Soil water deficit effects on soil inorganic nitrogen in alternate-furrow flood irrigated Australian cotton production systems
Ben C. T. Macdonald A * , Graeme D. Schwenke
A * , Graeme D. Schwenke  B , Annabelle McPherson C , Clarence Mercer B , Jonathan Baird C and Gunasekhar Nachimuthu
B , Annabelle McPherson C , Clarence Mercer B , Jonathan Baird C and Gunasekhar Nachimuthu  C
C
A CSIRO Agriculture and Food, Black Mountain, Canberra, ACT 2601, Australia.
B NSW Department of Primary Industries, Tamworth Agricultural Institute, Calala, NSW 2340, Australia.
C NSW Department of Primary Industries, Australian Cotton Research Institute, Narrabri, NSW, Australia.
Soil Research 60(2) 137-146 https://doi.org/10.1071/SR20223
Submitted: 12 August 2020 Accepted: 12 August 2021 Published: 4 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Predicting the nitrogen (N) mineralisation from soil organic matter is a key aid to fertiliser decision-making and improving the N fertiliser use efficiency of a crop.
Aims and methods: Field experiments were conducted to assess the amount of inorganic N derived from soil organic matter mineralisation over two seasons (2017–2018 and 2018–2019) across treatments differing in irrigation frequency and amount. During both seasons, the plant line soil in each treatment was sequentially sampled at each irrigation event.
Key results: There was an effect of the soil water deficit on the measured accumulated soil inorganic N derived from mineralisation in both measurement years. It was observed that soil inorganic N accumulated in the plant line rather than in other hillside and furrow positions for all soil moisture deficit treatments in both years. In 2017–2018, N accumulated in the plant was significantly greater than the measured accumulated inorganic N (0–300 mm).
Conclusions and implications: The sequential soil sampling approach was challenging in irrigated systems and we propose a hybrid measurement of pre-season available soil N and/or plant N uptake in nil N fertiliser plots as a means of estimating N derived from soil organic matter mineralisation.
Keywords: cotton, nitrogen mineralisation, vertosol furrow irrigation.
Introduction
Nitrogen (N) fertiliser use efficiency research in the Australian cotton industry has shown that only 30–60% of the synthetic fertiliser N applied prior to sowing is taken up by the plant (Constable and Rochester 1988; Humphreys et al. 1990; Rochester et al. 1993; Rochester 2011, 2012; Macdonald et al. 2017; Brackin et al. 2019). The research has shown that cotton plants also utilise N mineralised from the soil organic matter (SOM) reserves. A proportion of the applied fertiliser and soil mineralised N is lost from the cropping system via gaseous and irrigation runoff losses, while some is also temporarily immobilised by soil microbiota. Fertiliser N use efficiency in the Australia cotton industry has not improved in recent times (Macdonald et al. 2018), and the measurement and prediction of nitrogen source from SOM mineralisation is difficult (Mary and Recous 1994), which may explain why it is not utilised in fertiliser decisions. Nonetheless, SOM mineralisation is important N source for plant nutrition and in Australian cotton soils can exceed 120 kg N ha−1 (Rochester and Bange 2016). The rate of SOM mineralisation is influenced by multiple factors, including soil temperature and moisture, clay content, porosity and SOM content and characteristics.
The Australian cotton industry relies heavily on irrigation as well as N fertiliser to achieve the crop’s high lint yield potential (Roth et al. 2013; Macdonald et al. 2018). Irrigation scheduling is often triggered by the measurement of soil water deficits (ranging from 50 to 100 mm) created by crop water use. The use of different soil water deficits triggers alters the frequency and amount of irrigation water applied per event, which will in turn affect SOM mineralisation and N dynamics within irrigated cotton soils. The cycle of wetting and drying does produce a flush of SOM mineralisation and N release and in lab experiments the enhanced mineralisation ceases after three cycles (Mary and Recous 1994). Also, in furrow-irrigated systems where partial field irrigation (alternate-furrow irrigation) is employed, N mineralisation and N losses will vary across the irrigated furrow-plant line-non-irrigated furrow transect. This paper examines variations in soil N mineralisation in various positions in the irrigated paddock plant-bed (hill) and furrow system under two contrasting soil water deficits in 2 years of cotton production. The irrigation frequencies relied on soil water measurements averaged across treatments reaching 50-mm and 70-mm deficit in the 2017–2018, and 60-mm and 90-mm deficit in the 2018–2019 season.
Materials and methods
Soil measurements
The experimental site was located on a Grey Vertosol at the Australian Cotton Research Institute (ACRI), north-west NSW (30°20′S, 149°59′E). The soil has a neutral to alkaline pHw throughout (7.2–9.1 in 0–1200 mm), low organic carbon (1.0% in 0–150 mm; 0.7% in 150–300 mm) and medium clay content (43–58% in 0–1200 mm). The field experiments were conducted during the 2017–2018 and the 2018–2019 summer cotton cropping seasons. In-field net soil inorganic N sourced from SOM mineralisation was quantified in nil-N-fertilised field strip plots (8 × 1 m rows × 130–200 m) of both 50-mm and 70-mm soil water deficits treatments in 2017–2018 and 60-mm and 90-mm deficits (×3 reps) in 2018–2019. In both years, the experimental fields featured other N management treatments that were not sampled in this work. The soil water deficit triggers changed between years due to the increasingly limited availability of irrigation water in the second year. The cotton crop, Bollgard III variety, Sicot 748B3F (CSD, Australia), was grown in both years in a furrow irrigated system where alternate furrows were supplied with water during each irrigation event. No other fertiliser was applied to the crop.
Soil samples were collected throughout the growing season, from sowing to picking (October 2017–April 2018; October 2018–May 2019). Once field preparation and sowing were completed, an initial sample (t0) was collected. At the next time of sampling (and all following times), two soil samples were collected: (1) another soil sample was collected from the previous sampling area, approximately 0.1 m from the first core hole; and (2) a soil sample was collected from a new location established approximately 1 m from the first sampling point. At the new sampling position, all aboveground plants biomass in a 1 m length of the plant line (centred on the soil sample) were removed to prevent root uptake of newly mineralised N in between sampling events. Lateral roots from plants surrounding the 1 m sampling zone (1 m × 1 m) were cut using a spade along the perimeter to prevent the uptake of mineral N by neighbouring plants in between sampling times. Sampling was conducted prior to each irrigation event throughout the whole season. Therefore, every soil sampling after the initial event involved re-sampling soil at the previous sampling location, and sampling soil at a new nearby sampling location. In 2017–2018, this equated to 11 (50-mm deficit) and nine (70-mm deficit) sampling events. In 2018–2019, this equated to 12 (60-mm deficit) and nine (90-mm deficit) sampling events.
Soil samples were collected using a 50-mm diameter steel coring tube pushed to a depth of 300 mm. The soil sample was divided in the field into 0–150 mm and 150–300 mm depth segments, bagged and placed in an insulated box with ice-bricks. Only the plant line units were soil sampled in the three replicated treatments to determine the accumulation of inorganic N sourced from SOM mineralisation. The remaining field units, irrigated furrow, irrigated hill-side, non-irrigated hillside and non-irrigated furrow were sampled in only one treatment replicate to provide an observational data set.
The measurement of in situ net soil inorganic N and linkage to SOM mineralisation is challenging and the approach used in this study was based on the underlying theories of Raison et al. (1987). We did not use ‘Raison tubes’ because of the soil moisture artefacts such as prolonged saturation that would be generated after the crop irrigation. Instead, our approach was one of unconfined sequential coring of the undisturbed soil. In our study, aboveground plants were removed and the roots of the cotton plant were severed around the edge of the sampling location at the beginning of each measurement. The effect of foliage removal and root severance on net soil N mineralisation is not clear. Microbial immobilisation of N may result from the decomposition of severed roots (Adams et al. 1989), N release due to premature decomposition (Hatch et al. 1990) and modification of the rhizophere (Jussy et al. 2004). All of the net soil N mineralisation measurements occurred within a 14-day period, except the first and last measurements. This time length has been identified as the optimum time period to reduce the influence of the severed roots on N turnover (Adams et al. 1989).
Once field sampling was completed (<5 h), the soil samples were returned to the ACRI soil laboratory and stored in the refrigerator (4–6°C) overnight. The day after field sampling, the soil samples were weighed, and two sub-samples were collected. The first sub-sample (∼75 g) was oven-dried (105°C) for a week and reweighed to determine the soil water content. The dry bulk density of each sample was calculated using the corer volume and oven-dry soil weight (McKenzie et al. 2002). The second sub-sample (∼30 g dry weight equivalent) was used to determine exchangeable nitrate (NO3−-N) + nitrite (NO2−-N) (reported here simply as NO3-N, and ammonium (NH4+-N). This sample was mixed with 1 M KCl at a 1:5 ratio on an end-over-end shaker for 1 h. After the suspension settled for 0.5 h, the supernatant was filtered (Whatman No. 42) and frozen until analysis. Soil NO3-N and NH4-N were determined using method 7C2b (Rayment and Higginson 2011) using a LACHAT Quick Chem 8500 Series 2 flow injection analyser (Lachat Instruments, Milwaukee USA).
Net soil inorganic N content
The net soil inorganic N content for each sampling interval for each field location was determined from the change in the soil inorganic-N pool size over time (t):
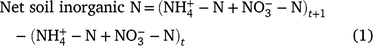
where t is the sum of the measured soil NO3-N and NH4-N (kg N ha−1) at the beginning of the season or prior to an irrigation and t + 1 is the sum of the measured soil NO3-N and NH4-N (kg N ha−1) prior to the subsequent irrigation event. The N results are reported in kg N ha−1. The net soil inorganic N is the amount of N sourced from SOM mineralisation remaining in the profile after denitrification, run-off, leaching (below the 300 mm sample depth) and irrigation additions, during each sampling interval.
The area weighted amount of the net soil inorganic N content at each sampling location (MinN) was determined according to Eqn 2.
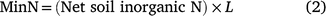
where L is the % area of the sampled field unit viz irrigated furrow (25%), irrigated hill-side (12.5%), plant-line (25%), non-irrigated hillside (12.5%) and non-irrigated furrow (25%), and reported as kg N ha−1.
Accounting for N leaching losses
During the 2018–2019 season, KBr tracer was used to estimate the amount of N lost (Kessavalou et al. 1996) from the 0–300 mm soil layer at each furrow-plant-bed position during the period between each paired soil sampling. Immediately after the collection of the initial soil sample at time t, 5 mL of 0.7 M Br− L−1 was injected to a depth of 75 mm approximately 0.1 m along the row from the initial soil core location. At the injection site, a marking flag was used to accurately position the sampling tube on the injection site at the subsequent soil sampling (t + 1). The Br−1 concentration in the subsequent soil sample was determined from the same KCl extract used for the N concentrations using Method 4500-Br−1 B (Rice et al. 2012) for UV-Vis spectrophotometer (Shimadzu UV-2700). The recovery and loss of Br in the soil after an irrigation event was calculated and the mass balance computed. The ratio of Br recovered in the soil was calculated as:

where Brt−1 is the soil Br concentration after the irrigation and Brt is the amount of Br added to the soil at time t. Br is biologically stable and it can be assumed that amount lost from the surface soil by leaching represents the maximum NO3-N leaching potential of the system (Kessavalou et al. 1996). It is possible to utilise Z, the ratio of Br recovered, to estimate the net N mineralisation by accounting for the leached N from sample time t according to Eqn 4.
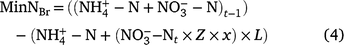
where x = 0.75 and is a factor to account for the difference in the leaching potentials of Br−1 and NO3− (Clay et al. 2004). To estimate the MinNBr during the 2017–2018 season, the MinN value was multiplied by the MinN/MinNBr ratio for the 2018–2019 season.
Irrigation and N run-off
San Dimas flumes (200 mm; Wilm et al. 1936) were used to measure the runoff volume of the sampled plots. The galvanised steel flumes were manufactured as outlined by Walkowiak (2008). Separate flumes were installed outside the actual cropping area in the tail end of each of the two soil water deficit treatments in each year. Runoff water from four inter-rows in each treatment was directed into the flume for flow measurement and flow-weighted water sample collection. The standard flow calibration equation (Nachimuthu et al. 2017) for converting flow height into flow discharge for a 200 mm San Dimas flume is:
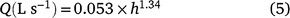
where Q is discharge and h is water height in the flume (mm). The flow height was measured using a Teledyne ISCO 730 bubbler module connected to a Teledyne ISCO 6712 standard portable water sampler, which logged the flow height in min intervals. The module uses a differential pressure transducer and a flow of bubbles to measure liquid levels to determine flow height. The samplers were programmed to capture a representative aliquot of all runoff from an irrigation event. After each runoff event, samples were collected and transported to laboratory the next morning. NO3-N and NH4-N were determined using method 7C2b (Rayment and Higginson 2011) using a LACHAT Quick Chem 8500 Series 2 flow injection analyser (Lachat Instruments). Field in-flow was measured with an ‘Irrimate Series 9’ siphon paddle-wheel meter. The N run-off losses were calculated with the following equation:

where A is the cross sectional area (m2), V is the average velocity of the water (ms−1) and C is the N concentration of the run-off water (g m3) (Wigginton et al. 2012).
Plant N uptake
The aboveground plant uptake of N in each treatment replicate was measured prior to each irrigation and prior to defoliation at the end of the season for the 1 m (linear) of plants that were removed prior to each soil sampling event. The main plant stalk was cut at the soil surface and the aboveground biomass placed in a bag. Immediately after cutting, the plants were returned to the site laboratory, weighed and the samples were dried in a dehydrator for 1 week at 70°C. The samples were re-weighed and then chopped and coarsely ground. Sub-samples were finely ground and total N concentration (N%) was measured by combustion analyser (EA1112, Thermo Finnigan, San Jose, CA, USA). The plant uptake N represents the apparent net soil mineralised and residual inorganic N left in the system (PlantN) and is the amount of N that the plant accumulates during the cropping season (Villar et al. 2014).
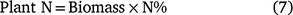
where biomass is the total dried mass of the sampled plant and N% is the N content of the plant sample.
An estimate of plant N uptake can be calculated from lint yield (Rochester and Bange 2016) using Eqn 8:
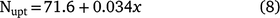
where x is the lint yield harvested from plots that have not received N fertiliser.
Apparent net inorganic N
An alternative method for estimating whole-of-season net inorganic N was calculated using pre- and post-season soil inorganic mineral N and maximum plant N uptake (0 N fertiliser plots) at harvest (Brackin et al. 2019).
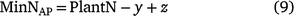
where y is pre-season soil N and z is the post-harvest N in the stored in the soil profile to a depth of 1.2 m. The pre-season soil N represents fertiliser carry over N from the previous season and any N sourced from mineralised SOM between each cropping periods. The weakness here was that the contribution of carry-over fertiliser cannot be determined. MinNAP does not account for soil N that was lost from the root zone during the season via leaching, runoff or denitrification or that which accumulated in the plant roots.
N content of the biomass at harvest is not routinely measured in commercial cotton fields. An estimated plant uptake N and hence, an estimated total inorganic N derived from SOM mineralisation (EMinNAP) was determined from the Nupt using Eqn 8. This estimate was examined to see if it would be a suitable rule of thumb approach.
Water use management
The varied irrigation rates were scheduled using soil water deficits; i.e. the amount of water extracted from the plant available water in the soil profile below full plant available water capacity (PAWC). The PAWC was calculated using site specific drained upper limits (DUL) and crop lower limits (cotton specific) as outlined in APSIM (Holzworth et al. 2014). The irrigation treatments included scheduling irrigation events at deficits that included 50, 60, 70 and 90 mm below the soil’s PAWC. Actual plant available water (PAW) was measured prior to and following each irrigation event throughout the growing season using a Neutron Moisture Meter (NMM) (CPN International, USA) at 150 mm increments from 150 to 900 mm and at 300 mm increments between 900 and 1200 mm.
A calibrated regression equation for each soil depth increment was developed for the site. Firstly, soil bulk density was measured following methods for shrink swell vertosols (course soil fragments) (Cresswell and Hamilton 2002) at a wet pond and dry soil site established in 2017 on an adjacent field. When the wet pond reached the DUL, a number of cores were removed. Along with soil removed from the dry soil site, the soil volumetric water (%) was calculated and calibrated to the NMM following methods outlined by Cull (1979) and (Dalgliesh and Foale 1998). For the 0–150 mm increment, the soil moisture was measured gravimetrically by soil sampling and oven drying (105°C, 48 h). At each sampling date, the recording rate of the NMM was calibrated via water (drum) counts (×36) and an air count at each aluminium access tube within each plot before measuring.
Actual water (Wi, mm) input to the soil from each irrigation event was calculated by the difference of soil PAW pre-irrigation and the measured PAW after each irrigation event.
Treatment evapotranspiration (ET, mm) values were calculated by:
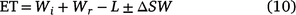
where Wi is the applied irrigation water, Wr is the in-crop rainfall, L is the amount of water lost to sub soil leaching (with the soil’s high clay content and low amount of natural leaching this was considered negligible) and ΔSW is the difference between the sowing soil moisture and the plant harvest soil moisture (Tennakoon and Milroy 2003).
Soil moisture readings at 35 cm – EM38MKII methods
Soil moisture at a depth of 350 mm was measured periodically throughout the growing season using an electromagnetic induction (EMI) device – EM38MKII (Genomics Ltd, Canada). Apparent bulk electrical conductivity (ECa) was converted to soil water content (mm) and PAW (mm) following the method outlined by (Huth and Poulton 2007). At each sampling date, the EM38MkII was calibrated to environmental conditions, and a temperature coefficient factor as outlined in (Huth and Poulton 2007) was used to correct for localised climatic conditions. To account for EC variability within the field, each plot was calibrated independently for soil PAWC.
Statistical analysis
The general least square method with autocorrelation function (nlme package) within the R statistical software was used to analyse the relationship between soil water deficit and time with cumulative inorganic N content and plant N uptake. The standard t-test method with the R statistical software package was used to determine statistical difference between the different irrigation management strategies for the lint yield and seed and lint N.
Seasonal day degree
Cumulative day degree for both seasons were calculated using the method outlined by Constable and Shaw (1988):
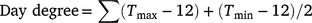
where Tmax is daily maximum temperature (below 36°C), Tmin is daily minimum temperature.
Results and discussion
Effect of irrigation on measured net soil inorganic N in the plant line
There was a significant interaction between deficit treatment and irrigation event in the amount of cumulative MinN measured in the plant line during both the 2017–2018 (F = 4.85, d.f. = 7, P < 0.05) and 2018–2019 (F = 2.63, d.f. = 7, P < 0.05) seasons. In both years, the lower deficit irrigation had greater cumulative MinN content in the plant line relative to the higher deficit (Fig. 1). In both seasons, there was more of the mineralised N retained in the soil between irrigations in the lower deficits (50-mm and 60-mm) than in the corresponding higher deficit treatments (Fig. 1). The MinN accumulation in the plant line from SOM mineralisation (Fig. 1) likely exceeded N losses via runoff, denitrification and immobilisation in the early season of both years. There were no deficit treatment effects early in the season as the different irrigation regimes only commenced once the plants were large enough (mid-December) to extract significant amounts of soil water (Fig. 2). From mid-December to early January, N losses outside the plant line were higher than the N accumulation in the plant line which led to a net decline in cumulative net soil inorganic N in the 70-mm and 90-mm treatments.

|
In both the higher deficits (70- and 90-mm), there was a period of reduced MinN accumulation during January where N losses via denitrification, leaching and runoff outweighed the net gains from SOM mineralisation. For example, during the month of January 2018, the N losses in the 70-mm deficit exceeded the amount of N accumulated from the SOM mineralisation process. This was likely due to the greater irrigation volume (4.3 ML ha−1) applied in that month compared to that applied in the 50-mm deficit treatment (3.4 ML ha−1; Fig. 2). During this period, the 50-mm deficit had more irrigation events, but less water was applied in total. The greater volume of water applied in fewer irrigation events in the 70-mm deficit likely increased waterlogging conditions and subsequent denitrification losses.
Previous research at the same site found that up to 85% of soil NO3-N can be lost from the soil via denitrification when the soil was waterlogged for 10 days (Rochester and Constable 2000). In 2017–2018, the more frequent wetting, and drying cycles in the 50-mm deficit compared to the 70-mm deficit led to greater net soil N mineralisation during January 2018. Patterns of NO3-N accumulation and immobilisation in these soils have been previously explained by variations in soil temperature and soil water deficit conditions (Rochester et al. 1991) so the alteration of soil moisture regime due to different soil water deficits would logically lead to the observed differences in MinN.
Effect of irrigation on measured plant N and cotton yield
The effect soil water deficit on plant N uptake, from plants sampled within the linear metre prior to the irrigation, was not significant in the 2017–2018 season (Fig. 3). This was due to the inherent variability in measured plant N content caused by the spatial variation in plot SOM mineralisation and plant growth. However, the plot lint and seed N at harvest was significantly different (t = 1.95, d.f. = 2, P < 0.10) between the two soil water deficits and the cotton yield was also greater in the 50-mm deficit (t = 2.19; d.f. = 2, P < 0.10) than the 70-mm deficit (Table 1). The plot scale measurements of seed and lint N measurements masked the inherent variability in SOM mineralisation, which affected linear metre biomass cuts and N measurements.

|

|
The plant N uptake was significantly greater (Tables 1 and 2) than the cumulative end of season MinN for both the 50-mm (t = 3.79, d.f. = 2, P < 0.1) and 70-mm deficits (t = 7.32, d.f. = 2, P < 0.05). The observed MinNAP was greater than the measured MinN for both the 50-mm and 70-mm soil water deficits during the 2017–2018 season (Table 2). The average net soil N mineralisation rate determined from the MinNAP over the season in the top 0–300 mm was 0.98–1.21 kg N ha−1 day−1, which was similar to other cotton soils in the region (Brackin et al. 2019).

|
During the 2018–2019 season, a hailstorm struck the crop in December 2018 and totally defoliated the aboveground biomass. This is evident in the plant N uptake between each season (Fig. 2) and the measured yields (Table 1). There was no effect of the soil water deficit treatment on the yield or the MinNAP or the lint and seed N (2018–2019). In both seasons, there was a synchronicity between crop N uptake and soil N mineralisation (Figs 1 and 3).
Observed net soil inorganic N variation in the plant line-furrow transect
In both the 2017–2018 and 2018–2019 cotton-growing seasons, the release and retention of inorganic N from SOM mineralisation was not uniform across the furrow-hill transect (Fig. 4). There were large observed differences in cumulative mineral N (NO3− + NH4+) in the 0–300 mm depth of the soil at the various sampled positions (Fig. 1). The MinN was greatest in the middle of the plant bed (the plant-line) followed by the non-irrigated hillside position, while least N accumulated in the irrigated furrow soils. We acknowledge that the reduced replication of sol samples out of the plant line limits interpretation; however, in furrow irrigated systems, our observations are consistent with the fact that N is concentrated into the plant line (Rauschkolb and Hornsby 1994). In alternate irrigation furrow systems, the applied water moves through the hill and into the non-irrigated furrow. This causes the movement of N from the irrigated side to the non-irrigated side of the hill (Macdonald et al. 2016; Macdonald et al. 2020). The approach used to estimate plant line MinN will still be influenced by the movement of N because the t sample occurs before the irrigation. The MinNBr, which accounts for potential N leaching, indicates that the net amount of N that sourced from SOM mineralisation was greater than determined by the MinN approach (Table 2).
Management implications
There was a definite effect of the different irrigation strategies on yield and lint and seed N uptake and inorganic N accumulation in the plant line during 2017–2018 season. This was not observed during the 2018–2019 season due to the hailstorm damage to the crop. While not conclusive over the 2-year measurement period, the 2017–2018 results indicate that different deficit irrigation will result in varied MinN and seed and lint N uptake. Denitrification losses, which have been estimated to be the main loss pathway in irrigation cotton systems (Macdonald et al. 2017; Brackin et al. 2019; Macdonald et al. 2020), and redistribution of N in the hill (Macdonald et al. 2020) could explain the variation in the end of season MinN between the deficit treatments. Strategies that reduce N redistribution in the hill or waterlogging such as with smaller soil water deficits should be examined. The results show that MinNAp sourced from SOM mineralisation was between 60 and 96 kg N ha−1 under the conditions of both measurement years. The management of SOM is important for N nutrition and should involve strategic residue management, minimum tillage of the hill (Hulugalle et al. 2020) and cover cropping.
In-field measurement of soil N mineralisation using the sequential sampling approach alone or coupled with KBr tracer is impractical for in-season N management by growers, agronomists and researchers. The logistical effort to make the measurements is a significant barrier. However, the use of zero N fertiliser strips or sections within a field is an opportunity for growers and agronomists to estimate soil N mineralisation and N carry-over.
Lint yield (t ha−1) from plants grown in the nil N strip can be used to estimate Nupt using Eqn 8. If this is coupled to pre-season soil N measurements, then EMinNAP can be calculated and potential N mineralisation estimated. The Nupt for the 70-mm deficit (t = 2.90, d.f. = 2, P < 0.1), 60-mm (t = 7.72, d.f. = 2, P < 0.01) and 90-mm deficit (t = 10.74, d.f. = 2, P < 0.01) was significantly greater than the measured PlantN (Table 1) and the observed EMinNAP is greater than the MinNAP (Table 2). This approach is far simpler than the use of soil methods employed in this study and does over come the in-field variation due to plot harvesting. While based on harvested yield, it will be too late to use this information to ameliorate any N deficiency in the crop. However, it does allow for the annual mineralisation rate to be determined and this information could then be used to modify fertilisation practice for future seasons.
Conclusions
Field experiments were conducted to assess the amount of inorganic N derived from soil organic matter mineralisation over two seasons (2017–2018 and 2018–2019) across treatments differing in irrigation frequency and amount. There was a significant difference in the yield between the 50- and 70-mm soil water deficit treatments in the 2017–18 season but not between the 60- and 90-mm soil water deficit treatments 2018–19. In the 2018–2019 season, a hailstorm caused significant damage to the crop. None-the-less during both seasons season there was a significant difference in the cumulative MinN plant line content between the and the soil water deficits. This indicates that N accumulation in the top 300 mm in the plant line from SOM mineralisation is affected by irrigation management. The management of SOM pool is critical for the ongoing delivery of mineralised N annually.
It was observed that the plant line had a greater inorganic N content relative to the rest of the field and was a potentially a hotspot for SOM mineralisation and/or inorganic N accumulation. It was observed that mineralisation of SOM within the top 300 mm supplied between 60 and 90 kg N ha−1 to the crop during the field experiment from the furrow-plant line transect.
In-field measurement of net soil N mineralisation in a flood furrow irrigation system using sequential paired soil sampling is challenging and subject to both temporary and permanent N loss processes. For growers and agronomists, estimates of apparent soil N mineralisation can be derived from the measured yields in zero N strips and pre- and post-season soil N measurements at the end of each season. Local calibration of yield and biomass N are needed improve this relationship. Over time, an estimate of average soil mineralisation potential could be developed.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was undertaken as a sub-project of the More Profit from Nitrogen Program, funded by the Australian Government Department of Agriculture, Water and the Environment as part of its Rural R&D for Profit programme and Cotton Research and Development Corporation.
Acknowledgements
We thank all the technical and casual staff from NSW DPI and CSIRO for their support with the trials and laboratory analysis, including Tim Grant, Stacey Cunningham, Wayne McPherson, Hugh Coman, Joseph Palmer, Murray Scott, Seija Tuomi and the ACRI farm staff.
References
Adams MA, Polglase PJ, Attiwill PM, Weston CJ (1989) In situ studies of nitrogen mineralization and uptake in forest soils; some comments on methodology. Soil Biology and Biochemistry 21, 423–429.| In situ studies of nitrogen mineralization and uptake in forest soils; some comments on methodology.Crossref | GoogleScholarGoogle Scholar |
Brackin R, Buckley S, Pirie R, Visser F (2019) Predicting nitrogen mineralisation in Australian irrigated cotton cropping systems. Soil Research 57, 247–256.
| Predicting nitrogen mineralisation in Australian irrigated cotton cropping systems.Crossref | GoogleScholarGoogle Scholar |
Clay DE, Zheng Z, Liu Z, Clay S, Trooien T (2004) Bromide and nitrate movement through undisturbed soil columns. Journal of Environmental Quality 33, 338–342.
| Bromide and nitrate movement through undisturbed soil columns.Crossref | GoogleScholarGoogle Scholar | 14964388PubMed |
Constable GA, Rochester IJ (1988) Nitrogen application to cotton on a clay soil: timing and soil testing. Agronomy Journal 80, 498–502.
| Nitrogen application to cotton on a clay soil: timing and soil testing.Crossref | GoogleScholarGoogle Scholar |
Constable GA, Shaw AJ (1988) Temperature requirements for cotton. Agfact P5.3.5.. Division of Plant Industries, New South Wales Department of Agriculture. Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/710796/Agfact-P5.3.5-Temperature-requirements-for-cotton.pdf
Cresswell H, Hamilton G (2002) Bulk density and pore space relations. In ‘Soil physical measurement and interpretation fro land evaluation’. (Eds N McKenzie, K Coughlan, H Cresswell) pp. 35–58. (CSIRO Publishing: Melbourne, Vic., Australia)
Cull P (1979) Irrigation scheduling of cotton by computer. PhD thesis, University of New England, Armidale, NSW, Australia.
Dalgliesh N, Foale M (1998) Soil Matters—Monitoring soil water and nutrients in dryland farming systems.
Hatch DJ, Jarvis SC, Philipps L (1990) Field measurement of nitrogen mineralization using soil core incubation and acetylene inhibition of nitrification. Plant and Soil 124, 97–107.
| Field measurement of nitrogen mineralization using soil core incubation and acetylene inhibition of nitrification.Crossref | GoogleScholarGoogle Scholar |
Holzworth DP, Huth NI, deVoil PG, Zurcher EJ, Herrmann NI, McLean G, Chenu K, van Oosterom EJ, Snow V, Murphy C, Moore AD, Brown H, Whish JPM, Verrall S, Fainges J, Bell LW, Peake AS, Poulton PL, Hochman Z, Thorburn PJ, Gaydon DS, Dalgliesh NP, Rodriguez D, Cox H, Chapman S, Doherty A, Teixeira E, Sharp J, Cichota R, Vogeler I, Li FY, Wang E, Hammer GL, Robertson MJ, Dimes JP, Whitbread AM, Hunt J, van Rees H, McClelland T, Carberry PS, Hargreaves JNG, MacLeod N, McDonald C, Harsdorf J, Wedgwood S, Keating BA (2014) APSIM – Evolution towards a new generation of agricultural systems simulation. Environmental Modelling & Software 62, 327–350.
| APSIM – Evolution towards a new generation of agricultural systems simulation.Crossref | GoogleScholarGoogle Scholar |
Hulugalle NR, Nachimuthu G, Kirkby K, Lonergan P, Heimoana V, Watkins MD, Finlay LA (2020) Sowing maize as a rotation crop in irrigated cotton cropping systems in a vertosol: effects on soil properties, greenhouse gas emissions, black root rot incidence, cotton lint yield and fibre quality. Soil Research 58, 137–150.
| Sowing maize as a rotation crop in irrigated cotton cropping systems in a vertosol: effects on soil properties, greenhouse gas emissions, black root rot incidence, cotton lint yield and fibre quality.Crossref | GoogleScholarGoogle Scholar |
Humphreys E, Freney JR, Constable GA, Smith JWB, Lilley D, Rochester IJ (1990) The fate of your N fertiliser. In ‘Fifth Australian cotton conference: the Australian cotton industry under the microscope, August 8th–9th 1990, Broadbeach, Queensland’. Vol. 452, pp. 161–170. (Australian Cotton Growers’ Research Association: Broadbeach, Qld, Australia)
Huth NI, Poulton PL (2007) An electromagnetic induction method for monitoring variation in soil moisture in agroforestry systems. Australian Journal of Soil Research 63–72.
| An electromagnetic induction method for monitoring variation in soil moisture in agroforestry systems.Crossref | GoogleScholarGoogle Scholar |
Jussy J-H, Ranger J, Bienaimé S, Dambrine E (2004) Effects of a clear-cut on the in situ nitrogen mineralisation and the nitrogen cycle in a 67-year-old Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) plantation. Annals of Forest Science 61, 397–408.
| Effects of a clear-cut on the in situ nitrogen mineralisation and the nitrogen cycle in a 67-year-old Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) plantation.Crossref | GoogleScholarGoogle Scholar |
Kessavalou A, Doran JW, Powers WL, Kettler TA, Qian JH (1996) Bromide and nitrogen-15 tracers of nitrate leaching under irrigated corn in central Nebraska. Journal of Environmental Quality 25, 1008–1014.
| Bromide and nitrogen-15 tracers of nitrate leaching under irrigated corn in central Nebraska.Crossref | GoogleScholarGoogle Scholar |
Macdonald BCT, Chang YF, Nadelko A, Tuomi S, Glover M (2017) Tracking fertiliser and soil nitrogen in irrigated cotton: uptake, losses and the soil N stock. Soil Research 55, 264–272.
| Tracking fertiliser and soil nitrogen in irrigated cotton: uptake, losses and the soil N stock.Crossref | GoogleScholarGoogle Scholar |
Macdonald BCT, Latimer JO, Schwenke GD, Nachimuthu G, Baird JC (2018) The current status of nitrogen fertiliser use efficiency and future research directions for the Australian cotton industry. Journal of Cotton Research 1, 15
| The current status of nitrogen fertiliser use efficiency and future research directions for the Australian cotton industry.Crossref | GoogleScholarGoogle Scholar |
Macdonald BCT, Nachimuthu G, Chang YF, Nadelko AJ, Tuomi S, Watkins M (2020) Nitrogen composition in furrow irrigated run-off water. Agricultural Water Management 242, 106399
| Nitrogen composition in furrow irrigated run-off water.Crossref | GoogleScholarGoogle Scholar |
Macdonald BCT, Nadelko A, Chang Y, Glover M, Warneke S (2016) Contribution of the cotton irrigation network to farm nitrous oxide emissions. Soil Research 54, 651–658.
| Contribution of the cotton irrigation network to farm nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar |
Mary B, Recous S (1994) Measurement of nitrogen mineralization and immobilization fluxes in soil as a means of predicting net mineralization. European Journal of Agronomy 3, 291–300.
| Measurement of nitrogen mineralization and immobilization fluxes in soil as a means of predicting net mineralization.Crossref | GoogleScholarGoogle Scholar |
McKenzie N, Coughlan K, Cresswell H (2002) ‘Soil physical measurement and interpretation for land evaluation’. (CSIRO Publishing: Collingwood, Vic., Australia)
Nachimuthu G, Halpin NV, Bell MJ (2017) Impact of practice change on runoff water quality and vegetable yield—an on-farm case study. Agriculture 7, 30
| Impact of practice change on runoff water quality and vegetable yield—an on-farm case study.Crossref | GoogleScholarGoogle Scholar |
Raison RJ, Connell MJ, Khanna PK (1987) Methodology for studying fluxes of soil mineral-N in situ. Soil Biology and Biochemistry 19, 521–530.
| Methodology for studying fluxes of soil mineral-N in situ.Crossref | GoogleScholarGoogle Scholar |
Rauschkolb RS, Hornsby AG (1994) ‘Nitrogen management in irrigated agriculture’. (Oxford University Press: New York, NJ, USA)
Rayment GE, Higginson FR (2011) ‘Soil chemical methods – Australasia’. (Inkarta Press: Melbourne, Vic., Australia)
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) ‘Standard methods for the examination of water and wastewater’. 22th edn. (American Public Health Association - American Water Works Association - Water Environment Federation: Baltimore, MD, USA)
Rochester IJ (2011) Assessing internal crop nitrogen use efficiency in high-yielding irrigated cotton. Nutrient Cycling in Agroecosystems 90, 147–156.
| Assessing internal crop nitrogen use efficiency in high-yielding irrigated cotton.Crossref | GoogleScholarGoogle Scholar |
Rochester IJ (2012) Using seed nitrogen concentration to estimate crop N use-efficiency in high-yielding irrigated cotton. Field Crops Research 127, 140–145.
| Using seed nitrogen concentration to estimate crop N use-efficiency in high-yielding irrigated cotton.Crossref | GoogleScholarGoogle Scholar |
Rochester IJ, Bange M (2016) Nitrogen fertiliser requirements of high-yielding irrigated transgenic cotton. Crop and Pasture Science 67, 641–648.
| Nitrogen fertiliser requirements of high-yielding irrigated transgenic cotton.Crossref | GoogleScholarGoogle Scholar |
Rochester IJ, Constable GA (2000) Denitrification and immobilisation in flood-irrigated alkaline grey clays as affected by nitrification inhibitors, wheat straw, and soil texture. Soil Research 38, 633–642.
| Denitrification and immobilisation in flood-irrigated alkaline grey clays as affected by nitrification inhibitors, wheat straw, and soil texture.Crossref | GoogleScholarGoogle Scholar |
Rochester IJ, Constable GA, Macleod DA (1993) Cycling of fertilizer and cotton crop residue nitrogen. Soil Research 31, 597–609.
| Cycling of fertilizer and cotton crop residue nitrogen.Crossref | GoogleScholarGoogle Scholar |
Rochester IJ, Constable GA, MacLeod DA (1991) Mineral nitrogen dynamics in a fallow grey clay. Australian Journal of Experimental Agriculture 31, 237–244.
| Mineral nitrogen dynamics in a fallow grey clay.Crossref | GoogleScholarGoogle Scholar |
Roth G, Harris G, Gillies M, Montgomery J, Wigginton D (2013) Water-use efficiency and productivity trends in Australian irrigated cotton: a review. Crop and Pasture Science 64, 1033–1048.
| Water-use efficiency and productivity trends in Australian irrigated cotton: a review.Crossref | GoogleScholarGoogle Scholar |
Tennakoon SB, Milroy S (2003) Crop water use and water use efficiency on irrigated cotton farms in Australia. Agricultural Water Management 61, 179–194.
| Crop water use and water use efficiency on irrigated cotton farms in Australia.Crossref | GoogleScholarGoogle Scholar |
Villar N, Aizpurua A, Castellón A, Ortuzar MA, Moro MBG, Besga G (2014) Laboratory methods for the estimation of soil apparent N mineralization and wheat N uptake in calcareous soils. Soil Science 179, 84–94.
| Laboratory methods for the estimation of soil apparent N mineralization and wheat N uptake in calcareous soils.Crossref | GoogleScholarGoogle Scholar |
Walkowiak DK (Ed.) (2008) ‘ISCO open channel flow measurement handbook’. (Teledyne ISCO)
Wigginton D, Pendergast L, Foley JL (2012) Metering. In ‘WATERPak: a guide for irrigation management in cotton and grain farming systems’ 3rd edn. (Ed D Wigginton) pp. 92−112. (Cotton Research and Development Corporation)
Wilm HG, Cotton JS, Storey HC (1936) Experiments with critical-depth flumes for measurement of flow in debris-laden streams. Eos, Transactions American Geophysical Union 17, 528
| Experiments with critical-depth flumes for measurement of flow in debris-laden streams.Crossref | GoogleScholarGoogle Scholar |




