Global travel and HIV/STI epidemics among men who have sex with men: what does the future hold?
Veronica C. Lee A , Patrick S. Sullivan A C and Stefan D. Baral BA Department of Epidemiology, Rollins School of Public Health, Emory University, 1518 Clifton Road NE, 4th Floor GCR Building, Atlanta, GA 30322, USA.
B Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, 615 N. Wolfe Street, Room E7146, Baltimore, MD 21205, USA.
C Corresponding author. Email: pssulli@emory.edu
Sexual Health 14(1) 51-58 https://doi.org/10.1071/SH16099
Submitted: 31 May 2016 Accepted: 27 September 2016 Published: 6 January 2017
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Infectious disease epidemics occur within dynamic systems and environments that shape risk and, ultimately, the spread of infectious diseases. Gay men and other men who have sex with men (MSM) are disproportionately impacted by HIV everywhere in the world. Several emerging trends present risks for sustained or increased HIV acquisition and transmission, and the growth of global travel in the context of emerging online platforms for social/sexual networking is discussed here. Four factors associated with travel that could potentiate HIV transmission are highlighted: different patterns of sexual risk behaviours during travel; the growth of online tools to meet sex partners more efficiently; the global heterogeneity of HIV strains; and the potential for diassortative mixing of men from high- and low-HIV prevalence areas. Prevention tools and services must rise to these challenges, and innovative mobile applications and programs have played, and will continue to play, an important role in supporting MSM at risk for or living with HIV during their periods of travel.
Introduction
Travel shapes the social and sexual networks and, consequently, the HIV and sexually transmissible infections (STI) acquisition risks of gay men and other men who have sex with men (MSM), and global travel is, and will continue, increasing. In the past decade alone, global travel has increased steadily by ~3–5% annually.1 In this context of increasing population mobility, travel by lesbian, gay, bisexual and transgender (LGBT) people has similarly increased as travel destinations become increasingly LGBT-friendly. The most recent annual tourism and hospitality survey conducted among LGBT in the United States (US) found that more than half of the LGBT individuals surveyed had taken one to three trips for leisure, vacation or holiday in the past year.2 In other settings, travel of MSM may result from forced migration due to conflict or local government-sponsored homonegative policies, including the criminalisation of consensual adult same-sex practices or overt violence affecting LGBT people.3,4
Regardless of the reason for travel, movement and mixing of populations has implications for HIV and STI epidemiology and prevention. Examining HIV risk and transmission in the context of increasing travel is important for understanding the epidemiology of HIV among gay men and other MSM and how best to respond. The changing landscape in which HIV/STI acquisition risks occur due to increased connectedness through travel is essential for informing the development of HIV prevention and treatment interventions for MSM. In this review, we consider the current knowledge about travel and HIV/STI risks, examine mechanisms through which travel influences HIV/STI risks, and suggest opportunities for HIV/STI prevention services and tools in the future.
Current knowledge about travel and STI/HIV risk
Patterns of behaviour during travel
The risk of acquiring STIs and HIV during travel has been examined in adult populations,5,6 including MSM. MSM may be more likely to engage in higher-risk sexual and drug use behaviours for HIV and STIs – including condomless anal intercourse (CAI) with HIV status unknown or serodiscordant sexual partners, concurrent multiple sex partners during travel, or engaging in alcohol and substance use in combination with sexual intercourse – while travelling.7–11 MSM were also less likely to disclose or discuss HIV status with their sex partners while on travel or vacation.7 This risk may be further amplified when the reason for travel is to attend group sex events; men who attended these events were more likely to report having multiple sex partners, engaging in CAI with sexual partners of unknown HIV status, and engaging in alcohol and substance use.9,10,12
Recent data suggest that the relationship between travel and sexual risk may be more complex than earlier studies suggested. How MSM adapt their behaviours when traveling to destinations perceived to be higher or lower HIV risk is not well understood; studies have been limited given the inherent challenges of sampling the particular MSM that travel more than others. One study of MSM travellers who reside in San Francisco found that men were less likely to report engaging in CAI in their international sexual partnerships, as compared with those that occurred during domestic travel and while in San Francisco; this association remained after controlling for HIV status, age, relationship status and type of partnership.13 These findings indicate that MSM may adapt their sexual behaviours while traveling based on their knowledge or perceptions of risk in that travel destination.
Increasing access to sex partners during travel through online social networking
Historically, some MSM travelled to destinations specifically to attend organised events, including those designed to facilitate meeting sex partners.14,15 In the future, travel by MSM will likely continue to expand as countries make concerted efforts to attract gay tourists.16 Online social networking, including geosocial-networking smartphone applications, has altered the ways in which MSM seek out and identify sexual partners, and this is especially relevant in the context of travel. Use of these networking platforms has grown across the world and will likely become commonplace in the future. Already, mobile applications like Grindr, Hornet and Tinder have many millions of users, residing in all 196 countries of the world.17–19 In areas where smartphone usage is less prevalent, there are other web-based venues to meet sex partners, such as Facebook groups, Manhunt or Planet Romeo.20
For MSM in particular, online social networking has made meeting sexual partners during travel increasingly feasible. For example, among MSM attending a gay pride event in Denver, Colorado, 12% of men interviewed sought sexual partners online before travel. These men who sought partners before travel were more likely to have a new sexual partner and report more sexual partners during travel, compared with survey respondents who did not use the Internet to seek out partners before travel.7 Similar patterns of utilising online social networking platforms to identify potential sexual partners at the travel destination before travel have also been observed in Asia and Africa. In Vietnam, Nguyen et al. found that of the 41% of MSM who reported a new or casual sexual partner while abroad, approximately half found their partner from the Internet.21 In Swaziland and Lesotho, MSM reporting higher levels of social stigma were more likely to seek and find sexual partners online and were more likely to be living with HIV.22 This trend of utilising geosocial networking applications to facilitate sexual connections among MSM will only increase in the future in all parts of the globe.
Using Internet-based social networking websites or mobile social networking applications for partner identification is especially common among young men who have sex with men (YMSM). In recent years, geosocial networking applications have become a primary way to identify sex partners among YMSM.23 The Healthy Young Men’s study, a longitudinal cohort study among US YMSM, found that the Internet was the most common way that study participants found their last sexual partners.24 One YMSM study in Los Angeles found that two-thirds of respondents who used Grindr reported using the smartphone application to meet a sex partner.25
Based on these available data, sex partners met during travel may be more likely to have met online than in person. This is important, because MSM who seek sex partners through online platforms are more likely to report engaging in CAI and having multiple sexual partnerships, as well as being less likely to know the HIV status of these partners, compared with those who meet their partners offline.7,20,26 A meta-analysis of 11 observational studies found the odds of CAI were higher in online-initiated sexual encounters compared with sexual partnerships conducted offline among MSM, suggesting that online platforms make partner identification for CAI easier.26 This relationship may be explained by the ease in which sexual partners can be identified, especially for those willing to engage in high-risk sexual behaviours like CAI with a partner of unknown HIV-status or unknown viral load suppression status. Studies conducted in the US and in East and South-East Asia have shown that the elevated HIV risk may not be due to seeking partners online per se, but to the ways in which online social networking platforms provide increased opportunities for engaging in multiple partnerships, larger sexual networks, CAI or concurrent alcohol use during intercourse.27,28 While greater engagement in risk-taking behaviours among MSM during travel has been documented in several studies,8–10,12 other studies have shown that MSM may adopt protective behaviours to reduce their HIV and STI risk based on their travel destination,13 and those who seek partners through the Internet before travel may not engage in higher levels of CAI compared with those who sought their partners offline.7
It is important to note that the significance of meeting sex partners online may evolve over time. Even a decade ago, online platforms were more commonly used for sex work than finding partners for the average person. And while sex work has continued to move from the streets to the Internet, so too has partner-finding for many gay men. Consequently, re-evaluating the relationships between online partner identification and HIV-related risks will be important to assess whether these relationships have been sustained. In addition, the significance of meeting sex partners online varies greatly by region, and understanding how it differs in different contexts will be informative for understanding those relationships between online partner-seeking and risk-taking behaviours. In more stigmatising settings, meeting sex partners online was independently associated with increased experienced stigma and challenges with meeting partners in physical venues.22
Travel influences STI/HIV risks: mixing strains, risk levels and biological susceptibility
Local strains/local epidemics with different characteristics
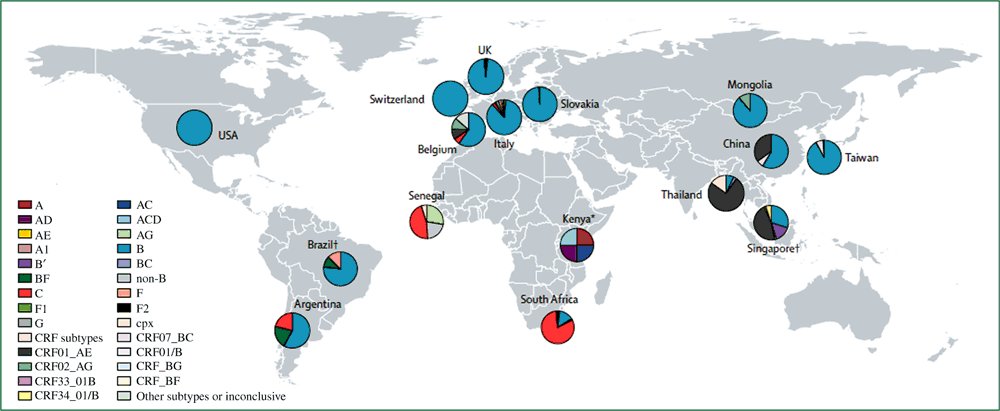
The increased ease in which sexual partnerships can occur as a result of social networking applications in various parts of the globe poses significant implications for the spread of diverse HIV strains and resistant strains of HIV and STIs. The current global picture of the HIV pandemic among MSM demonstrates the existing diversity of the virus (Fig. 1). Subtype B predominates among MSM populations in several parts of the world, but there is significant global HIV diversity, particularly in South-East Asia and Africa.29 Even in regions where subtype B is dominant, the HIV viruses of MSM may already have considerable genetic variation. A phylogenetic analysis of HIV-1 genetic diversity among HIV-infected individuals in the UK found that strains traditionally associated with heterosexual transmission in East Africa were present in local transmission networks among MSM, most likely due to travel and migration.30 In a US-based cohort of MSM with acute HIV infection, MSM were twice as likely as heterosexual individuals to be infected with multiple viruses compared with a single virus.31 Wagner et al. found a 14% prevalence of dual infection (defined as two distinct viral strains within the same host) among their MSM study participants.32 The Thai A/E recombinant was initially highly localised in Thailand,33 but has more recently been reported in other Asian cities, suggesting that intra-Asia travel, with Bangkok as a central connecting point for sexual contact, has supported the regional spread of the strain.34
|
The changes in the genetic composition of the virus and the emergence of new strains as a result of the globalisation of sexual partnerships among MSM may influence the diagnosis of HIV, susceptibility to antiretroviral treatment and the measurement of viral load.35,36 Secondary drug resistance mediated through the transmission of primary HIV-1 drug resistance is a concern, with the geographic expansion of sexual networks of MSM. In a cohort of MSM newly diagnosed with HIV (n = 64), Truong et al. found that eight of the study participants had primary HIV-1 drug resistance, of whom seven reported traveling internationally in the period between their last HIV-negative test and their first positive test, and four had recently been infected.37 Acquiring a HIV strain with pre-existing resistance is especially consequential for men who live in low- and middle-income countries where pre-therapy HIV resistance testing is not standard practice. These men are more likely to experience virological failure if the conventional first-line antiretroviral therapy contains an agent or class against which their virus has resistance. Furthermore, HIV genetic diversity also presents considerable challenges for HIV vaccine development, particularly in determining which isolate sequences should be included to produce a globally effective vaccine.38,39
The expansion of men’s sexual networks facilitated by international travel and social networking applications also has implications for other STIs and antimicrobial resistance. The shift in treatment guidelines for Neisseria gonorrhoeae (NG) from fluoroquinolones to cephalosporins for MSM in the last decade reflects the increasing concern over the emergence of broader resistance to NG, and this may be of particular importance for MSM. In their global systematic review and meta-analysis, Yu et al. compared cefixime susceptibility rates of NG isolates across 21 studies and found that NG isolates from men had a lower cefixime susceptibility rate than isolates from the mixed group of men and women and the group where biological sex was not provided.40 Among MSM specifically, gonococcal surveillance in the US from 2005 to 2010 and from 2006 to 2012 showed that MSM exhibited higher prevalence of microbial resistance compared with men who have sex exclusively with women.41,42 Kirkcaldy et al. hypothesised this higher level of resistance could be attributed to exposure to sexual partnerships during international travel or at group events where participants travelled from various geographic locations.42 These data indicating increasing resistance among isolates of NG among MSM, especially among those who have travelled, reinforces the severity of these concerns, particularly given the limited additional treatment options for an otherwise curable STI. The international spread of anti-microbial resistance has been ongoing for decades;43 most recently, resistant shigellosis among MSM has been linked to increased mobility across geographic regions. Using whole genomic sequencing, Baker et al. determined that an emergent strain of this enteric infection had spread globally in less than 20 years to regions traditionally considered low risk for shigellosis through male-to-male sexual transmission.44
Disassortativity: mixing risk levels and biological susceptibility
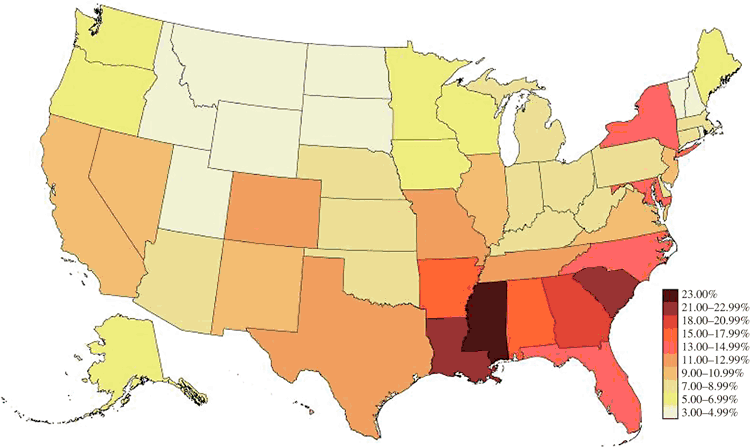
A main driver of infectious disease epidemics is the mixing of high-risk/high-prevalence populations and low-risk/low-prevalence populations; that is, disassortativity. Assortativity (the opposite of disassortativity) refers to the tendency of people, when forming partnerships or networks, to affiliate with people who are similar to them in meaningful ways. From an infectious disease perspective, disassortativity is important because infectious disease transmission is optimised when persons living with an infectious disease have transmission contact with a pool that contains a high proportion of susceptible people. For example, the proportion of MSM living with HIV in Mississippi is ~28%, and in North Dakota it is ~4% (Fig. 2).45 For a man living in North Dakota, all other aspects of sexual contact being equal, his risk of acquiring HIV through sexual contact with another man would be higher when travelling to Mississippi, where his probability of selecting a partner living with HIV would be greater. Consequently, choosing partners from populations with dissimilar risks – disassortativity – potentiates infectious disease transmission.
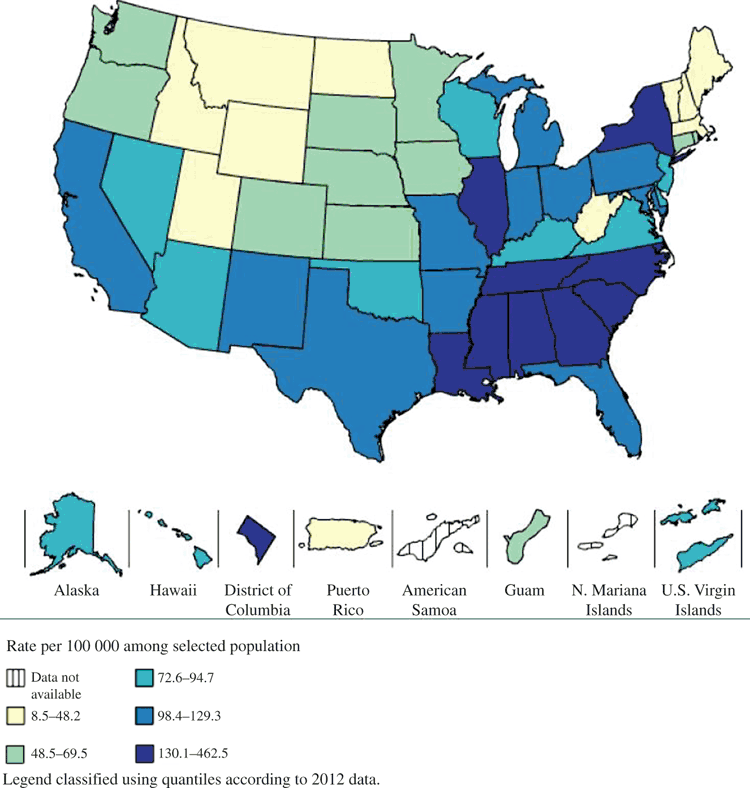
The potential for disassortative mixing can also be illustrated graphically by examining the prevalence or rate of diagnoses of STIs across geographies. For example, gonorrhoea is diagnosed at much higher rates in the US South and some parts of the Northeast, compared with the Mountain West after adjusting for testing levels (Fig. 3). Among 26 high-income countries globally, there is also substantial heterogeneity; in a review of HIV prevalence in 2011, country-specific prevalence ranged from 6 per 100 000 in Japan to 761 per 100 000 in Estonia.46
|
The potential for assortativity to potentiate HIV transmissions among MSM operates across both variations in prevalence, and, conceptually, across variations in HIV strains globally. Travelling from a location with low HIV prevalence, low STI prevalence and a homogenous HIV subtype B epidemic to a place with a higher prevalence of HIV and STIs, and with more substantial strain variation, may increase the likelihood of exposure to HIV; likelihood of exposure to STIs, which can increase subsequent risk of HIV infection;47 and likelihood of exposure to foreign strains, which can result in HIV superinfection for those already living with HIV. Disssortativity is itself potentiated by the increased ease of meeting sex partners during travel, as discussed above; decades ago, men traveling for short periods of time might have had fewer opportunities to find and meet disassortative male partners.
Opportunities for HIV prevention and treatment intervention strategies among MSM
The challenges of increasing global travel for HIV prevention and the treatment of those living with HIV are substantial, but so too are the opportunities. The same digital platforms that facilitate easier sexual connections for men while travelling could also be used to provide information to empower men to understand how their risks may change in different settings and how they can manage those risks accordingly. For example, mobile phone apps can be used to provide tailored information about risks48 and recommended prevention tools for men.49,50 Although not currently offered, there is potential to provide personalised information through mobile phones about the local HIV prevalence as men arrive in new destinations, combined with recommendations for appropriate HIV and STI prevention options. Similarly, just as mobile apps can facilitate meeting sex partners in new locations, mobile apps should also be developed to easily locate local HIV prevention resources, such as free condom distribution locations, clinics that provide post-exposure prophylaxis (PEP) and pre-exposure prophylaxis (PrEP), and HIV and STI testing and management services. Some geosocial networking apps, like Grindr,51 Tinder52 and Hornet,53 have already begun to include HIV testing locators within their respective platforms. Condom finder apps exist in some urban locations (e.g. NYC Condom Finder, Cares Community Health Condom Finder and Philadelphia’s iCondom app), but national or global repositories for publicly supported condom distribution locations, and other HIV prevention resources, could also have significant potential.
PrEP 54 may also have a role in mitigating increased risks of HIV transmission associated with travel. PrEP has been identified as an intervention that is uniquely suited to provide additional protection against HIV acquisition during ‘seasons of risk’, and short-term PrEP has been proposed for vacation periods during which sexual risks are anticipated.55 Non-occupational post-exposure prophylaxis56 (nPEP), despite its limited uptake in the US and many parts of the world,57 has been widely used by MSM in recent European PrEP trials,58,59 and also may have a role for managing HIV exposures that occur during travel to high prevalence areas. For men living with HIV, travel may present certain unique challenges, such as issues with optimal timing of antiretroviral dosing when crossing time zones,60 concerns about carrying antiretroviral medications in some countries61 or challenges in timing refills for antiretroviral medications during travel. Recent concerns about Zika virus transmission through male–male sex62 highlight the rapidly changing environment of travel-related sexual health risks for MSM, and emphasise the need to have established channels to provide timely information related to the risks of STIs while travelling to men. It is also important to develop clear recommendations and tools for dosing during travel that crosses time zones,60 and to make provisions through public health departments or other providers to facilitate access to replacement or refill medications for men living with HIV and nPEP and PrEP for men at risk for HIV during travel.
Conclusions
Travel among MSM for leisure or business will likely continue to increase in the future, and social networking platforms to identify potential sexual partners will become effectively ubiquitous. Taken together, these shifting risk contexts should similarly shift the way in which we think, and ultimately implement, HIV and STI prevention and treatment strategies for gay men and other MSM. In other words, times have changed, and effective public health services must similarly evolve to remain effective. To do so, we must first understand how increasing connectedness through travel and technology alters HIV/STI risk behaviours and transmissions, and ultimately the composition of the global HIV epidemic among MSM.
Global travel is a reality and may be challenging to public health, but it also presents several opportunities for novel HIV prevention approaches. Ease of access to sexual partners and mixing of populations of men across HIV subtypes and HIV prevalence pools increases the potential for HIV and STI transmissions, for exposure to resistant HIV strains, and for HIV superinfection with foreign strains. At the same time, prevention tools, including antiretroviral tools for HIV treatment and prevention and mobile apps to customise HIV prevention information and referrals, offer opportunities for targeted provision of information and prevention resources that are adapted to the local risk and prevention environment. Ensuring that innovation in HIV prevention approaches keeps pace with increasingly common global travel among gay men will be one of the tests of public health services in changing the trajectory of HIV and STI risks, and ultimately the HIV burden, among gay men and other MSM.
Conflicts of interest
None declared.
References
[1] World Travel Markets. WTM global trends report 2014. 2014. Available online at: http://www.wtmlondon.com/RXUK/RXUK_WTMLondon/2015/documents/WTM-Global-Trends-2014.pdf [verified 26 May 2016].[2] Community Marketing and Insights. 20th annual survey on LGBT tourism and hospitality: U.S. overview report. 2015. Available online at: http://communitymarketinginc.com /20th-annual-lgbt-tourism-hospitality-survey/ [verified 26 May 2016].
[3] Drame FM, Peitzmeier S, Lopes M, et al Gay men and other men who have sex with men in West Africa: evidence from the field. Cult Health Sex 2013; 15 7–21.
| Gay men and other men who have sex with men in West Africa: evidence from the field.Crossref | GoogleScholarGoogle Scholar |
[4] Poteat T, Diouf D, Drame FM, et al HIV risk among MSM in Senegal: a qualitative rapid assessment of the impact of enforcing laws that criminalize same sex practices. PLoS One 2011; 6 e28760
| HIV risk among MSM in Senegal: a qualitative rapid assessment of the impact of enforcing laws that criminalize same sex practices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xis1SisQ%3D%3D&md5=8699eb667e61d6b9c3d4978b2abfc9a5CAS |
[5] Richens J. Sexually transmitted infections and HIV among travellers: a review. Travel Med Infect Dis 2006; 4 184–95.
| Sexually transmitted infections and HIV among travellers: a review.Crossref | GoogleScholarGoogle Scholar |
[6] Memish ZA, Osoba AO. International travel and sexually transmitted diseases. Travel Med Infect Dis 2006; 4 86–93.
| International travel and sexually transmitted diseases.Crossref | GoogleScholarGoogle Scholar |
[7] Benotsch EG, Martin AM, Espil FM, Nettles CD, Seal DW, Pinkerton SD. Internet use, recreational travel, and HIV risk behaviors in men who have sex with men. J Commun Healthc 2011; 36 398–405.
| Internet use, recreational travel, and HIV risk behaviors in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[8] Benotsch EG, Mikytuck JJ, Ragsdale K, Pinkerton SD. Sexual risk and HIV acquisition among men who have sex with men travelers to Key West, Florida: a mathematical modeling analysis. AIDS Patient Care STDS 2006; 20 549–56.
| Sexual risk and HIV acquisition among men who have sex with men travelers to Key West, Florida: a mathematical modeling analysis.Crossref | GoogleScholarGoogle Scholar |
[9] Cheung DH, Lim SH, Guadamuz TE, Koe S, Wei C. The potential role of circuit parties in the spread of HIV among men who have sex with men in Asia: a call for targeted prevention. Arch Sex Behav 2015; 44 389–97.
| The potential role of circuit parties in the spread of HIV among men who have sex with men in Asia: a call for targeted prevention.Crossref | GoogleScholarGoogle Scholar |
[10] Fisher MP, Ramchand R, Bana S, Iguchi MY. Risk behaviors among HIV-positive gay and bisexual men at party-oriented vacations. J Stud Alcohol Drugs 2013; 74 158–67.
| Risk behaviors among HIV-positive gay and bisexual men at party-oriented vacations.Crossref | GoogleScholarGoogle Scholar |
[11] Vanden Berghe W, Nostlinger C, Hospers H, Laga M. International mobility, sexual behaviour and HIV-related characteristics of men who have sex with men residing in Belgium. BMC Public Health 2013; 13 968
| International mobility, sexual behaviour and HIV-related characteristics of men who have sex with men residing in Belgium.Crossref | GoogleScholarGoogle Scholar |
[12] Grov C, Rendina HJ, Breslow AS, Ventuneac A, Adelson S, Parsons JT. Characteristics of men who have sex with men (MSM) who attend sex parties: results from a national online sample in the USA. Sex Transm Infect 2014; 90 26–32.
| Characteristics of men who have sex with men (MSM) who attend sex parties: results from a national online sample in the USA.Crossref | GoogleScholarGoogle Scholar |
[13] Truong HM, Fatch R, Grasso M, et al Gay and bisexual men engage in fewer risky sexual behaviors while traveling internationally: a cross-sectional study in San Francisco. Sex Transm Infect 2015; 91 220–5.
| Gay and bisexual men engage in fewer risky sexual behaviors while traveling internationally: a cross-sectional study in San Francisco.Crossref | GoogleScholarGoogle Scholar |
[14] Colfax GN, Mansergh G, Guzman R, et al Drug use and sexual risk behavior among gay and bisexual men who attend circuit parties: a venue-based comparison. J Acquir Immune Defic Syndr 2001; 28 373–9.
| Drug use and sexual risk behavior among gay and bisexual men who attend circuit parties: a venue-based comparison.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnovVOqtg%3D%3D&md5=1fedd023d923d69a53fb090e97e9b467CAS |
[15] Mansergh G, Colfax GN, Marks G, Rader M, Guzman R, Buchbinder S. The Circuit Party Men’s Health Survey: findings and implications for gay and bisexual men. Am J Public Health 2001; 91 953–8.
| The Circuit Party Men’s Health Survey: findings and implications for gay and bisexual men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzhvVyqsQ%3D%3D&md5=96998a6555adcb52b4d4c546da2afba8CAS |
[16] UN World Tourism Organization. Global report on LGBT tourism. 2012. Available online at: http://www.e-unwto.org/doi/pdf/10.18111/9789284414581 [verified 29 May 2016].
[17] Grindr. Grindr fact sheet. 2015. Available online at: http://www.grindr.com/press/ [verified 25 May 2016].
[18] Hornet. Hornet acquires Vespa to become #1 gay App. 2016. Available online at: http://love.hornetapp.com/blog/2016/7/5/hornet-acquires-vespa-to-become-1-gay-app [verified 8 September 2016].
[19] Tinder. About Tinder. 2016. Available online at: https://www.gotinder.com/press [verified 25 May 2016].
[20] Stahlman S, Nowak RG, Liu H, et al Online sex-seeking among men who have sex with men in Nigeria: implications for online intervention. AIDS Behav 2016;
| Online sex-seeking among men who have sex with men in Nigeria: implications for online intervention.Crossref | GoogleScholarGoogle Scholar |
[21] Nguyen H, Nguyen HQ, Colby DJ. HIV knowledge and risks among Vietnamese men who have sex with men travelling abroad. Int J STD AIDS 2014; 25 643–9.
| HIV knowledge and risks among Vietnamese men who have sex with men travelling abroad.Crossref | GoogleScholarGoogle Scholar |
[22] Stahlman S, Grosso A, Ketende S, et al Characteristics of men who have sex with men in Southern Africa who seek sex online: a cross-sectional study. J Med Internet Res 2015; 17 e129
| Characteristics of men who have sex with men in Southern Africa who seek sex online: a cross-sectional study.Crossref | GoogleScholarGoogle Scholar |
[23] Holloway IW, Rice E, Gibbs J, Winetrobe H, Dunlap S, Rhoades H. Acceptability of smartphone application-based HIV prevention among young men who have sex with men. AIDS Behav 2014; 18 285–96.
| Acceptability of smartphone application-based HIV prevention among young men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[24] Kubicek K, Carpineto J, McDavitt B, Weiss G, Kipke MD. Use and perceptions of the internet for sexual information and partners: a study of young men who have sex with men. Arch Sex Behav 2011; 40 803–16.
| Use and perceptions of the internet for sexual information and partners: a study of young men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[25] Rice E, Holloway IW, Winetrobe H, et al Sex risk among young men who have sex with men who use Grindr, a smartphone geosocial networking application. J AIDS Clin Res 2012; S4 005
| Sex risk among young men who have sex with men who use Grindr, a smartphone geosocial networking application.Crossref | GoogleScholarGoogle Scholar |
[26] Lewnard JA, Berrang-Ford L. Internet-based partner selection and risk for unprotected anal intercourse in sexual encounters among men who have sex with men: a meta-analysis of observational studies. Sex Transm Infect 2014; 90 290–6.
| Internet-based partner selection and risk for unprotected anal intercourse in sexual encounters among men who have sex with men: a meta-analysis of observational studies.Crossref | GoogleScholarGoogle Scholar |
[27] Jenness SM, Neaigus A, Hagan H, Wendel T, Gelpi-Acosta C, Murrill CS. Reconsidering the internet as an HIV/STD risk for men who have sex with men. AIDS Behav 2010; 14 1353–61.
| Reconsidering the internet as an HIV/STD risk for men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[28] Wei C, Lim SH, Guadamuz TE, Koe S. Virtual versus physical spaces: which facilitates greater HIV risk taking among men who have sex with men in East and South-East Asia? AIDS Behav 2014; 18 1428–35.
| Virtual versus physical spaces: which facilitates greater HIV risk taking among men who have sex with men in East and South-East Asia?Crossref | GoogleScholarGoogle Scholar |
[29] Beyrer C, Baral SD, van Griensven F, et al Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380 367–77.
| Global epidemiology of HIV infection in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[30] Gifford RJ, de Oliveira T, Rambaut A, et al Phylogenetic surveillance of viral genetic diversity and the evolving molecular epidemiology of human immunodeficiency virus type 1. J Virol 2007; 81 13050–6.
| Phylogenetic surveillance of viral genetic diversity and the evolving molecular epidemiology of human immunodeficiency virus type 1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtl2jtbrF&md5=f179b8b90d5ccb86d2d2d5b3ace44852CAS |
[31] Li H, Bar KJ, Wang S, et al High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog 2010; 6 e1000890
| High multiplicity infection by HIV-1 in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[32] Wagner GA, Pacold ME, Kosakovsky Pond SL, et al Incidence and prevalence of intrasubtype HIV-1 dual infection in at-risk men in the United States. J Infect Dis 2014; 209 1032–8.
| Incidence and prevalence of intrasubtype HIV-1 dual infection in at-risk men in the United States.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXktl2ksbg%3D&md5=9aed1d648e91623b211a1733de43b7f1CAS |
[33] Angelis K, Albert J, Mamais I, et al Global dispersal pattern of HIV Type 1 subtype CRF01_AE: a genetic trace of human mobility related to heterosexual sexual activities centralized in Southeast Asia. J Infect Dis 2015; 211 1735–44.
| Global dispersal pattern of HIV Type 1 subtype CRF01_AE: a genetic trace of human mobility related to heterosexual sexual activities centralized in Southeast Asia.Crossref | GoogleScholarGoogle Scholar |
[34] Huang SW, Wang SF, Cowo AE, et al Molecular epidemiology of HIV-1 infection among men who have sex with men in Taiwan in 2012. PLoS One 2015; 10 e0128266
| Molecular epidemiology of HIV-1 infection among men who have sex with men in Taiwan in 2012.Crossref | GoogleScholarGoogle Scholar |
[35] Hemelaar J. The origin and diversity of the HIV-1 pandemic. Trends Mol Med 2012; 18 182–92.
| The origin and diversity of the HIV-1 pandemic.Crossref | GoogleScholarGoogle Scholar |
[36] Hemelaar J. Implications of HIV diversity for the HIV-1 pandemic. J Infect 2013; 66 391–400.
| Implications of HIV diversity for the HIV-1 pandemic.Crossref | GoogleScholarGoogle Scholar |
[37] Truong HM, Kellogg T, Schwarcz S, et al Frequent international travel by men who have sex with men recently diagnosed with HIV-1: potential for transmission of primary HIV-1 drug resistance. J Travel Med 2008; 15 454–6.
| Frequent international travel by men who have sex with men recently diagnosed with HIV-1: potential for transmission of primary HIV-1 drug resistance.Crossref | GoogleScholarGoogle Scholar |
[38] Gao F, Morrison SG, Robertson DL, et al Molecular cloning and analysis of functional envelope genes from human immunodeficiency virus type 1 sequence subtypes A through G. The WHO and NIAID Networks for HIV Isolation and Characterization. J Virol 1996; 70 1651–67.
| 1:CAS:528:DyaK28XpvFamsA%3D%3D&md5=3d24c97dc415dd569c9dccc9efd2991aCAS |
[39] Gordon M, De Oliveira T, Bishop K, et al Molecular characteristics of human immunodeficiency virus type 1 subtype C viruses from KwaZulu-Natal, South Africa: implications for vaccine and antiretroviral control strategies. J Virol 2003; 77 2587–99.
| Molecular characteristics of human immunodeficiency virus type 1 subtype C viruses from KwaZulu-Natal, South Africa: implications for vaccine and antiretroviral control strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpvVGmug%3D%3D&md5=718b7d3e5d01590c37a220bf109bf2c0CAS |
[40] Yu RX, Yin Y, Wang GQ, et al Worldwide susceptibility rates of Neisseria gonorrhoeae isolates to cefixime and cefpodoxime: a systematic review and meta-analysis. PLoS One 2014; 9 e87849
| Worldwide susceptibility rates of Neisseria gonorrhoeae isolates to cefixime and cefpodoxime: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[41] Kirkcaldy RD, Kidd S, Weinstock HS, Papp JR, Bolan GA. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: the Gonococcal Isolate Surveillance Project (GISP), January 2006-June 2012. Sex Transm Infect 2013; 89 iv5–10.
| Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: the Gonococcal Isolate Surveillance Project (GISP), January 2006-June 2012.Crossref | GoogleScholarGoogle Scholar |
[42] Kirkcaldy RD, Zaidi A, Hook EW, et al Neisseria gonorrhoeae antimicrobial resistance among men who have sex with men and men who have sex exclusively with women: the Gonococcal Isolate Surveillance Project, 2005–2010. Ann Intern Med 2013; 158 321–8.
| Neisseria gonorrhoeae antimicrobial resistance among men who have sex with men and men who have sex exclusively with women: the Gonococcal Isolate Surveillance Project, 2005–2010.Crossref | GoogleScholarGoogle Scholar |
[43] Centers for Disease Control and Prevention Fluoroquinolone-resistant Neisseria gonorrhoeae–San Diego, California, 1997. MMWR Morb Mortal Wkly Rep 1998; 47 405–8.
[44] Baker KS, Dallman TJ, Ashton PM, et al Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study. Lancet Infect Dis 2015; 15 913–21.
| Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study.Crossref | GoogleScholarGoogle Scholar |
[45] Rosenberg ES, Grey JA, Sanchez TH, Sullivan PS. Rates of prevalent HIV infection, prevalent diagnoses, and new diagnoses among men who have sex with men in US States, metropolitan statistical areas, and counties, 2012–2013. JMIR Public Health Surveill 2016; 2 e22
| Rates of prevalent HIV infection, prevalent diagnoses, and new diagnoses among men who have sex with men in US States, metropolitan statistical areas, and counties, 2012–2013.Crossref | GoogleScholarGoogle Scholar |
[46] Sullivan PS, Jones JS, Baral SD. The global north: HIV epidemiology in high-income countries. Curr Opin HIV AIDS 2014; 9 199–205.
| The global north: HIV epidemiology in high-income countries.Crossref | GoogleScholarGoogle Scholar |
[47] Kelley CF, Vaughan AS, Luisi N, et al The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of black and white men who have sex with men in Atlanta, Georgia. AIDS Res Hum Retroviruses 2015; 31 587–92.
| The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of black and white men who have sex with men in Atlanta, Georgia.Crossref | GoogleScholarGoogle Scholar |
[48] Sullivan PS, Grey JA, Simon Rosser BR. Emerging technologies for HIV prevention for MSM: what we have learned, and ways forward. J Acquir Immune Defic Syndr 2013; 63 S102–7.
| Emerging technologies for HIV prevention for MSM: what we have learned, and ways forward.Crossref | GoogleScholarGoogle Scholar |
[49] Goldenberg T, McDougal SJ, Sullivan PS, Stekler JD, Stephenson R. Preferences for a mobile HIV prevention app for men who have sex with men. JMIR Mhealth Uhealth 2014; 2 e47
| Preferences for a mobile HIV prevention app for men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[50] Goldenberg T, McDougal SJ, Sullivan PS, Stekler JD, Stephenson R. Building a mobile HIV prevention app for men who have sex with men: an iterative and community-driven process. JMIR Public Health Surveill 2015; 1 e18
[51] Grindr. Grindr – World AIDS Day 2015. 2015. Available online at: http://www.grindr.com/blog/world-aids-day-2015/ [verified 25 May 2016].
[52] Ranosa T. Tinder adds HIV and STD testing locator on its website. 2016. Available online at: http://www.techtimes.com/articles/126756/20160122/tinder-adds-hiv-and-std-testing-locator-on-its-website.htm#sthash.KnVkt2Ma.dpuf [verified 25 May 2016].
[53] Hornet. Find the closest HIV testing location with Hornet. 2014. Available online at: http://love.hornetapp.com/blog/2014/9/23/find-the-closest-hiv-testing-location-with-hornet [verified 26 May 2016].
[54] Grant RM, Lama JR, Anderson PL, et al Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363 2587–99.
| Preexposure chemoprophylaxis for HIV prevention in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXit1ansg%3D%3D&md5=0341ee2cfaa1dd3a056b90f732e92218CAS |
[55] Elsesser SA, Oldenburg CE, Biello KB, et al Seasons of risk: anticipated behavior on vacation and interest in episodic antiretroviral pre-exposure prophylaxis (PrEP) among a large national sample of U.S. men who have sex with men (MSM). AIDS Behav 2016; 20 1400–7.
| Seasons of risk: anticipated behavior on vacation and interest in episodic antiretroviral pre-exposure prophylaxis (PrEP) among a large national sample of U.S. men who have sex with men (MSM).Crossref | GoogleScholarGoogle Scholar |
[56] McDougal SJ, Alexander J, Dhanireddy S, Harrington RD, Stekler JD. Non-occupational post-exposure prophylaxis for HIV: 10-year retrospective analysis in Seattle, Washington. PLoS One 2014; 9 e105030
| Non-occupational post-exposure prophylaxis for HIV: 10-year retrospective analysis in Seattle, Washington.Crossref | GoogleScholarGoogle Scholar |
[57] Sullivan PS, Jones J, Kishore N, Stephenson R. The roles of technology in primary HIV prevention for men who have sex with men. Curr HIV/AIDS Rep 2015; 12 481–8.
| The roles of technology in primary HIV prevention for men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[58] McCormack S, Dunn DT, Desai M, et al Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387 53–60.
| Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial.Crossref | GoogleScholarGoogle Scholar |
[59] Molina JM, Capitant C, Spire B, et al On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373 2237–46.
| On-demand preexposure prophylaxis in men at high risk for HIV-1 infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XntFSnsLc%3D&md5=e79e5715fed31994b8c74acf262635a6CAS |
[60] Lewis JM, Volny-Anne A, Waitt C, Boffito M, Khoo S. Dosing antiretroviral medication when crossing time zones: a review. AIDS 2016; 30 267–71.
| Dosing antiretroviral medication when crossing time zones: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitV2nsbjK&md5=5d501a211eaf91c96559f60b86632ac9CAS |
[61] Koole O, Denison JA, Menten J, et al Reasons for missing antiretroviral therapy: results from a multi-country study in Tanzania, Uganda, and Zambia. PLoS One 2016; 11 e0147309
| Reasons for missing antiretroviral therapy: results from a multi-country study in Tanzania, Uganda, and Zambia.Crossref | GoogleScholarGoogle Scholar |
[62] Oster AM, Brooks JT, Stryker JE, et al Interim guidelines for prevention of sexual transmission of Zika Virus – United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65 120–1.
| Interim guidelines for prevention of sexual transmission of Zika Virus – United States, 2016.Crossref | GoogleScholarGoogle Scholar |