Priorities in the implementation of partner services for HIV/STIs in high-income nations: a narrative review of evidence and recommendations
Matthew R. Golden A , Jo Gibbs B , Charlotte Woodward B * and Claudia S. Estcourt C
B * and Claudia S. Estcourt C
A Center for AIDS and STD, University of Washington, and Public Health – Seattle & King County, Seattle, WA, USA.
B Centre for Population Research in Sexual Health & HIV, Institute for Global Health, University College London, London, UK.
C School of Health & Life Science, Glasgow Caledonian University, Glasgow, UK.
Sexual Health - https://doi.org/10.1071/SH22060
Submitted: 11 April 2022 Accepted: 30 June 2022 Published online: 16 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Partner notification (PN) remains a crucial prevention tool to reduce sexually transmitted infection (STI) transmission and prevent STI-related morbidity. Although there have been a variety of different approaches taken to facilitate the notification, testing and management of sexual contacts of STIs and HIV, there is an increasing acknowledgement that these interventions are unscalable and have relatively little impact on disease transmission. At the same time, an expanding body of evidence supports a shift in the emphasis of STI outreach-related work from an exclusive focus on PN to an approach that incorporates epidemiologic data collection, case management, and PN, an approach that is sometimes called partner services (PS). In this review, we appraise the current evidence base for different PN interventions for STIs in high-income nations, make recommendations for best practices, present a schema for how public health programs might prioritise PS for different programs, and identify priority research questions related to PN.
Keywords: contact tracing, HIV/AIDS, partner notification, STIs.
Introduction
Partner notification (PN) is a longstanding component of clinical and public health efforts to control sexually transmitted infections (STIs), including HIV infection.1,2 The primary objective of PN is to ensure that potentially exposed sex and needle-sharing partners learn of their exposure, test for STIs, and receive recommended treatment. Due largely to variability in the severity of morbidity associated with different STIs and the scale of different STI epidemics, the approaches taken to achieve this goal have varied between infections (Table 1). Many nations have invested substantial resources to provide assisted partner notification services (APS) to persons with HIV and syphilis, relatively morbid and uncommon infections, while relying on less resource-intensive methods to promote PN for STIs such as gonorrhoea and chlamydia, which are more prevalent and associated with relatively low morbidity.

|
There is increasing recognition that our approach to PN is antiquated and inadequate. The public health PN system in the US, UK, and many other high-income nations was designed to confront the syphilis epidemic in the wake of the Second World War. In some nations, it was subsequently expanded to affect people with HIV infection and, less frequently, gonorrhoea and chlamydia. But the traditional approach to APS is resource-intensive, unscalable for most bacterial STIs, increasingly recognised as ineffective in promoting partner testing and treatment, and ill-suited to a world in which large numbers of people with STIs meet and communicate with their sex partners online and some sexually transmitted pathogens exhibit increased antimicrobial resistance (AMR).
The current system is not what we need, but that is not to say that PN is unimportant or should be abandoned altogether. The problem is that our current approach is unnecessarily narrow in scope and characterised by a poverty of imagination and resources. We have failed to consistently bring innovations to scale (e.g. expedited partner therapy (EPT)) and to define the value of new technologies in promoting PN and then deploy those technologies effectively. Moreover, traditional public health PN efforts fail to capitalise on opportunities to achieve wider public health objectives (e.g. promotion of HIV pre-exposure prophylaxis [PrEP]). Increasing recognition of this reality has prompted a shift in STI outreach activities and an associated change in the terminology used to describe these activities from PN to partner services (PS) or field services, terms that include a wider array of interventions affecting both index cases and their contacts.
The need to modify the content and organisation of STI PS has accelerated in the context of the ongoing coronavirus disease 2019 (COVID-19) pandemic. Early experience with the COVID-19 pandemic highlighted the need for a trained and versatile workforce capable of effective contact tracing in response to emerging threats to the public’s health. The staff employed to provide STI and HIV field services should be a central part of that workforce.
In this paper, we summarise evidence related to different approaches to PN for STIs in high-income nations, make recommendations related to STI PS best practices, and identify priority research questions related to STI PS. We do not address network interventions here and concentrate primarily on STIs other than HIV and on PS in high-income nations, recognising that the intervention is also important in low- and middle-income (LMIC) nations, a topic that has been reviewed elsewhere.3
Traditional, assisted partner notification
The scientific literature typically describes three main approaches to PN: patient referral, provider referral, and conditional referral. However, these terms mask substantial heterogeneity in the delivery of each of the interventions. For example, patient referral involves the index patient contacting their sex partner(s) to inform them of the need for testing and treatment and to refer them to a clinical/sexual health service. But, in some instances, clinics support index patients by providing them with online or paper information and resources or serial telephone calls to encourage PN. This type of patient referral has been termed enhanced or assisted patient referral. Provider referral involves the clinical service/public health team contacting sex partners directly and is usually done anonymously in that the provider does not disclose the index patient’s identity. Here too, there is substantial variance in the intervention provided. Some health departments send public health workers to index cases’ homes or workplaces, investing many hours of work in each case, whereas others rely primarily on phone calls, sometimes simply offering index cases assistance with little follow up.
Although recent controlled studies from sub-Saharan Africa support the efficacy of provider and conditional referral for HIV,4–7 the data supporting the efficacy of provider referral from high-income nations are limited and dated. Two randomised trials conducted in the 1980s, only one of which was published, found that provider referral increased partner treatment among male sexually transmitted disease clinic patients,8,9 a finding also observed in a small trial of HIV PN.10 However, other non-randomised experimental and quasi-experimental studies from the 1970s to the 1980s reported mixed results.11–15 Moreover, several studies suggest that the effectiveness of traditional PS has eroded over time, and that many of the partners defined as treated through PN would have been treated in the absence of any intervention.16–18 Summarising data from US studies of syphilis APS published between 1995 and 2003, Brewer reported a median brought-to-treatment index of 0.22, whereas a recent study of syphilis APS in seven US jurisdictions from 2015 to 2017 reported an index of 0.15.16,19 HIV PN effectiveness also appears to have declined over time in both the US and UK.19–23 Overall, these studies suggest that provider or conditional referral can increase PN and treatment, but the magnitude of that effect is uncertain, and most recent studies suggest the impact of the intervention is small.
Expedited partner therapy and accelerated partner therapy
Starting in the 1990s, recognition that traditional PN was not scalable for gonorrhoea, chlamydia and trichomonas prompted a series of studies designed to improve partner treatment using lower-intensity interventions.24–27 The basic idea behind these studies was to make it easier and quicker for the sex partner to access treatment and, in some cases, testing and treatment. These interventions are known as EPT. EPT involves a healthcare professional giving the index patient antibiotics or a prescription for their partner(s) and has been used for PN in people with chlamydia, gonorrhoea, and Trichomonas vaginalis. A systematic review found that EPT results in lower proportions of index cases experiencing repeated curable STIs than simple patient referral,28 though the impact of EPT on rates of recurrent gonorrhoea appears to be much greater than the impact on chlamydia or trichomonas.25 A study from Washington State in the US found that EPT could be brought to scale when free EPT medication was made widely available and suggested that the intervention may have reduced chlamydia and gonorrhoea rates at the population level.29 But efforts to widely implement EPT in other parts of the US have met with more limited success, owing to failure of medical providers to offer index cases the intervention, electronic medical records that have no mechanism to facilitate prescriptions for partners, and the fragmented nature of US healthcare financing, which discourages insurers from paying for medication for sex partners who are insured through other entities.30
EPT, as practised in the US, does not require that sex partners directly communicate with medical providers and does not comply with prescribing guidance in many countries. This led to the development of accelerated partner therapy (APT), an adaptation of EPT, which has been almost exclusively used in the UK to date.31,32 APT complies with UK prescribing guidance because a healthcare professional assesses the appropriateness of prescribing antibiotics for the partner. In brief, the healthcare professional performs a telephone consultation with the sex partner in private during the index patient’s clinic attendance. If medically safe, the index patient receives an APT pack, containing antibiotics and self-sampling kits for STI and HIV to deliver to their sex partner(s), or the clinic may post the APT pack to the sex partner. In pilot studies, APT resulted in faster sex partner treatment and greater overall numbers of sex partners treated when compared with routine care, but lower levels of testing for HIV and other STIs when offered without HIV testing as part of the pack.31,32
A recent large randomised controlled trial of APT for people with chlamydia showed that the offer of APT as an additional contact tracing method to usual care likely caused a small reduction in repeat chlamydia infection 12–24 weeks after treatment and an increase in proportion of sex partners treated, compared with usual care alone. The authors concluded that APT can be safely offered as a cost-saving contact tracing option for heterosexual people with chlamydia, and might reduce the risk of repeat infection, particularly for those in emotionally connected relationships, although uptake needs to be improved and novel approaches are needed for one-off partners.33–35
Digital partner notification interventions for STIs and HIV
As global communication becomes increasingly digitalised, digital interventions could improve the effectiveness and efficiency of PN processes. The majority of digital PN interventions aim to improve notification rates (the proportion of sex partners informed) and include anonymised notification.36 A variety of different digital health technologies have been used, including SMS, email, social media and PN apps. These are usually provided in addition to traditional PN methods. A low number of apps allows index patients to share their test results with sex partners and healthcare professionals.37
Many of the published evaluations of digital PN interventions are hypothetical preference studies. Of the studies included in a recent review,36 very few were based on real-world PN for people with gonorrhoea, chlamydia, and/or syphilis,38–41 and few reported ‘downstream’ sex partner outcomes, such as the number or proportion of partners tested or treated.39,40,42–46 Data from Australia suggest that a digital PN intervention that allows users to notify contacts (either anonymously or by name) using short-message service (SMS) can be brought to scale with increased use over time.47 Many studies of digital PN do not include a control group. The most common measure of effectiveness was number of partners notified and there is very little evidence on, or recognition of the importance of, health economic parameters and equity indicators or digital exclusion.
Acceptability of digital interventions to index patients is associated with relationship type.39,48–50 This is not surprising given that some digital interventions involve anonymised sex partner messaging, and numerous PN studies have shown that index patients prefer telling their established (more emotionally connected) sex partners in person.51–53 However, it is unclear whether people liked anonymous digital PN because of the anonymous aspect, or because the anonymous aspect was delivered digitally. Barriers to index patient uptake of digital PN interventions include concern that sex partners would not trust (or act on) the digital notification and worries about privacy and confidentiality.48,54,55 Low trust in the received notification message is also a concern for sex partners and, to some extent, healthcare professionals,55–57 and could affect subsequent care-seeking decisions.54
There remains considerable potential for digital interventions to enhance PN beyond simply notifying a greater proportion of exposed sex partners rapidly. More ambitious digital PN interventions could include comprehensive partner management,37 including linking people with wider prevention interventions such as HIV PrEP, vaccinations, and risk reduction more broadly. One UK exploratory study of people with chlamydia managed within an online eSexual health clinic enabled sex partners to do an online automated consultation and collect antibiotics from a community pharmacy.58 Partner uptake was low, but those who accessed the PN and management system found it highly acceptable.
Digital PN interventions have intuitive appeal as they are responsive to how people meet partners within an increasingly digital world and could be rolled out at scale. However, although these interventions show promise in increasing the number of sex partners informed of their exposure to infection, the ‘active ingredient’ in many digital interventions is opaque and we do not know whether they lead to more partners getting tested and or treated. More work is needed to determine the place, if any, of digital PN interventions. This will ideally include controlled evaluations of sex partner and index patient outcomes such as numbers of partners tested and treated and health economic and equity analyses. We know that people make careful choices of preferred methods of PN for different infections and different partner types. Inclusive, co-designed, evidence-based digital interventions will be needed as part of a wider menu of PN options if we are to adequately meet people’s needs and expectations and impact STI and HIV transmission.
Use of empirical treatment within PN
In many countries, antibiotics are recommended for sex partners of index patients for a range of STIs, either as well as or instead of testing. Historically, this made sense because nucleic acid amplification tests were available if routine testing options lacked sensitivity. In the era of highly sensitive tests for many STI pathogens and the emergence of AMR in pathogens such as Neisseria gonorrhoeae and Mycoplasma genitalium, some believe that this practice should be abandoned or reassessed59,60 (syphilis is an exception here because serological tests are not highly sensitive in persons with incubating and primary syphilis). Such a change in practice would also potentially influence EPT and APT, both of which involve empiric treatment of partners. However, structural barriers such as access to care, the need to pay for testing in addition to treatment, the need to wait out the appropriate window period before testing, and the need to abstain from sex for a longer period may make this a challenge. Data from diverse populations on the safety and effectiveness of a test before treatment approach to partners are needed before the current practice of empiric therapy should be abandoned.
Integrating outcomes other than STI treatment into partner notification
One way to improve the effectiveness and cost-effectiveness of PN may be to expand the intervention beyond STI case-finding, to shift from an exclusive emphasis on PN to a more expansive model of PS.61 STI PSpresent an opportunity to link index cases and their partners to HIV testing, PrEP, HIV medical care, and other services. In a country such as the US, where most STIs are diagnosed outside of specialised sexual health clinics, integrating HIV testing into population-based PS for bacterial STIs can significantly increase the proportion of index cases who test for HIV.62 In North Carolina and Mississippi, 1.6% and 2.3% of index cases receiving syphilis PS were newly diagnosed with HIV infection when HIV testing was made an explicit PS goal.17,63 Studies from Seattle, Chicago, and Iowa suggest that between one-quarter and one-third of HIV-negative men who have sex with men (MSM) who are not on PrEP can be successfully linked to PrEP through PS.64–66 Data on the success of PS in relinking out-of-care HIV-positive persons to care are limited, but a study of syphilis and gonorrhoea PS in six US jurisdictions found that 29% of previously diagnosed HIV-positive persons receiving PS for syphilis or gonorrhoea in 2016–17 – 17 574 people – were out of care or not virally suppressed, suggesting that PS could play a large role in increasing engagement in HIV care.67 In general, the marginal cost of integrating these outcomes into the services provided to people who are already receiving PS is low.68,69 Whether these non-traditional outcomes can justify the expansion of PS programs beyond their current scope will depend on the relative cost-effectiveness of competing alternatives to achieve similar outcomes.
Best practices and priority questions
Existing data suggest that the efficacy of traditional approaches to APS has eroded over time; EPT and APT have yet to go to scale, are not relevant to syphilis, and may not be ideal in an era of increasing AMR and concern related to antibiotic stewardship; and digital approaches, though intuitively appealing, are not known to be effective. These conclusions should animate an effort to modernise our approach to PS through an integrated program of public health practice innovation and research. There are many outstanding questions about how to organise and implement PS, but the existing literature and experience suggest some principles that should guide public health authorities as they revise and implement PS programs better suited to the contemporary HIV/STI landscape (Table 2). PN is voluntary and requires that workers maintain strict confidentiality, never revealing the identity of index cases to the partners they name. At the same time, APS is not a study nor is it a medical procedure; it’s a public health activity. As such, the rules of informed consent applied to research studies and medical procedures do not necessarily apply. The United States President’s Emergency Plan for AIDS Relief (PEPFAR), which funds activities to control the HIV pandemic in over 50 countries, recommends obtaining formal consent before enquiring about sexual partners and biological children. This is not routinely done in the US, Western European or Australia. Whether a formal consent process acts as a barrier to PN or a welcome reassurance is unknown. Different countries and local jurisdictions have different perspectives on the balance between ensuring that PN is non-coercive and protecting the public’s health. Good APS programs and workers respect the voluntary nature of APS, but also actively encourage index cases to ensure that their partners are notified, tested, and treated.

|
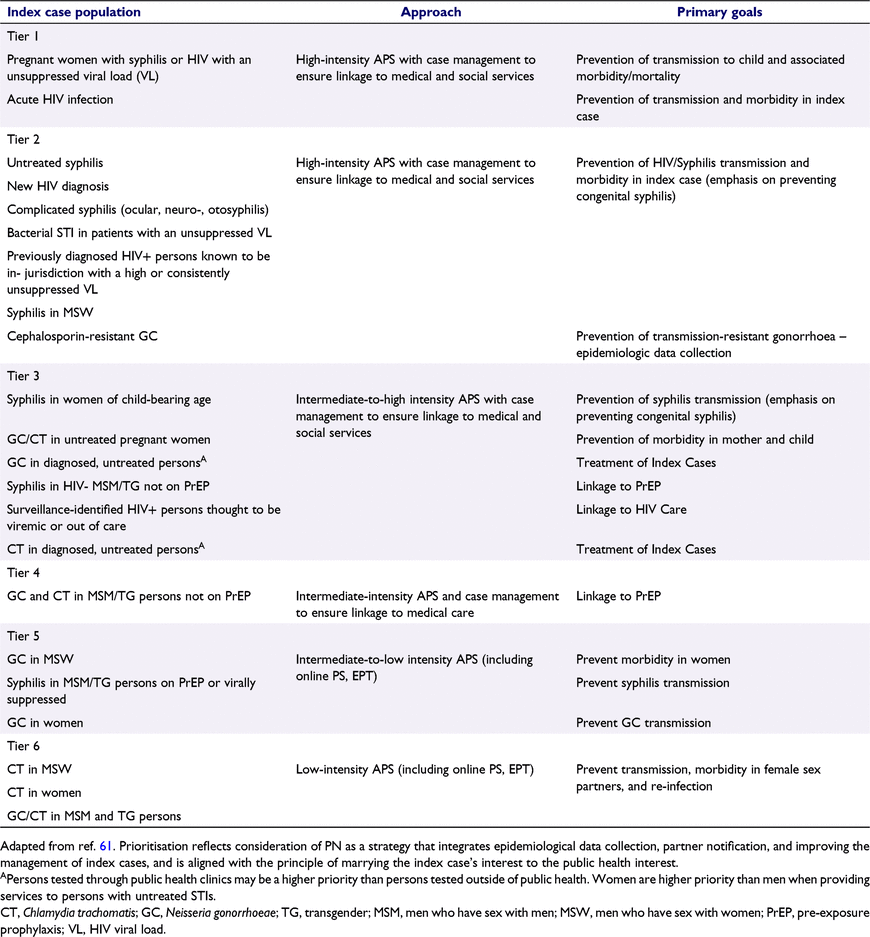
Ideally, APS programs develop a comprehensive, population-based approach to PS that employs an array of methods of variable cost and intensity based on the scale of different STI epidemics, the morbidity associated with different infections, evidence on the effectiveness and cost-effectiveness of interventions, and the resources available. The content of PS interventions should vary, but in almost all instances should seek to integrate epidemiologic data collection to fulfill important surveillance functions (Gibbs et al.70), activities that benefit index cases (e.g. linkage to HIV and PrEP), and activities designed to prevent disease transmission and/or benefit cases’ sex and/or needle-sharing partners. The diverse objectives of PS programs and the frequent need to triage cases based on characteristics such as gender, gender of sex partners, and HIV status requires an interdisciplinary team that includes epidemiologists, clinicians, and disease investigators skilled in aspects of case management. Table 3 presents an example of how a program might prioritise services, focusing the most intensive and costly interventions on pregnant women with HIV and syphilis (Tier 1 in the example) while providing low-cost, low-intensity interventions (e.g. EPT, online notification) for highly prevalent infections with less associated morbidity and AMR concerns, such as chlamydia (Tier 6). These priorities will vary depending on the epidemiology of different infections in different settings, as well as the objectives PS programs seek to advance. As shown in the table, in many instances, PN and treatment may not be the primary objective of PS. For example, among HIV- negative MSM with syphilis, promoting increased use of PrEP among index cases may be more important than PN, particularly given evidence that our current approach to PN among MSM yields very modest benefits.
|
Good field services programs give index cases and partners choices. In high-income nations, most index patients prefer to tell their sex partners directly,52 but preferences, where choices exist and/or in hypothetical preference studies, are associated with sex partner type.50,52 In high-income nations, provider referral tends to be restricted to one-off or less emotionally connected (casual) partners. Giving patients alternatives, so that they can choose the most acceptable PN method, which might differ for different sex partners, is considered optimal practice.71
PN programs need to be able to change in response to changing needs and circumstances. In some countries, the COVID-19 pandemic required programs to transfer many STI disease investigators (public health staff who investigate cases of STI and provide APS) to new roles related to COVID-19 case investigation and contact tracing. This required reprioritising STI PN work and highlighted the importance of trained disease investigators in a public health emergency. Some nations, such as the US, are now investing substantial new resources to build larger teams of disease investigators, some of whom will focus on STIs. Although generic skills underpin all contact tracing practice, STIs and HIV present additional challenges due to the stigma associated with these infections and training needs to address the additional sensitivities in this field. This reality should prompt an effort to define core disease investigator competencies as well as the more specialised skills needed for different contact tracing activities. Such training programs should prepare investigators to focus on specific areas of communicable disease while ensuring that they are prepared to shift work in response to an emergency.
Although the existing body of scientific data and long experience allows one to define some best practices related to PN, a great deal of PN work lacks a sound scientific footing. Developing a more effective approach to this basic aspect of HIV/STI disease control requires a robust research agenda. Table 4 presents some key outstanding questions in PN -related research. These include the need for more and better data on all components of PN discussed above: traditional APS, EPT/APT, digital interventions, and outcomes other than case-finding and partner treatment.

|
PN remains a cornerstone of efforts to control HIV/STIs. The current system used in most high-income nations is outdated, but the field is not bereft of promising opportunities to improve PN outcomes. Achieving that objective will require investments in an integrated program of research and public health practice improvement and an openness to changes in how programs are organised, staffed, and monitored.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
Claudia Estcourt and Matthew Golden are guest Editors of Sexual Health, but were blinded from the peer-review process for this paper. All other authors declare that they have no relevant conflicts of interest.
Declaration of funding
Research reported in this publication was supported by the University of Washington/Fred Hutch Center for AIDS Research, an NIH-funded program under award number AI027757, which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK.
References
[1] Althaus CL, Turner KME, Mercer CH, Auguste P, Roberts TE, Bell G, Herzog SA, Cassell JA, Edmunds WJ, White PJ, Ward H, Low N. Effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable sexually transmitted infections: observational study, systematic reviews and mathematical modelling. Health Technol Assess 2014; 18 1–100.| Effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable sexually transmitted infections: observational study, systematic reviews and mathematical modelling.Crossref | GoogleScholarGoogle Scholar |
[2] Golden M, Faxelid E, Low N. Partner notification for sexually transmitted infections including HIV infection: an evidence-based assessment. In: Holmes KK, editor. Sexually transmitted diseases. New York, NY: McGraw Hill; 2008. pp. 965–83.
[3] Alam N, Chamot E, Vermund SH, Streatfield K, Kristensen S. Partner notification for sexually transmitted infections in developing countries: a systematic review. BMC Public Health 2010; 10 19
| Partner notification for sexually transmitted infections in developing countries: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[4] Cherutich P, Golden MR, Wamuti B, Richardson BA, Asbjornsdottir KH, Otieno FA, Ng’ang’a A, Mutiti PM, Macharia P, Sambai B, Dunbar M, Bukusi D, Farquhar C, aPS Study Group Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV 2017; 4 e74–82.
| Assisted partner services for HIV in Kenya: a cluster randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[5] Myers RS, Feldacker C, Cesar F, Paredes Z, Augusto G, Muluana C, Citao S, Mboa-Ferrao C, Karajeanes E, Golden MR. Acceptability and effectiveness of assisted human immunodeficiency virus partner services in mozambique: results from a pilot program in a public, urban clinic. Sex Transm Dis 2016; 43 690–5.
| Acceptability and effectiveness of assisted human immunodeficiency virus partner services in mozambique: results from a pilot program in a public, urban clinic.Crossref | GoogleScholarGoogle Scholar |
[6] Rosenberg NE, Mtande TK, Saidi F, Stanley C, Jere E, Paile L, Kumwenda K, Mofolo I, Ng’ambi W, Miller WC, Hoffman I, Hosseinipour M. Recruiting male partners for couple HIV testing and counselling in Malawi’s option B+ programme: an unblinded randomised controlled trial. Lancet HIV 2015; 2 e483–91.
| Recruiting male partners for couple HIV testing and counselling in Malawi’s option B+ programme: an unblinded randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[7] Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, Dominik RC, Kaufman JS, Mapanje C, Martinson F, Cohen MS, Hoffman IF. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr 2011; 56 437–42.
| HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention.Crossref | GoogleScholarGoogle Scholar |
[8] Katz BP, Danos CS, Quinn TS, Caine V, Jones RB. Efficiency and cost-effectiveness of field follow-up for patients with Chlamydia trachomatis infection in a sexually transmitted diseases clinic. Sex Transm Dis 1988; 15 11–6.
| Efficiency and cost-effectiveness of field follow-up for patients with Chlamydia trachomatis infection in a sexually transmitted diseases clinic.Crossref | GoogleScholarGoogle Scholar |
[9] Cleveland JQ. A cost-effective study of alternative methods for Gonorrhea contact referral and rescreening. Unpublished manuscript reviewed in: Oxman AD, Scott EAF, Sellors JW, et al. Partner notification for sexually transmitted diseases: an overview of the evidence. Can J Public Health 1994; 85 127–32.
[10] Landis SE, Schoenbach VJ, Weber DJ, Mittal M, Krishan B, Lewis K, Koch GG. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med 1992; 326 101–6.
| Results of a randomized trial of partner notification in cases of HIV infection in North Carolina.Crossref | GoogleScholarGoogle Scholar |
[11] Montesinos L, Frisch LE, Greene BF, Hamilton M. An analysis of and intervention in the sexual transmission of disease. J Appl Behav Anal 1990; 23 275–84.
| An analysis of and intervention in the sexual transmission of disease.Crossref | GoogleScholarGoogle Scholar |
[12] Woodhouse DE, Potterat JJ, Muth JB, Pratts CI, Rothenberg RB, Fogle JS. A civilian-military partnership to reduce the incidence of gonorrhea. Public Health Rep 1985; 100 61–5.
[13] Judson FN, Wolf FC. Tracing and treating contacts of gonorrhea patients in a clinic for sexually transmitted diseases. Public Health Rep 1978; 93 460–3.
[14] Potterat JJ, Rothenberg R. The case-finding effectiveness of self-referral system for gonorrhea: a preliminary report. Am J Public Health 1977; 67 174–6.
| The case-finding effectiveness of self-referral system for gonorrhea: a preliminary report.Crossref | GoogleScholarGoogle Scholar |
[15] Hammar H, Ljungberg L. Factors affecting contact tracing of gonorrhoea. Acta Derm Venereol 1972; 52 233–240.
[16] Cope AB, Bernstein KT, Matthias J, Rahman M, Diesel JC, Pugsley RA, Schillinger JA, Chew Ng RA, Klingler EJ, Mobley VL, Samoff E, Peterman TA. Effectiveness of Syphilis partner notification after adjusting for treatment dates, 7 jurisdictions. Sex Transm Dis 2022; 49 160–5.
| Effectiveness of Syphilis partner notification after adjusting for treatment dates, 7 jurisdictions.Crossref | GoogleScholarGoogle Scholar |
[17] Avoundjian T, Stewart J, Peyton D, Lewis C, Johnson K, Glick SN, Golden MR, Khosropour CM. Integrating human immunodeficiency virus testing into syphilis partner services in mississippi to improve human immunodeficiency virus case finding. Sex Transm Dis 2019; 46 240–5.
| Integrating human immunodeficiency virus testing into syphilis partner services in mississippi to improve human immunodeficiency virus case finding.Crossref | GoogleScholarGoogle Scholar |
[18] Golden MR, Stekler J, Kent JB, Hughes JP, Wood RW. An evaluation of HIV partner counseling and referral services using new disposition codes. Sex Transm Dis 2009; 36 95–101.
| An evaluation of HIV partner counseling and referral services using new disposition codes.Crossref | GoogleScholarGoogle Scholar |
[19] Brewer DD. Case-finding effectiveness of partner notification and cluster investigation for sexually transmitted diseases/HIV. Sex Transm Dis 2005; 32 78–83.
| Case-finding effectiveness of partner notification and cluster investigation for sexually transmitted diseases/HIV.Crossref | GoogleScholarGoogle Scholar |
[20] Bull L, Apea V, Wiggins H, Davies S, Saxon C, Hughes A, Curtis H, Sullivan A, on behalf of the members of the British Society for Sexual Health and HIV National Audit Group BASHH 2018 UK national audit of HIV partner notification. Int J STD AIDS 2021; 32 872–7.
| BASHH 2018 UK national audit of HIV partner notification.Crossref | GoogleScholarGoogle Scholar |
[21] Golden MR, AugsJoost B, Bender M, Brady KA, Collins LS, Dombrowski JD, Ealey J, Garcia C, George D, Gilliard B, Harris T, Johnson C, Khosropour CM, Rumanes SF, Surita K, Tabidze I, Udeagu CN, Walker-Baban C, Cramer NO. The organization, content, and case-finding effectiveness of HIV assisted partner services in high HIV morbidity areas of the U.S. J Acquir Immune Defic Syndr 2022; 89 498–504.
| The organization, content, and case-finding effectiveness of HIV assisted partner services in high HIV morbidity areas of the U.S.Crossref | GoogleScholarGoogle Scholar |
[22] Rayment M, Curtis H, Carne C, McClean H, Bell G, Estcourt C, Roberts J, Wilkins E, Estreich S, Morris G, Phattey J, Sullivan AK, members of the British Society for Sexual Health and HIV National Audit Group, and the British HIV Association Audit and Standards Subcommittee An effective strategy to diagnose HIV infection: findings from a national audit of HIV partner notification outcomes in sexual health and infectious disease clinics in the UK. Sex Transm Infect 2017; 93 94–9.
| An effective strategy to diagnose HIV infection: findings from a national audit of HIV partner notification outcomes in sexual health and infectious disease clinics in the UK.Crossref | GoogleScholarGoogle Scholar |
[23] Golden MR, Hogben M, Potterat JJ, Handsfield HH. HIV partner notification in the United States: a national survey of program coverage and outcomes. Sex Transm Dis 2004; 31 709–12.
| HIV partner notification in the United States: a national survey of program coverage and outcomes.Crossref | GoogleScholarGoogle Scholar |
[24] Kissinger P, Schmidt N, Mohammed H, Leichliter JS, Gift TL, Meadors B, Sanders C, Farley TA. Patient-delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sex Transm Dis 2006; 33 445–50.
| Patient-delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[25] Golden MR, Whittington WLH, Handsfield HH, Hughes JP, Stamm WE, Hogben M, Clark A, Malinski C, Helmers JRL, Thomas KK, Holmes KK. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 2005; 352 676–85.
| Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection.Crossref | GoogleScholarGoogle Scholar |
[26] Kissinger P, Mohammed H, Richardson-Alston G, Leichliter JS, Taylor SN, Martin DH, Farley TA. Patient-delivered partner treatment for male urethritis: a randomized, controlled trial. Clin Infect Dis 2005; 41 623–9.
| Patient-delivered partner treatment for male urethritis: a randomized, controlled trial.Crossref | GoogleScholarGoogle Scholar |
[27] Schillinger JA, Kissinger P, Calvet H, Whittington WLH, Ransom RL, Sternberg MR, Berman SM, Kent CK, Martin DH, Oh MK, Handsfield HH, Bolan G, Markowitz LE, Fortenberry JD. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis 2003; 30 49–56.
| Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial.Crossref | GoogleScholarGoogle Scholar |
[28] Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev 2013; 2013 CD002843
| Strategies for partner notification for sexually transmitted infections, including HIV.Crossref | GoogleScholarGoogle Scholar |
[29] Golden MR, Kerani RP, Stenger M, Hughes JP, Aubin M, Malinski C, Holmes KK. Uptake and population-level impact of expedited partner therapy (EPT) on Chlamydia trachomatis and Neisseria gonorrhoeae: The Washington state community-level randomized trial of EPT. PLoS Med 2015; 12 e1001777
| Uptake and population-level impact of expedited partner therapy (EPT) on Chlamydia trachomatis and Neisseria gonorrhoeae: The Washington state community-level randomized trial of EPT.Crossref | GoogleScholarGoogle Scholar |
[30] Schillinger JA, Gorwitz R, Rietmeijer C, Golden MR. The expedited partner therapy continuum: a conceptual framework to guide programmatic efforts to increase partner treatment. Sex Transm Dis 2016; 43 S63–75.
| The expedited partner therapy continuum: a conceptual framework to guide programmatic efforts to increase partner treatment.Crossref | GoogleScholarGoogle Scholar |
[31] Estcourt CS, Sutcliffe LJ, Copas A, Mercer CH, Roberts TE, Jackson LJ, Symonds M, Tickle L, Muniina P, Rait G, Johnson AM, Aderogba K, Creighton S, Cassell JA. Developing and testing accelerated partner therapy for partner notification for people with genital Chlamydia trachomatis diagnosed in primary care: a pilot randomised controlled trial. Sex Transm Infect 2015; 91 548–54.
| Developing and testing accelerated partner therapy for partner notification for people with genital Chlamydia trachomatis diagnosed in primary care: a pilot randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[32] Estcourt C, Sutcliffe L, Cassell J, Mercer CH, Copas A, James L, Low N, Horner P, Clarke M, Symonds M, Roberts T, Tsourapas A, Johnson AM. Can we improve partner notification rates through expedited partner therapy in the UK? Findings from an exploratory trial of Accelerated Partner Therapy (APT). Sex Transm Infect 2012; 88 21–6.
| Can we improve partner notification rates through expedited partner therapy in the UK? Findings from an exploratory trial of Accelerated Partner Therapy (APT).Crossref | GoogleScholarGoogle Scholar |
[33] Althaus CL, Mercer CH, Cassell JA, Estcourt CS, Low N. The cost-effectiveness of 333 accelerated partner therapy (APT) compared to standard contact tracing for people with 334 chlamydia: an economic evaluation based on the LUSTRUM population-based chlamydia 335 transmission model. Medrxiv 2021;
[34] Estcourt CS, Copas A, Low N, Mapp F, Stirrup O, Cassell JA. Accelerated partner therapy contact tracing for people with chlamydia: the LUSTRUM cluster cross-over randomised controlled trial. Medrxiv 2021;
| Accelerated partner therapy contact tracing for people with chlamydia: the LUSTRUM cluster cross-over randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[35] Williams EV, Ogwulu OCB, Estcourt CS, Howarth AR, Copas A, Low N, Althaus C, Mapp F, Woode OM, Symonds M, Roberts TE. The cost-effectiveness of accelerated partner therapy (APT) compared to standard contact tracing for people with chlamydia: an economic evaluation based on the LUSTRUM population-based chlamydia transmission model. Medrxiv 2021;
| The cost-effectiveness of accelerated partner therapy (APT) compared to standard contact tracing for people with chlamydia: an economic evaluation based on the LUSTRUM population-based chlamydia transmission model.Crossref | GoogleScholarGoogle Scholar |
[36] Woodward C, Lloyd KC, Bloch S, Saunders J, Estcourt C, Gibbs J. O12 The use of digital partner notification in sexually transmitted infections (STIs) including HIV: a scoping review. Sex Transm Infect 2022; 98 A6–7.
[37] Balán IC, Rios JL, Lentz C, Arumugam S, Dolezal C, Kutner B, Rael CT, Ying AW, Macar OU, Sia SK. Acceptability and use of a dual HIV/Syphilis rapid test and accompanying smartphone app to facilitate self- and partner-testing among cisgender men and transgender women who have sex with men. AIDS Behav 2022; 26 35–46.
| Acceptability and use of a dual HIV/Syphilis rapid test and accompanying smartphone app to facilitate self- and partner-testing among cisgender men and transgender women who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[38] Parkes-Ratanshi R, Mbazira Kimeze J, Nakku-Joloba E, Hamill MM, Namawejje M, Kiragga A, Kayogoza Byamugisha J, Rompalo A, Gaydos C, Manabe YC. Low male partner attendance after syphilis screening in pregnant women leads to worse birth outcomes: the Syphilis Treatment of Partners (STOP) randomised control trial. Sex Health 2020; 17 214–22.
| Low male partner attendance after syphilis screening in pregnant women leads to worse birth outcomes: the Syphilis Treatment of Partners (STOP) randomised control trial.Crossref | GoogleScholarGoogle Scholar |
[39] Clark JL, Segura ER, Oldenburg CE, Salvatierra HJ, Rios J, Perez-Brumer AG, Gonzales P, Sheoran B, Sanchez J, Lama JR. Traditional and web-based technologies to improve partner notification following syphilis diagnosis among men who have sex with men in Lima, Peru: pilot randomized controlled trial. J Med Internet Res 2018; 20 e232
| Traditional and web-based technologies to improve partner notification following syphilis diagnosis among men who have sex with men in Lima, Peru: pilot randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[40] Götz HM, van Rooijen MS, Vriens P, Op de Coul E, Hamers M, Heijman T, van den Heuvel F, Koekenbier R, van Leeuwen AP, Voeten HA. Initial evaluation of use of an online partner notification tool for STI, called ‘suggest a test’: a cross sectional pilot study. Sex Transm Infect 2014; 90 195–200.
| Initial evaluation of use of an online partner notification tool for STI, called ‘suggest a test’: a cross sectional pilot study.Crossref | GoogleScholarGoogle Scholar |
[41] Bilardi JE, Fairley CK, Hopkins CA, Hocking JS, Sze JK, Chen MY. Let them know: evaluation of an online partner notification service for chlamydia that offers E-mail and SMS messaging. Sex Transm Dis 2010; 37 563–5.
| Let them know: evaluation of an online partner notification service for chlamydia that offers E-mail and SMS messaging.Crossref | GoogleScholarGoogle Scholar |
[42] Mobley V, Cope A, Dzialowy N, Maxwell J, Foust E, Samoff E. A comparison of syphilis partner notification outcomes by reported use of internet-based apps to meet sex partners in North Carolina, 2013–2016. Sex Transm Dis 2018; 45 823–8.
| A comparison of syphilis partner notification outcomes by reported use of internet-based apps to meet sex partners in North Carolina, 2013–2016.Crossref | GoogleScholarGoogle Scholar |
[43] Pennise M, Inscho R, Herpin K, Owens J, Bedard BA, Weimer AC, Kennedy BS, Younge M. Using smartphone apps in STD interviews to find sexual partners. Public Health Rep 2015; 130 245–52.
[44] Hunter P, Oyervides O, Grande KM, Prater D, Vann V, Reitl I, Biedrzycki PA. Facebook-augmented partner notification in a cluster of syphilis cases in Milwaukee. Public Health Rep 2014; 129 43–9.
[45] Kerani RP, Fleming M, DeYoung B, Golden MR. A randomized, controlled trial of inSPOT and patient-delivered partner therapy for gonorrhea and chlamydial infection among men who have sex with men. Sex Transm Dis 2011; 38 941–6.
| A randomized, controlled trial of inSPOT and patient-delivered partner therapy for gonorrhea and chlamydial infection among men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[46] Ehlman DC, Jackson M, Saenz G, Novak DS, Kachur R, Heath JT, Furness BW. Evaluation of an innovative internet-based partner notification program for early syphilis case management, Washington, DC, January 2007–June 2008. Sex Transm Dis 2010; 37 478–85.
| Evaluation of an innovative internet-based partner notification program for early syphilis case management, Washington, DC, January 2007–June 2008.Crossref | GoogleScholarGoogle Scholar |
[47] Htaik K, Fairley CK, Bilardi JE, Chow EPF, Ong JJ, Chen MY. Evaluation of the online partner messaging service for sexually transmitted infections let them know. Sex Transm Dis 2022; 49 12–4.
| Evaluation of the online partner messaging service for sexually transmitted infections let them know.Crossref | GoogleScholarGoogle Scholar |
[48] Wang AL, Peng R-R, Tucker JD, Chakraborty H, Cohen MS, Chen X-S. Optimizing partner notification programs for men who have sex with men: factorial survey results from south China. PLoS One 2016; 11 e0157749
| Optimizing partner notification programs for men who have sex with men: factorial survey results from south China.Crossref | GoogleScholarGoogle Scholar |
[49] Clark JL, Segura ER, Perez-Brumer AG, Reisner SL, Peinado J, Salvatierra HJ, Sanchez J, Lama JR. Potential impact and acceptability of Internet partner notification for men who have sex with men and transgender women recently diagnosed as having sexually transmitted disease in Lima, Peru. Sex Transm Dis 2014; 41 43–5.
| Potential impact and acceptability of Internet partner notification for men who have sex with men and transgender women recently diagnosed as having sexually transmitted disease in Lima, Peru.Crossref | GoogleScholarGoogle Scholar |
[50] Golden MR, Whittington WLH, Handsfield HH, Malinski C, Clark A, Hughes JP, Gorbach PM, Holmes KK. Partner management for gonococcal and chlamydial infection: expansion of public health services to the private sector and expedited sex partner treatment through a partnership with commercial pharmacies. Sex Transm Dis 2001; 28 658–65.
| Partner management for gonococcal and chlamydial infection: expansion of public health services to the private sector and expedited sex partner treatment through a partnership with commercial pharmacies.Crossref | GoogleScholarGoogle Scholar |
[51] Reed JL, Huppert JS, Gillespie GL, Taylor RG, Holland CK, Alessandrini EA, Kahn JA. Adolescent patient preferences surrounding partner notification and treatment for sexually transmitted infections. Acad Emerg Med 2015; 22 61–6.
| Adolescent patient preferences surrounding partner notification and treatment for sexually transmitted infections.Crossref | GoogleScholarGoogle Scholar |
[52] Balfe M, Brugha R, O’Donovan D, O’Connell E, Vaughan D. Young women’s decisions to accept chlamydia screening: influences of stigma and doctor-patient interactions. BMC Public Health 2010; 10 425
| Young women’s decisions to accept chlamydia screening: influences of stigma and doctor-patient interactions.Crossref | GoogleScholarGoogle Scholar |
[53] Sutcliffe L, Brook MG, Chapman JL, Cassell JM, Estcourt CS. Is accelerated partner therapy a feasible and acceptable strategy for rapid partner notification in the UK?: a qualitative study of genitourinary medicine clinic attenders. Int J STD AIDS 2009; 20 603–6.
| Is accelerated partner therapy a feasible and acceptable strategy for rapid partner notification in the UK?: a qualitative study of genitourinary medicine clinic attenders.Crossref | GoogleScholarGoogle Scholar |
[54] Gkatzidou V, Hone K, Sutcliffe L, Gibbs J, Sadiq ST, Szczepura A, Sonnenberg P, Estcourt C. User interface design for mobile-based sexual health interventions for young people: design recommendations from a qualitative study on an online Chlamydia clinical care pathway. BMC Med Inform Decis Mak 2015; 15 72
| User interface design for mobile-based sexual health interventions for young people: design recommendations from a qualitative study on an online Chlamydia clinical care pathway.Crossref | GoogleScholarGoogle Scholar |
[55] Hopkins CA, Temple-Smith MJ, Fairley CK, Pavlin NL, Tomnay JE, Parker RM, Bowden FJ, Russell DB, Hocking JS, Chen MY. Telling partners about chlamydia: how acceptable are the new technologies? BMC Infect Dis 2010; 10 58
| Telling partners about chlamydia: how acceptable are the new technologies?Crossref | GoogleScholarGoogle Scholar |
[56] Cope AB, Seña AC, Eagle C, Pol A, Rahman M, Peterman TA. Assessing patient opinions about electronic messaging for gonorrhea and chlamydia result notification and partner services, Durham, North Carolina. Sex Transm Dis 2019; 46 625–8.
| Assessing patient opinions about electronic messaging for gonorrhea and chlamydia result notification and partner services, Durham, North Carolina.Crossref | GoogleScholarGoogle Scholar |
[57] Iturrieta-Guaita NG, Temple-Smith MJ, Tomnay J. Using electronic communication technologies for improving syphilis partner notification in Chile: healthcare providers’ perspectives – a qualitative case study. Sex Health 2019; 16 377–82.
| Using electronic communication technologies for improving syphilis partner notification in Chile: healthcare providers’ perspectives – a qualitative case study.Crossref | GoogleScholarGoogle Scholar |
[58] Estcourt CS, Gibbs J, Sutcliffe LJ, Gkatzidou V, Tickle L, Hone K, Aicken C, Lowndes CM, Harding-Esch EM, Eaton S, Oakeshott P, Szczepura A, Ashcroft RE, Copas A, Nettleship A, Sadiq ST, Sonnenberg P. The eSexual Health Clinic system for management, prevention, and control of sexually transmitted infections: exploratory studies in people testing for Chlamydia trachomatis. Lancet Public Health 2017; 2 e182–90.
| The eSexual Health Clinic system for management, prevention, and control of sexually transmitted infections: exploratory studies in people testing for Chlamydia trachomatis.Crossref | GoogleScholarGoogle Scholar |
[59] Qian S, Foster R, Bourne C, Vickers T, McIver R, McNulty A. Neisseria gonorrhoeae positivity in clients presenting as asymptomatic contacts of gonorrhoea at a sexual health centre. Sex Health 2020; 17 187–91.
| Neisseria gonorrhoeae positivity in clients presenting as asymptomatic contacts of gonorrhoea at a sexual health centre.Crossref | GoogleScholarGoogle Scholar |
[60] Pearce E, Chan DJ, Smith DE. Empiric antimicrobial treatment for asymptomatic sexual contacts of sexually transmitted infection in the era of antimicrobial resistance: time to rethink? Int J STD AIDS 2019; 30 137–9.
| Empiric antimicrobial treatment for asymptomatic sexual contacts of sexually transmitted infection in the era of antimicrobial resistance: time to rethink?Crossref | GoogleScholarGoogle Scholar |
[61] Golden MR, Katz DA, Dombrowski JC. Modernizing field services for human immunodeficiency virus and sexually transmitted infections in the United States. Sex Transm Dis 2017; 44 599–607.
| Modernizing field services for human immunodeficiency virus and sexually transmitted infections in the United States.Crossref | GoogleScholarGoogle Scholar |
[62] Katz DA, Dombrowski JC, Kerani RP, Aubin MR, Kern DA, Heal DD, Bell TR, Golden MR. Integrating HIV testing as an outcome of STD partner services for men who have sex with men. AIDS Patient Care STDS 2016; 30 208–14.
| Integrating HIV testing as an outcome of STD partner services for men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[63] Samoff E, Cope AB, Maxwell J, Thomas F, Mobley VL. The number of interviews needed to yield new syphilis and human immunodeficiency virus cases among partners of people diagnosed with syphilis, North Carolina, 2015. Sex Transm Dis 2017; 44 451–6.
| The number of interviews needed to yield new syphilis and human immunodeficiency virus cases among partners of people diagnosed with syphilis, North Carolina, 2015.Crossref | GoogleScholarGoogle Scholar |
[64] Howren MB, Francis SL, Polgreen LA, Shafer C, Hoth A, Ohl ME. Predictors of HIV preexposure prophylaxis initiation among public health clients in rural and small urban areas in Iowa. Public Health Rep 2021; 136 172–82.
| Predictors of HIV preexposure prophylaxis initiation among public health clients in rural and small urban areas in Iowa.Crossref | GoogleScholarGoogle Scholar |
[65] Teixeira da Silva D, Bouris A, Ramachandran A, Blocker O, Davis B, Harris J, Pyra M, Rusie LK, Brewer R, Pagkas-Bather J, Hotton A, Ridgway JP, McNulty M, Bhatia R, Schneider JA. Embedding a Linkage to preexposure prophylaxis care intervention in social network strategy and partner notification services: results from a pilot randomized controlled trial. J Acquir Immune Defic Syndr 2021; 86 191–9.
| Embedding a Linkage to preexposure prophylaxis care intervention in social network strategy and partner notification services: results from a pilot randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[66] Katz DA, Dombrowski JC, Bell T, Golden MR. STD partner services to monitor and promote PrEP use among men who have sex with men. Conference on Retroviruses and Opportunistic Infections 2017; Seattle, WA; 2017. Available at https://www.natap.org/2017/CROI/croi_188.htm
[67] Norkin SK, Benson S, Civitarese AM, Reich A, Chomsky Albright M, Convery C, Kasarskis IM, Cassidy-Stewart H, Howe K, Wang X, Golden MR, Khosropour CM, Glick SN, Kerani RP. Inadequate engagement in HIV care among people with HIV newly diagnosed with a sexually transmitted disease: a multijurisdictional analysis. Sex Transm Dis 2021; 48 601–5.
| Inadequate engagement in HIV care among people with HIV newly diagnosed with a sexually transmitted disease: a multijurisdictional analysis.Crossref | GoogleScholarGoogle Scholar |
[68] Digre P, Avoundjian T, Johnson K, Peyton D, Lewis C, Barnabas RV, Golden MR, Khosropour CM. Barriers, facilitators, and cost of integrating HIV-related activities into sexually transmitted disease partner services in Jackson, Mississippi. Sex Transm Dis 2021; 48 145–51.
| Barriers, facilitators, and cost of integrating HIV-related activities into sexually transmitted disease partner services in Jackson, Mississippi.Crossref | GoogleScholarGoogle Scholar |
[69] Silverman RA, Katz DA, Levin C, Bell TR, Spellman D, St John L, Manley Rodriguez E, Golden MR, Barnabas RV. Sexually transmitted disease partner services costs, other resources, and strategies across jurisdictions to address unique epidemic characteristics and increased incidence. Sex Transm Dis 2019; 46 493–501.
| Sexually transmitted disease partner services costs, other resources, and strategies across jurisdictions to address unique epidemic characteristics and increased incidence.Crossref | GoogleScholarGoogle Scholar |
[70] Gibbs J, Solomon D, Jackson L, Mullick S, Burns F, Shahmanesh M. Measuring and evaluating sexual health in the era of digital health: challenges and opportunities. Sexual Health 2022;
| Measuring and evaluating sexual health in the era of digital health: challenges and opportunities.Crossref | GoogleScholarGoogle Scholar |
[71] Trelle S, Shang A, Nartey L, Cassell JA, Low N. Improved effectiveness of partner notification for patients with sexually transmitted infections: systematic review. BMJ 2007; 334 354
| Improved effectiveness of partner notification for patients with sexually transmitted infections: systematic review.Crossref | GoogleScholarGoogle Scholar |
[72] Wayal S, Estcourt CS, Mercer CH, Saunders J, Low N, McKinnon T, Symonds M, Cassell JA. Optimising partner notification outcomes for bacterial sexually transmitted infections: a deliberative process and consensus, United Kingdom, 2019. Euro Surveill 2022; 27 2001895
| Optimising partner notification outcomes for bacterial sexually transmitted infections: a deliberative process and consensus, United Kingdom, 2019.Crossref | GoogleScholarGoogle Scholar |


