science & Technology 54, 15883–15892.
| Influences of chemical properties, soil properties, and solution pH on soil–water partitioning coefficients of per- and polyfluoroalkyl substances (PFASs).Crossref | GoogleScholarGoogle Scholar |
Nickerson A, Rodowa AE, Adamson DT, Field JA, Kulkarni PR, Kornuc JJ, Higgins CP (2021) Spatial trends of anionic, zwitterionic, and cationic PFASs at an AFFF-impacted site. Environmental Science & Technology 55, 313–323.
| Spatial trends of anionic, zwitterionic, and cationic PFASs at an AFFF-impacted site.Crossref | GoogleScholarGoogle Scholar |
Oliver DP, Navarro D, Baldock J, Simpson SL, Kookana RS (2020a) Sorption behaviour of per- and polyfluoroalkyl substances (PFASs) as affected by the properties of coastal estuarine sediments. Science of the Total Environment 720, 137263
| Sorption behaviour of per- and polyfluoroalkyl substances (PFASs) as affected by the properties of coastal estuarine sediments.Crossref | GoogleScholarGoogle Scholar |
Oliver DP, Li Y, Orr R, Nelson P, Barnes M, McLaughlin MJ, Kookana RS (2020b) Sorption behaviour of per- and polyfluoroalkyl substances (PFASs) in tropical soils. Environmental Pollution 258, 113726
| Sorption behaviour of per- and polyfluoroalkyl substances (PFASs) in tropical soils.Crossref | GoogleScholarGoogle Scholar |
Organisation for Economic Co-operation and Development (OECD) (2018) Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs). Summary report on updating the OECD 2007 list of per- and polyfluoroalkyl substances (PFASs). Series on Risk Management 39, Organisation for Economic Co-operation and Development (OECD), pp. 1–24.
Qi Y, Cao H, Pan W, Wang C, Liang Y (2022) The role of dissolved organic matter during per- and polyfluorinated substance (PFAS) adsorption, degradation, and plant uptake: a review. Journal of Hazardous Materials 436, 129139
| The role of dissolved organic matter during per- and polyfluorinated substance (PFAS) adsorption, degradation, and plant uptake: a review.Crossref | GoogleScholarGoogle Scholar |
Qian J, Shen M, Wang P, Wang C, Hou J, Ao Y, Liu J, Li K (2017) Adsorption of perfluorooctane sulfonate on soils: effects of soil characteristics and phosphate competition. Chemosphere 168, 1383–1388.
| Adsorption of perfluorooctane sulfonate on soils: effects of soil characteristics and phosphate competition.Crossref | GoogleScholarGoogle Scholar |
Rayner JL, Slee D, Falvey S, Kookana R, Bekele E, Stevenson G, Lee A, Davis GB (2022) Laboratory batch representation of PFAS leaching from aged field soils: intercomparison across new and standard approaches. Science of the Total Environment 838, 156562
| Laboratory batch representation of PFAS leaching from aged field soils: intercomparison across new and standard approaches.Crossref | GoogleScholarGoogle Scholar |
Sharifan H, Bagheri M, Wang D, Burken JG, Higgins CP, Liang Y, Liu J, Schaefer CE, Blotevogel J (2021) Fate and transport of per- and polyfluoroalkyl substances (PFASs) in the vadose zone. Science of the Total Environment 771, 145427
| Fate and transport of per- and polyfluoroalkyl substances (PFASs) in the vadose zone.Crossref | GoogleScholarGoogle Scholar |
Silva JAK, Martin WA, Johnson JL, McCray JE (2019) Evaluating air-water and NAPL-water interfacial adsorption and retention of perfluorocarboxylic acids within the vadose zone. Journal of Contaminant Hydrology 223, 103472
| Evaluating air-water and NAPL-water interfacial adsorption and retention of perfluorocarboxylic acids within the vadose zone.Crossref | GoogleScholarGoogle Scholar |
Stults J, Illangasekare T, Higgins CP (2021) The mass transfer index (MTI): a semi-emperical approach for quantifying transport of solutes in variably saturated porous media. Journal of Contaminant Hydrology 242, 103842
| The mass transfer index (MTI): a semi-emperical approach for quantifying transport of solutes in variably saturated porous media.Crossref | GoogleScholarGoogle Scholar |
Umeh AC, Naidu R, Shilpi S, Boateng EB, Rahman A, Cousins IT, Chadalavada S, Lamb D, Bowman M (2021) Sorption of PFOS in 114 well-characterized tropical and temperate soils: application of multivariate and artificial neural network analyses. Environmental Science & Technology 55, 1779–1789.
| Sorption of PFOS in 114 well-characterized tropical and temperate soils: application of multivariate and artificial neural network analyses.Crossref | GoogleScholarGoogle Scholar |
United Nations Environment Program (UNEP) (2009) Stockholm Convention on Persistent Organic Pollutants (POPs) as amended in 2009. SC-4/17 listing of perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride. Available at http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx [Accessed 14 October 2022]
Wallis I, Hutson J, Davis G, Kookana R, Rayner J, Prommer H (2022) Model-based identification of vadose zone controls on PFAS mobility under semi-arid climate conditions. Water Research 225, 119096
| Model-based identification of vadose zone controls on PFAS mobility under semi-arid climate conditions.Crossref | GoogleScholarGoogle Scholar |
Wei C, Song X, Wang Q, Hu Z (2017) Sorption kinetics, isotherms and mechanisms of PFOS on soils with different physicochemical properties. Ecotoxicology and Environmental Safety 142, 40–50.
| Sorption kinetics, isotherms and mechanisms of PFOS on soils with different physicochemical properties.Crossref | GoogleScholarGoogle Scholar |
Xiao F, Jin B, Golovko SA, Golovko MY, Xing B (2019) Sorption and desorption mechanisms of cationic and zwitterionic per- and polyfluoroalkyl substances in natural soils: thermodynamics and hysteresis. Environmental Science & Technology 53, 11818–11827.
| Sorption and desorption mechanisms of cationic and zwitterionic per- and polyfluoroalkyl substances in natural soils: thermodynamics and hysteresis.Crossref | GoogleScholarGoogle Scholar |
You C, Jia C, Pan G (2010) Effect of salinity and sediment characteristics on the sorption and desorption of perfluorooctane sulfonate at sediment-water interface. Environmental Pollution 158, 1343–1347.
| Effect of salinity and sediment characteristics on the sorption and desorption of perfluorooctane sulfonate at sediment-water interface.Crossref | GoogleScholarGoogle Scholar |
Zareitalabad P, Siemens J, Hamer M, Amelung W (2013) Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater – a review on concentrations and distribution coefficients. Chemosphere 91, 725–732.
| Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater – a review on concentrations and distribution coefficients.Crossref | GoogleScholarGoogle Scholar |
Zhang R, Yan W, Jing C (2014) Mechanistic study of PFOS adsorption on kaolinite and montmorillonite. Colloids and Surfaces A: Physicochemical and Engineering Aspects 462, 252–258.
| Mechanistic study of PFOS adsorption on kaolinite and montmorillonite.Crossref | GoogleScholarGoogle Scholar |
Zhang R, Yan W, Jing C (2015) Experimental and molecular dynamic simulation study of perfluorooctane sulfonate adsorption on soil and sediment components. Journal of Environmental Sciences 29, 131–138.
| Experimental and molecular dynamic simulation study of perfluorooctane sulfonate adsorption on soil and sediment components.Crossref | GoogleScholarGoogle Scholar |
Zhao L, Zhang Y, Fang S, Zhu L, Liu Z (2014) Comparative sorption and desorption behaviors of PFHxS and PFOS on sequentially extracted humic substances. Journal of Environmental Sciences 26, 2517–2525.
| Comparative sorption and desorption behaviors of PFHxS and PFOS on sequentially extracted humic substances.Crossref | GoogleScholarGoogle Scholar |
Zhu X, Song X, Schwarzbauer J (2021) First insights into the formation and long-term dynamic behaviors of nonextractable perfluorooctanesulfonate and its alternative 6:2 chlorinated polyfluorinated ether sulfonate residues in a silty clay soil. Science of the Total Environment 761, 143230
| First insights into the formation and long-term dynamic behaviors of nonextractable perfluorooctanesulfonate and its alternative 6:2 chlorinated polyfluorinated ether sulfonate residues in a silty clay soil.Crossref | GoogleScholarGoogle Scholar |


