Jurassic primates, immobile ducks and other oddities: a reply to Heads’ review of The Monkey’s Voyage
Alan de QueirozDepartment of Biology, University of Nevada, Reno, NV 89557-0314, USA. Email: dequeiroza@gmail.com
Australian Systematic Botany 29(6) 403-423 https://doi.org/10.1071/SB16021
Submitted: 18 May 2016 Accepted: 15 December 2016 Published: 11 May 2017
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
In The Monkey’s Voyage, I focused on the issue of disjunct distributions, and, in particular, on the burgeoning support from molecular-dating studies for long-distance dispersal over vicariance as the most reasonable explanation for many (but by no means all) distributions broken up by oceans. Michael Heads’ assessment of the book is founded on his long-standing belief, following Croizat, that long-distance dispersal is an insignificant process and, therefore, that disjunctions are virtually always attributable to vicariance. In holding to these notions, Heads offered a series of unsound arguments. In particular, to preserve an ‘all-vicariance’ perspective, he presented a distorted view of the nature of long-distance dispersal, misrepresented current applications of fossil calibrations in molecular-dating studies, ignored methodological biases in such studies that often favour vicariance hypotheses, repeatedly invoked irrelevant geological reconstructions, and, most strikingly, showed a cavalier approach to evolutionary timelines by pushing the origins of many groups back to unreasonably ancient ages. The result was a succession of implausible histories for particular taxa and areas, including the notions that the Hawaiian biota is almost entirely derived from ancient (often Mesozoic) central Pacific metapopulations, that the disjunctions of extremely mobile organisms such as ducks rarely, if ever, result from long-distance dispersal, and that primates were widespread 120 million years before their first appearance in the fossil record. In contrast to Heads’ perspective, a central message of The Monkey’s Voyage is that explanations for disjunct distributions should be evaluated on the basis of diverse kinds of evidence, without strong a priori assumptions about the relative likelihoods of long-distance dispersal and vicariance.
Introduction
Explaining disjunct distributions is a central and contentious issue in historical biogeography. In my book, The Monkey’s Voyage (de Queiroz 2014), I described some of the history of thought about disjunctions, especially those involving distributions of terrestrial organisms broken up by oceans. I focused especially on the surge of molecular dating (=timetree) results that, when coupled with evidence about the timing of fragmentation of areas, frequently have supported relatively recent long-distance dispersal (LDD) as an explanation for disjunctions, including many cases that previously had been ascribed to more ancient vicariance events resulting from tectonic processes. This molecular evidence, I argued, has shifted historical biogeography towards a more balanced view, in which vicariance is not assumed to be the default explanation for disjunctions, and both LDD and vicariance are acknowledged as major factors in shaping distributions.
In his review of The Monkey’s Voyage, Heads (2014a) is pointedly critical, taking issue with my depiction of the history of the field and, at greater length, with the evidence presented and conclusions reached concerning the causes of disjunctions (unless otherwise noted, ‘Heads’ refers to Heads 2014a). Heads is among a minority of biogeographers who believe, on the one hand, that LDD is not an important process, and, on the other hand, that all or nearly all disjunctions must be explained by some form of vicariance (Heads 1985, 2005, 2009, 2010, 2011, 2012a, 2014b). In Heads’ case, these beliefs derive directly from the panbiogeography of Croizat (1958, 1962), and, especially, the claim that an objective examination of distribution patterns reveals the dominance of the fragmentation and conglomeration of areas as biogeographic processes. Tellingly, Heads once wrote (in an email message to me in 2010), ‘Apart from Croizat I don’t think there has been much development [in biogeography] over the last 50 years’ (de Queiroz 2014, p. 274). However, my argument here is not against Heads’ Croizatian views in general, but, rather, against the idea that LDD is an insignificant process in biogeography, an idea that has been expressed not only by Heads and other panbiogeographers (e.g. Craw 1979; Grehan and Schwartz 2009) but also by scientists not labelled as such (e.g. McCarthy 2003; Parenti 2006; Nelson and Ladiges 2009; Mazza 2014). (A related point is that not all researchers who use the panbiogeographic approach of track analysis dismiss LDD as insignificant; e.g. see Page and Lydeard 1994; Morrone 2015.)
Regarding the opposing views at the heart of the present paper, it is important to correct from the start an inaccurate impression given by Heads. Specifically, Heads cast myself and others who disagree with his perspective as ‘dispersalists’; he went so far as to use ‘de Queiroz’ to stand in for ‘modern dispersal theory’ (Heads, p. 282), which gave the impression that we believe LDD explains all or nearly all disjunctions. This representation is clearly misleading; I am not aware of any historical biogeographer who claims that vicariance is insignificant. Biogeographers such as the botanist Susanne Renner or the zoologists Steve Trewick and Miguel Vences, who Heads would surely label as ‘dispersalists’, have published multiple papers that support vicariance explanations (e.g. Trewick et al. 2000; Renner et al. 2010; Tolley et al. 2013). Similarly, the fact that I referred to only a few specific well supported cases of vicariance in the book reflects only my emphasis on establishing the importance of LDD, not a disbelief in the significance of fragmentation events. Just among publications from the past 10 years, I have seen scores of studies that present compelling evidence for vicariance, and I believe that thousands more such cases remain to be discovered.
This distinction between Heads’ perspective, entailing a wholesale rejection of long-distance colonisation as a significant process, and the more balanced views of those he is criticising, should be kept in mind in evaluating his review of The Monkey’s Voyage. As I will illustrate below, in case after case, the arguments he uses to support vicariance hypotheses and to cast doubt on LDD explanations strain credibility. Many of the points I raise here have been made by others in assessments of Heads’ work (e.g. Goswami and Upchurch 2010; O’Grady et al. 2012; Swenson et al. 2012; Lohman and Tsang 2014; Matzke 2015; McGlone 2015), or in general critiques of panbiogeography (e.g. Cox 1998; Briggs 2007; Waters et al. 2013), but I add to the deconstruction of Heads’ perspective through new arguments as well as discussions of many examples not considered elsewhere. In fact, my main purpose here is to use an abundance of detail, both in terms of pointing out diverse logical flaws and describing many case histories, to illustrate the problems with the anti-dispersal approach espoused by Heads.
My response is divided into five main sections. The first addresses Heads’ criticisms of my description of the history of the vicariance–dispersal debate, especially with respect to the situation in New Zealand since the early 1970s. In the second section, I evaluate his arguments against molecular-dating analyses. I argue that his conclusion that such analyses are extremely biased towards favouring LDD is based on mischaracterisations of the use of calibration points, lack of recognition of biases against LDD explanations, and an unsupported assumption about the mindset of investigators. In the third section, I describe several other specious arguments made by Heads in his review or in other relevant papers, including the erroneous inference that studies of normal dispersal demonstrate the implausibility of LDD events, and a misinterpretation of the relationship between LDD and speciation by genetic revolutions. The fourth section critiques Heads’ specific analyses of areas and of primates and ratite birds; these cases illustrate his unrealistic assumptions about the ages of groups and his repeated references to irrelevant or unsupported geologic reconstructions.
As I argued in the book, estimating divergence ages is critical to progress in historical biogeography; not having this information when trying to decipher the history of taxa is something like trying to make sense of events in human history, such as the Great Depression or the Vietnam War, without knowing when they occurred. In the fifth and final section, I turn from criticism of Heads’ arguments to more constructive thoughts about this central issue of timing. In particular, I briefly describe some of the key, often surprising, conclusions that have emerged from timetree studies with respect to distributions of terrestrial taxa broken up by oceans.
In the interest of space, I have not attempted to address every point raised by Heads in his review. For instance, I do not specifically go through his seven supposed ‘myths about biogeography’ (although I deal with most of them at least indirectly) and I do not examine all the analyses of areas that he critiques. Nonetheless, the many cases evaluated here should make clear the pattern of misleading claims and unjustified inferences that characterise Heads’ arguments, and, in doing so, illustrate why the conclusions he draws, about The Monkey’s Voyage and about historical biogeography in general, are deeply flawed.
Some historical revision, especially concerning beliefs of New Zealand scientists
Before getting to the heart of this reply, I briefly address what I consider Heads’ most nearly legitimate criticism of the book, namely, that I inaccurately claimed that biogeography in the 1970s through much of the 1990s was dominated by the vicariance viewpoint and that this was especially true in New Zealand. There is some truth to this criticism, although Heads’ alternative description of the situation in New Zealand is clearly incorrect, as I will show.
For the field in general, I did claim, in the book’s Introduction (p. 15), to be telling the story of the shift ‘from a view dominated by vicariance to a more balanced outlook.’ I admit that this is an overstatement; I think it is more reasonable to say that, while the importance of vicariance as a process became very widely accepted, the view that it is the dominant explanation for disjunctions, although common, was less widely held. It is easy to find statements from biogeographers during this period that are not in line with the view of vicariance as dominant (e.g. Mayr 1982; Brown and Gibson 1983; Goldblatt 1993).
A more accurate and nuanced perspective comes out in other sections of the book, somewhat contrary to that claim in the Introduction. For example, in Chapter 7, I noted that many botanists continued to believe in the importance of long-distance colonisation, especially because of the relative ease of seed dispersal. I specifically pointed to the botanist Susanne Renner as someone who never wavered from believing in the great importance of LDD. And in Chapter 11, I stated that vicariance biogeography ‘never came close to being universally embraced’ (p. 276); in fact, in that chapter, I used the observation that the vicariance view did not take over the field as part of my argument that historical biogeography has been in a ‘pre-paradigm’ state for the past 150 years. Those statements more accurately represent my views of the situation in the 1970s through most of the 1990s than does the statement in the book’s Introduction.
For the purposes of the book, the key, in any case, is not that the vicariance viewpoint became thoroughly dominant, but that many disjunctions, such as those involving the ratite birds, southern beeches, baobabs, and many lesser-known examples, were typically interpreted as products of area fragmentation, without strong supporting evidence. This reflected a belief, even among scientists who did not hold an extreme vicariance worldview, that range fragmentation was the default explanation for disjunctions involving areas that had been connected in the past.
With respect to what people thought in New Zealand, I think the situation was less extreme than either Heads’ description or mine indicated, although I argue here that my view was slightly inaccurate whereas Heads’ view was grossly so. I said (p. 100) that, in New Zealand, the vicariance worldview came ‘fairly close’ to dominating historical biogeography, ‘not in the consistent use of cladograms or Croizat’s tracks, but in the belief that an ancient vicariance event was the key to understanding the biota.’ In contrast, Heads claimed that in the 1970s and early 1980s, the only advocates of vicariance in New Zealand were he and three other PhD students and, a bit later, the National Museum zoologist F. Climo. Heads further argued that my claim that the vicariance view was dominant is contradicted by the fact that the New Zealand panbiogeographers were exiled by being fired or shut out from obtaining jobs in that country.
A part of the discrepancy here might be that Heads considered only panbiogeographers as having a vicariance viewpoint, whereas, as just noted, I included anyone who believed that Gondwanan breakup was a key to understanding the New Zealand biota. From the latter perspective (which has to be considered ‘correct,’ because the discussion is framed by how I defined the issue), Heads’ claims are clearly misleading. During the period in question, many scientists in New Zealand, other than those identified by Heads, expressed the view that its biota was made up substantially of Gondwanan relict lineages. For instance, Skipworth (1974), in a paper titled ‘Continental drift and the New Zealand biota’, argued for the Gondwanan origins of many taxa, including plants in the Podocarpaceae, Winteraceae, Proteaceae and Restionaceae, Sophora and Hebe, and, among animals, Peripatus, chironomid midges, Leiopelma frogs, the tuatara (Sphenodon) and the ratites. Cooper and Millener (1993) considered many of those same groups to be Gondwanan hold-overs in New Zealand, and added to the list geckos, skinks, wetas, some spiders, terrestrial gastropods, the kauri (Agathis australis), and many ferns, lycopods and other ‘lower’ plants. Along the same lines, Daugherty et al. (1993, p. 437) noted that New Zealand differs strongly from oceanic islands such as Hawaii and the Galápagos ‘because of the ancient continental origins of both the landmass and the biota.’ All six of the authors of those three papers were New Zealand scientists.
This Gondwanan-relict view even influenced C. A. Fleming, who Heads (p. 285) described as ‘a prominent dispersalist,’ and the leader of the New Zealand biogeographical establishment from the 1960s to the late 1980s. By the mid-1970s, Fleming (1975) had accepted that New Zealand was once part of Gondwana and that the origins of some New Zealand taxa traced back to that early continental history. In particular, Fleming interpreted some taxa in his ‘paleo-austral’ group as being Gondwanan hold-overs.
Furthermore, there were well known, vicariance-oriented technical papers written by scientists and others outside of the country but dealing with New Zealand taxa, as well as popular works that considered the origins of the country’s biota, and it is reasonable to assume that these influenced scientists within New Zealand to adopt the Gondwanan-relict view. Some of the prominent papers included Brundin (1966), Raven and Axelrod (1972), Cracraft (1974), Nelson (1975), Humphries (1981) and Melville (1981), and the books included Enting and Molloy’s (1982) The Ancient Islands, Flannery’s (1994) The Future Eaters and, especially, Bellamy et al.’s (1990) Moa’s Ark: the Voyage of New Zealand, which was also a popular documentary television series.
In light of this evidence for widespread acceptance of the idea that a large number of New Zealand taxa are Gondwanan relicts, the exiling of Heads, Craw and others, to the extent that it was related to their scientific views, should be seen, not as an attempt to squash the idea of vicariance in general, but as an indictment of the specific shortcomings of panbiogeography. (See McGlone 2015 for a more detailed discussion of the fate of the New Zealand panbiogeographers.)
In developing the argument that my description of biogeography in New Zealand was totally wrong, Heads (p. 285) also suggested that the three scientists who told me that the vicariance viewpoint was dominant in New Zealand, namely Dallas Mildenhall, Mike Pole and Steve Trewick, ‘were all dispersalists, and they have a vested interest in portraying themselves as independent critical thinkers.’ (I should point out that Mildenhall noted that neontologists tended to hold the Gondwanan-relict view whereas palaeontologists did not.) However, the references cited above, especially those that were written by New Zealand scientists, suggest that the memories of Mildenhall, Pole and Trewick are substantially correct. Could it be that Heads’ own vested interest has drastically influenced his recollections and interpretations of those times?
From the above, it should be clear that Heads’ depiction of the situation in New Zealand from the 1970s into the 1990s is grossly inaccurate. Nonetheless, I do not want to claim that everything I wrote in the book about this period was completely correct. In particular, there were a fair number of New Zealand scientists who maintained a belief through this time in the great (and, for some of them, dominant) importance of LDD. I recognised that fact in the book, mentioning Fleming, Mildenhall and Robert McDowall in that context, but I also de-emphasised their continuing influence through the period in question. Still, the works written at the time, given above, as well as perceptions of recent developments (Winkworth et al. 2002; Didham 2005; McGlone 2005; Waters and Craw 2006; Goldberg et al. 2008; Giribet and Boyer 2010; Trewick and Gibb 2010) indicate that, contrary to Heads’ claim, historical biogeography within New Zealand has undergone a major shift away from vicariance and towards dispersal explanations for the origins of the biota. It has not been a case of ‘dispersalism’ simply maintaining dominance the whole time, as Heads would have us believe.
The key issue of estimating divergence ages
I emphasised in my book the significance of molecular divergence-date studies in promoting a shift away from vicariance explanations and in favour of dispersal explanations for many disjunctions. Heads argued, as he has in other publications (e.g. Heads 2005, 2009, 2012b), that estimates from these molecular studies, when calibrated with fossils, provide only minimum ages for groups (or, more accurately, nodes in a phylogeny), and that these age estimates, therefore, are often gross underestimates. As a result, he claimed that support from these studies for recent LDD over ancient vicariance is illusory.
The problem of calibrations and, more generally, of accurately estimating ages using molecular data are certainly real and are widely recognised. However, Heads presented a distorted view of the problems; he mischaracterised the current practice of how calibrations are chosen and used, ignored biases or deliberately conservative practices that work against the acceptance of recent LDD, and mistakenly assumed that a longstanding bias favouring LDD pervades the field. I will deal with these issues in turn.
Mischaracterising the choice and use of calibrations
Many recent molecular studies have used Bayesian methods to produce timetrees (trees with age estimates for nodes). For each calibration, these methods use the age of the oldest fossil of a group in applying a calibration prior, that is, a probability distribution for the age of the node in question, with the fossil age as the minimum bound. Heads focused on the fact that authors can choose a prior that extends only a small number of years older than this minimum age. According to Heads (p. 286), ‘If authors choose a small number, the method is guaranteed to produce young clade ages, and this is what is usually done in practice’, with the result that ‘Unless a group has an exceptional fossil record, this methodology automatically rules out early clade ages and vicariance.’
However, this criticism focused on studies that ‘choose a small number’ for the range of the calibration prior. However, use of very narrow priors is not the current standard practice for timetree analyses. This is readily apparent in examining the extensive Fossil Calibration Database (Ksepka et al. 2015) in which the span between the minimum age of a node (the lower bound for the age of the fossil as indicated by stratigraphic, radiometric or other evidence) and the maximum age is often large in both an absolute sense (several tens of millions of years or more) and as a proportion of the minimum age. A perusal of recent timetree studies shows that such large ranges for calibration priors have become commonplace (e.g. Bell et al. 2010; Clarke et al. 2011; Meredith et al. 2011; Joyce et al. 2013; Rota-Stabelli et al. 2013; Prum et al. 2015). Further, many investigators use ‘soft maxima’ (Yang and Rannala 2006), which means that the prior probability distribution for the age of a node actually extends past the nominal maximum, albeit with a low probability density. In short, Heads’ claim about the use of narrow priors is misleading and does not apply to the most sophisticated recent timetree analyses.
On a related point, Heads assumed that the fossil record places no limit on the maximum age of any group. This is apparent from his extension of the age of many nodes to depths far beyond those considered reasonable by both palaeontologists and neontologists who specialise on the taxa in question (e.g. Heads 2005, 2010, 2012c). Heads’ claim amounts to saying that the cumulative, and massive volume of palaeontological work provides no clues (other than minimum ages) for when any group appeared on the planet.
That claim is untenable given a simple observation, namely, that the appearance of groups in the fossil record is often significantly correlated with the branching order within phylogenetic trees (Norell and Novacek 1992; Benton et al. 2000; Smith et al. 2006; Marjanović and Laurin 2007). (Even before the wide acceptance of evolution, a similar relationship was known, namely, the increasing resemblance of fossil taxa to living ones with a decreasing age of the fossils.) It is true that the correlation does not exist for many groups, but these tend to be ones for which divergence-age studies are absent or are viewed as especially tentative (Wills 2002; Smith et al. 2006; Sohn et al. 2015). Indeed, if there were no such correlation for any group, it is doubtful that fossil calibrations would ever have been widely adopted in timetree analyses.
This correlation is very broadly acknowledged, so much so that it forms a standard part of educational curricula on evolution. It seems likely that, if no such correlation existed, the idea of evolution would not be so widely accepted. In fact, creationists recognise the importance of the correlation, which is why they have tried to undermine it by claiming, for instance, that human footprints have been found in the same strata as non-avian dinosaur fossils. And, on the other side, J. B. S. Haldane supposedly quipped that his belief in evolution would be shattered if a Precambrian rabbit were discovered. Seen in this light, Heads’ view that the fossil record provides no information about the maximum ages of groups is extraordinary and, for an evolutionist, truly mystifying. His arguments imply that we should not be surprised at the discovery of Cretaceous humans, or of Precambrian lagomorphs, especially if their distributions could be explained by vicariance!
The relevance of the correlation between first appearances and branching order for inferring maximum ages of taxa can be appreciated if one considers the consequences of pushing ages for particular nodes to extreme depths, as Heads has done frequently. In a striking example of this, Heads (2012c) assumed that the separation of the New Zealand Abrotanella muscosa (Asteraceae) and its South American sister group, A. submarginata, occurred when the land connection between those areas was broken, c. 80–84 million years ago. Using this as a calibration point in a molecular-dating analysis, Swenson et al. (2012) estimated the age of crown-group Asteraceae as 1.456 billion years (95% HPD, highest posterior density: 0.77–2.36 billion years), which they rightly pointed out is absurd given the fossil record, not only of Asteraceae, but of land plants in general. This age estimate is implausible because even its lower bound predates the known fossil record of all land plants and, thus, renders coincidental the relationship between fossil first appearances and branching order for various major land-plant groups and major lineages of angiosperms. Under the scenario implied by Heads, Asteraceae was already an old group at the time of the first fossil appearances of land plants, bryophytes, lycopsids, ferns, gymnosperms and all branches deeper than Asteraceae within angiosperms (Silvestro et al. 2015); thus, the facts that fossils representing early branches within land plants are hundreds of millions of years older than the first angiosperm fossils, and that fossils of many deep branches within angiosperms are tens of millions of years older than the first fossils of Asteraceae become completely irrelevant, unrelated to the actual sequence of evolution. Emphasising the implausibility of Heads’ approach even further, Swenson et al. (2012, p. 530) noted that the point estimate derived from the tectonic calibration for Abrotanella places the origin of Asteraceae ‘at a time when the biosphere was nearly exclusively populated by microscopic marine organisms.’
Similarly, Heads’ scenario for the historical biogeography of primates (Heads 2010, 2012a; see Goswami and Upchurch 2010 for a detailed critique) assumes that crown-group primates are c. 180 million years old, more than 120 million years older than the oldest fossils for the group (O’Leary et al. 2013; Benton et al. 2015), and this, again, renders the correlation between first appearance and branching order for primates and deeper branches coincidental. For instance, in Heads’ scenario, the fact that several early branches in the mammalian tree, such as monotremes and marsupials, are represented by fossils much older than any primate fossil has nothing to do with the actual ages of the involved groups, because all of them were already in existence at the time.
None of this is to say that calibration points and calibration priors currently in use are unproblematic; virtually everyone who uses molecular divergence dating recognises that fossil calibrations are prone to substantial error, and that there is a degree of arbitrariness to the form of prior probability distributions (e.g. Clarke et al. 2011; Parham et al. 2012; Warnock et al. 2014). The recent emphasis on compiling calibration points and ranges (Benton et al. 2015; Ksepka et al. 2015), evaluating the effect of using different calibration points (Near and Sanderson 2004; Schaefer et al. 2009; Clarke et al. 2011; Rota-Stabelli et al. 2013; Garzón-Orduña et al. 2015) and developing new methods for incorporating fossils more fully within a probabilistic framework (Pyron 2010; Wilkinson et al. 2011; Ronquist et al. 2012; Heath et al. 2014; Claramunt and Cracraft 2015) reflects a general belief that the choice of fossils and their integration in timetree analyses are in need of improvement. Nonetheless, it does not seem an egregious leap of faith to assume that the first known fossils of a group do not grossly underestimate the actual age of the group, if the stratum containing those fossils is followed by younger strata in which the group is increasingly common, and is preceded by well sampled, geographically relevant strata not much older that contain close relatives of the group, but no members of the group itself. Couple such judicious choice of fossil calibrations with the practice of using many such calibration points, as well as evaluating sensitivity to using different samples of calibration points, and the effect of errors ought to be strongly reduced. Provisionally accepting the results of such analyses, while continuing to seek refinements through improved methods and new molecular and palaeontological data, seems far preferable to pushing the origin of primates into the Early Jurassic or the origin of sunflowers into the Proterozoic.
Conservative bias with respect to inferring long-distance dispersal in timetree analyses
Heads repeatedly insisted that the use of fossil calibrations biases timetrees towards young ages and, therefore, inflates the evidence for LDD. However, he conveniently ignored evidence that many such analyses may be biased to estimate ages of biogeographic events as too old, and might thus be more likely to fail to reject explanations based on ancient vicariance.
First, molecular estimates for many relatively deep divergences within mammals (Meredith et al. 2011; dos Reis et al. 2012), birds (Jetz et al. 2012; Jarvis et al. 2014; Mitchell et al. 2014a) and flowering plants (Bell et al. 2010; Zeng et al. 2014) typically are much older than the oldest known fossils of the groups in question (Friis et al. 2010; Mayr 2013; O’Leary et al. 2013). These groups have been key ones in debates over the importance of continental breakup v. overwater dispersal, so potential biases in estimations of their ages are clearly relevant to the issue at hand. Although the reasons for the age discrepancies remain controversial, plausible arguments have been made that molecular analyses have been biased to produce older divergence ages because of unrealistically old calibration priors (Mayr 2013; Prum et al. 2015), among-lineage rate heterogeneity (Magallón 2014; Beaulieu et al. 2015) and inadequate taxon sampling (Beaulieu et al. 2015). In any case, the key point here is that these examples are more likely to represent bias against rather than in favour of LDD explanations.
A second, possibly more widespread source of bias has to do with the logic of interpreting divergences between taxa in disjunct areas. Specifically, if the divergence between such taxa is old enough to have been caused by vicariance, then that explanation often is accepted (because, even today, vicariance is frequently treated as the default explanation). However, as pointed out by Poux et al. (2006) and others, such a divergence age provides only a maximum age for the existence of the taxa in both areas. Therefore, even if the estimated age is consistent with an ancient vicariance event, it may provide only weak support for that explanation.
Consider, for example, the divergence between the kauri (Agathis australis) of New Zealand and its living sister group in Australia. The molecular dating analyses of Knapp et al. (2007) gave an age for this split that is consistent with Agathis persisting in both areas since the opening of the Tasman Sea, some 80–84 million years ago (also see Wilf and Escapa 2015). However, because A. australis is the only New Zealand species in this clade, there is no evidence from these molecular studies for a deep divergence within New Zealand, and, thus, no strong support from this work that the lineage has been present in Zealandia since its separation from Australia. The fossil record also provides no clear evidence that Agathis was present soon after the separation; the earliest definitive New Zealand Agathis fossils are from the late Oligocene (Lee et al. 2007; Pole 2008). A plausible alternative is that the ancestors of A. australis dispersed from Australia to Zealandia after those landmasses separated, but that the Australian lineages closest to A. australis subsequently became extinct (Biffin et al. 2010).
In his ‘Analyses of areas’ section, Heads repeatedly assumed that the divergence age between an island lineage and its relatives elsewhere implies existence on the island or nearby prior land since that time, thus inflating the evidence for the involvement of ancient events. For example, he stated that the skink Afroablepharus annobonensis, endemic to the Gulf of Guinea island of Annobón, ‘has been dated as ~10 million years old’ (Heads, p. 290), and used this information to argue for the long existence of this lineage on Annobón or on nearby islands that no longer exist (and are, it should be pointed out, hypothetical). However, the 10-million-year estimate is for the divergence between A. annobonensis and its relatives on the islands of São Tomé and Príncipe, not within Annobón. The cited study (Jesus et al. 2007) found no sequence divergence at all within A. annobonensis, and, thus, no clear evidence for long persistence there or on those hypothetical prior islands. Similarly, all of the divergence ages Heads cited for the Chatham Islands are for splits between Chathams taxa and related groups elsewhere, not divergences within the Chathams (see section below on the Chathams). I am not aware of any studies that show divergences within the Chathams significantly greater than 3 million years old (the age I gave in the book for the emergence of the current islands).
To reiterate the general point, interpreting between-area divergence ages as clear indications of persistence since that time in the areas in question produces a bias favouring the involvement of ancient events. Many investigators, not just Heads, have interpreted divergences in this way (e.g. Ericson et al. 2002; Nagy et al. 2003; Vences et al. 2003; Allwood et al. 2010; Heenan et al. 2010). Thus, as with the probable bias of molecular methods to overestimate the ages of divergences in birds, mammals and flowering plants, this logical error, if anything, has led to underestimating the importance of LDD. Fortunately, estimating both maximum (between-area) and minimum (within-area) divergence ages is becoming more common. Nonetheless, the collective body of evidence that Heads claimed is heavily biased against vicariance, has actually suffered from a widespread bias in the other direction.
Heads also ignored the fact that some investigators have intentionally biased timetree analyses towards older ages, with the specific intention of rendering the results conservative with respect to supporting LDD. For example, in the book, I mentioned that Matt Lavin and colleagues had run analyses for woody legumes in which fossils thought to predate crown groups were treated as crown-group fossils, which should push estimates towards deeper ages. For the same reason, de Queiroz and Lawson (2008) treated fossils that might be stem-group gartersnakes (Thamnophis) and watersnakes (Nerodia) as members of crown groups. Similarly, Renner (2004) used a possible stem-group fossil as the calibration for crown-group Myrtaceae. It is also common for investigators to use different sets of calibration points or ranges, and to accept biogeographic interpretations only if they are supported by all analyses (e.g. de Queiroz and Lawson 2008; Schaefer et al. 2009; Hedges and Conn 2012, Springer et al. 2012). That practice does not specifically bias results to favour ancient events, but it makes interpretations conservative in general, by widening the estimated age ranges in both directions.
In summary, Heads’ claim of a consistent underestimation of divergence ages in timetree studies is refuted by consideration of (1) the likely overestimation of such ages for certain key taxa, (2) a widespread bias tied to the use of divergence ages between (rather than within) areas, and (3) intentionally conservative practices employed in many cases.
Is there a widespread investigator bias favouring long-distance dispersal?
Heads erroneously claimed that there is a strong a priori tendency among those who study historical biogeography to discount vicariance and favour LDD. For instance, he painted investigators as ‘dispersalists’ when, as I have shown above, these scientists generally have no problem favouring vicariance explanations when the evidence supports such hypotheses. In fact, as I described in the book, some of these investigators have admitted that they were initially biased to prefer vicariance explanations, but changed their beliefs because of evidence indicating that LDD also is extremely important. For example, Matt Lavin, who was studying woody legume taxa on both sides of the Atlantic Ocean, and Miguel Vences, working on amphibians in the Indian Ocean region, both started out focused on vicariance, but were convinced by timetree results that most of the involved disjunctions came about through LDD. Such personal histories call into question Heads’ (p. 300) statement that ‘the retention of chance dispersal is largely based on conservative prejudice and hold-overs from the Mayrian approach’. If these scientists have now come to agree with much (although certainly not all) of what Mayr and other ‘dispersalists’ believed, it is because the evidence made them reject the vicariance view on which they had been raised.
Further, one could make the case that the attributes of widely used biogeographic models, rather than indicating a dispersalist bias, suggest just the opposite. In particular, the models DIVA and DEC (Ronquist 1997; Ree and Smith 2008) do not specifically incorporate speciation by founder-event LDD, and are biased to support vicariance explanations for disjunctions (Matzke 2014, 2015).
If there is no pervasive investigator preference for LDD and if, as I have argued above, timetree analyses have not been consistently biased to favour that explanation, then we are left with the conclusion that I reached in the book, namely that evidence from many molecular-dating studies, coupled with fossil data, and geological reconstructions of landmass histories, point to the great importance of LDD in explaining disjunct distributions.
A trio of specious arguments against long-distance dispersal
In his review of my book and elsewhere, Heads incorrectly characterised the nature of chance, long-distance dispersal as envisioned by myself and many others, and also argued erroneously that population genetic studies refute the validity of speciation by small founding populations, as is assumed to occur in most cases of long-distance colonisation. These subjects provide more evidence of how Heads’ wholesale rejection of LDD and, thus, his critique of The Monkey’s Voyage, is based on specious arguments.
Concerning the nature of dispersal, Heads (p. 288) claimed that LDD as usually conceived involves ‘factors beyond our understanding’ and that such dispersal ‘can happen in any direction, at any time’. He went on to suggest that such rare dispersal events do occur, ‘but they do not explain distribution patterns that are repeated in many different groups with different means of dispersal and very different ecology’ (Heads, p. 289).
If LDD were conceived to be completely random with respect to routes and timing of colonisation, then Heads would have a legitimate point here; fully random dispersal would invalidate the claim, made by myself and many others, that LDD is expected to produce general patterns of linkage among areas. However, those arguing that LDD is important do not view it as even close to completely random. For instance, with respect to dispersal of land organisms over sea barriers, the probability of colonisation should decrease with increasing distance between areas, patterns of colonisation by rafting should be influenced by prevailing ocean currents, and, for many taxa, routes with stepping-stone islands should be used more than those without stepping stones. It is from such considerations that expectations of repeated patterns produced by LDD emerge.
A significant point here is that, although specific LDD events are not predictable, this does not mean that LDD in general is beyond all understanding and provides no expectations about the phylogenetic connections among biotas. Such expectations arise from considering the collection of possible dispersal events. This is a straightforward point that comes out of probabilistic thinking, but it is worth emphasising in the present context.
In the same section, Heads also made a flawed argument about what can be inferred about long-distance dispersal from studies of normal dispersal. He focused on the case of New Zealand Veronica shrubs, for which observations found an average seed-dispersal distance of 13 cm and a maximum distance of 1.1 m (Pufal and Garnock-Jones 2010). From these results he suggested that the inference, from the occurrence of conspecific populations in New Zealand and Australia, that two Veronica species dispersed over the Tasman Sea is implausible; when normal, observable, ecological dispersal operates only over metres, Heads argued, it is untenable to posit chance dispersal over hundreds of kilometres. More generally, this argument implies that dispersal distances much greater than those that have been observed can be discounted.
Superficially, it might seem reasonable to posit, for any particular taxon, only the kinds of dispersal events that have been documented for that group. However, many other observations have indicated that such an assumption is unrealistically restrictive. For example, natural rafts often have been observed far out at sea (Van Duzer 2004), providing a mechanism of chance, long-distance dispersal for many kinds of land organisms, and rafting colonisation by large iguanas has even been witnessed (Censky et al. 1998). Diverse plant seeds, as well as some small arthropods and molluscs, can become attached to birds and might disperse great distances in this way (McAtee 1914; Carlquist 1974; Aoyama et al. 2012). Many seeds and at least some snails also can survive in the digestive tracts of birds (Proctor 1968; Sousa 1993; Nogales et al. 2012; van Leeuwen et al. 2012), and there is direct evidence of dispersal of seeds by this mechanism over hundreds of kilometres of ocean (Viana et al. 2016). Beginning with Darwin (1859), investigators have shown that many kinds of seeds remain viable after extended exposure to seawater (Carlquist 1974; Guja et al. 2010; Aoyama et al. 2012), which would allow seeds inundated on rafts or even floating free in the ocean to colonise distant areas. Furthermore, explicit modelling of dispersal events, based on measurable physical and biological parameters, has validated the existence and importance of LDD (e.g. Nathan et al. 2008; Viana et al. 2013). The general point here is that plausible LDD mechanisms exist for a great diversity of taxa, even though direct observations of LDD have been made for only a few groups.
With respect to the Veronica shrubs cited by Heads, there is no good reason to believe that seed dispersal must be limited to distances similar to those seen in a study entirely focused on the standard dispersal mechanism, that is, raindrops displacing seeds out of the seed capsules. In fact, the authors of the study cited by Heads (Pufal and Garnock-Jones 2010) suggested that the lightweight seeds of Veronica might be carried long distances by strong winds and that, because the seeds become mucilaginous when wet, they might also be dispersed attached to the feet or feathers of birds. More generally, dispersal ecologists (who are mostly little concerned with the dispersal v. vicariance issue) have concluded that LDD events probably often occur by vectors that the species in question do not normally use (non-standard vectors), such as strong winds connected to extreme meteorological events, animal-mediated dispersal in organisms not adapted for such dispersal, and rafting (Berg 1983; Nathan et al. 2008; Viana et al. 2013). It follows that studies showing that standard vectors generate very short dispersal distances do not refute the occurrence of much longer-distance events. In short, Heads’ notion that measured normal dispersal shows that LDD explanations are implausible is based on an insupportably narrow view of the evidence for LDD.
Heads’ unrealistic rejection of LDD is also illustrated by his argument (Heads 2009, 2010) relating to founder effect speciation. Heads claimed that this mode of speciation, in which reproductive isolation is achieved partly through strong genetic drift tied to small founding population size, is not validated by experimental and other studies. He then went on to argue that the lack of support for founder effect speciation constitutes an argument against the occurrence of chance, long-distance colonisation by small numbers of individuals.
The importance of founder effect speciation remains controversial (Butlin et al. 2012), and, in fact, there is experimental evidence indicating that very small founding population size can promote reproductive isolation (Templeton 2008; Matute 2013). However, even if it were true that founder effect speciation is unimportant, this would provide no reason to reject colonisation of areas by small founding groups. Whether or not genetic drift leads to genetic revolutions and speciation, if founding populations persist in isolation, they are likely to become differentiated from source populations and, eventually, to become distinct species. For example, a small founding group might rapidly expand to a point at which genetic drift is relatively unimportant; however, this would not preclude divergence from the source population by natural or sexual selection. The logical flaw in Heads’ argument is conflating a particular mode of speciation that requires small founding populations and strong genetic drift with any kind of speciation that involves small founding populations. It is telling that Jerry Coyne, who Heads cited for doubting the existence of founder effect speciation (Coyne and Orr 2004), accepts the importance of colonisations by LDD (Coyne 2009).
To summarise this section, Heads presented flawed arguments about (1) the nature of LDD and its relationship to repeated patterns, (2) the range of LDD events that can be inferred from normal dispersal, and (3) the connection between population genetic studies of founder effect speciation and the plausibility of long-distance colonisation by small founding groups. As with much else in his review and other work, these arguments show a mindset bent on denying the importance of LDD.
Ancient islands, ancient monkeys
Much of Heads’ review was devoted to critiquing cases I presented involving continental and oceanic islands, as well as the historical biogeography of two taxonomic groups that I discussed in some detail, namely primates and ratite birds. These sections of Heads’ review demonstrate how his refusal to place limits on the maximum ages of groups is connected to unrealistic interpretations of evolutionary history. More generally, they indicate, once again, a biased evaluation of the evidence for alternative explanations of geographic distributions.
Several of the islands or island groups on which Heads focused are either purely volcanic (e.g. the Hawaiian Islands) or are volcanic in the sense that the emergence of the current islands is thought to have been caused by vulcanism, but the islands are composed partly of continental rock (e.g. the Chatham Islands). The standard explanation, and the one I followed in the book, is that the native land biotas of these islands are derived from long-distance, over-water dispersal. In contrast, Heads believes that the ancestors of current species existed on prior land in these areas and that they reached the modern islands by normal dispersal. If one accepts either timetree results or a straightforward argument about the separation of conspecific lineages (see below), Heads’ posited scenarios for these cases typically involve movements from land areas that are too ancient to have been involved in the origins of most or all of the taxa in question. Furthermore, for the majority of these cases, the earlier land areas emerged in the ocean as volcanic islands and were probably distant from other landmasses; thus, even if Heads were correct that these earlier land areas contained the progenitors of many species on the current islands, his scenarios beg the question of how those earlier areas were colonised.
In responding to Heads’ criticisms, I focus here on the two island groups that he considered in the greatest detail, namely São Tomé and Príncipe, and the Chatham Islands. I then briefly discuss some of the other island cases, to establish more generally the flaws in Heads’ analyses of areas. A key distinction between our views is that I consider scenarios untenable if they strongly conflict with the molecular divergence-date results, whereas, obviously, Heads does not constrain his argument in this way. (His major constraint is very different, namely, that LDD is not a viable explanation.) In my view, most of the geological evidence that Heads presented for ancient direct land connections or stepping-stone routes is irrelevant in the face of the timetree results, as I will indicate below.
São Tomé and Príncipe
São Tomé and Príncipe are volcanic islands in the Gulf of Guinea, respectively 255 and 220 km from the West African coast. They form part of the Cameroon Volcanic Line, a series of volcanic swells, some on the African continent and others arising from the ocean floor. It is generally believed that São Tomé and Príncipe have never been connected to the mainland, which has led to the belief that their biotas originated through over-water dispersal, mostly of the chance variety, but also, for organisms that can disperse easily over sea barriers, by normal dispersal. In the book, I described, in particular, the arguments of Measey et al. (2007) for long-distance colonisation of these islands by several lineages of amphibians, especially Ptychadena frogs, organisms for which a sea barrier even a few kilometres wide probably would be surmounted only rarely and with difficulty.
Heads argued, in contrast, that few if any of the native species of São Tomé and Príncipe arrived there by LDD, but instead reflect former land connections or stepping-stone routes by which normal dispersal could have taken place. He suggested that ‘the amphibians’ ancestors (not the modern species) were always in the region, before the islands were formed and even before the Atlantic opened’ (Heads, p. 289). Here, and for the ancestors of oceanic island taxa in general, Heads (2011, 2012a) envisioned ancestral metapopulations in which the individual populations may be on separated islands, but are connected to each other by normal dispersal. When islands become too widely separated, for example, because of submergence of some of them, metapopulations are fragmented and the now truly isolated parts are free to diverge. The process is therefore a vicariant one. For São Tomé and Príncipe, Heads cited not only the opening of the Atlantic c. 100 million years ago, but also alkaline intrusive magmatism along the Cameroon Volcanic Line from 65 to 30 million years ago, as well as more recent volcanic episodes, and he implied that all these geological events could have been directly involved in the origins of the biotas of the islands.
Obviously, the geological history of the region must be considered in studies of historical biogeography. However, the ages of evolutionary events for which explanations are sought are also critical; geological events that occurred long before the divergences in question are not relevant, at least not as processes that directly influenced those divergences. For the Ptychadena frogs of the Gulf of Guinea islands, the 16S rRNA sequence difference between the island species and the closest known relatives on the mainland suggests a divergence age of between 5.6 and 18.6 million years (Measey et al. 2007). Because the taxon sampling in Measey et al.’s (2007) study was limited (see Bell et al. 2015), it is possible that the actual closest mainland relatives were not included, which would make the estimate too old. (Recall also that divergences between islands and other areas are inherently biased high as estimates of the time of residence on the islands.) The only other Gulf of Guinea amphibian lineage for which an explicit divergence-age estimate is available is Hyperolius, with an estimated age of Pleistocene, based on the within-archipelago divergence, to late Miocene, based on the divergence between the island forms and their closest relative on the mainland (Bell et al. 2015). Additionally, Gulf of Guinea Phrynobatrachus are perhaps Miocene in age (Zimkus et al. 2010).
As far as I know, the only evidence of possible prior land in the Gulf of Guinea in the Miocene or later is the existence of two seamounts along the Cameroon Volcanic Line, one between Bioko and Príncipe and one between São Tomé and Annobón (Njome and de Wit 2014). It is unclear whether these volcanic structures were ever subaerial. However, even if they were, they likely would have formed islands that still would have required colonisation by chance (not normal) dispersal by amphibians.
Of course, it is possible that geological evidence will emerge that indicates stepping-stone islands (which would have to be very closely spaced to allow normal dispersal by amphibians) or an actual land connection to Africa that could explain the existence of Ptychadena, Hyperolius, Phrynobatrachus and other amphibians on the Gulf of Guinea islands, without involving LDD. However, agreeing with Darwin, ‘it shocks my philosophy to create land’ (Burkhardt and Smith 1989, p. 344) without any evidence. The fact that volcanic or other magmatic processes were occurring in the area is not a compelling argument without any specifics. Thus, it seems premature to invoke such hypotheses to account for the occurrence of amphibians on São Tomé and Príncipe.
In his section on São Tomé and Príncipe, Heads also suggested that similar ‘prior land’ hypotheses can account for all amphibian occurrences on oceanic islands, citing as an example Platymantis frogs on Fiji. ‘Although the individual islands of Fiji are young and have never been connected to a continent,’ Heads (p. 290) noted, ‘the structure producing them, the Pacific subduction zone, originated by a mainland.’ I am not aware of any biogeographic studies that have dealt with the origins of Fijian Platymantis; however, it is worth noting that herpetologists have hypothesised that the ability of Platymantis and other ceratobatrachids to persist and reproduce without standing fresh water has facilitated their colonisation of islands (Brown et al. 2015). In any case, there is now evidence from timetree analyses for many instances of over-water dispersal by amphibians, including colonisations of Madagascar, many Caribbean islands, Sulawesi, the Malukus, the Lesser Sundas, the Philippines, the Seychelles, the Comoros, the California Channel Islands, North America, South America, South Asia and Australasia, the latter possibly involving dispersal across the entire Pacific from South America (de Queiroz 2014 and references therein; Pyron 2014). Given this body of examples, over-water dispersal to São Tomé, Príncipe and Fiji is hardly disconcertingly unique.
Furthermore, it is worth noting that the amphibian timetree as a whole indicates, as one would expect from the dispersal abilities of these animals, that the large-scale, continental distribution of amphibians is mostly well explained by the breakup of Laurasia and Gondwana (Bossuyt et al. 2006; Pramuk et al. 2008; Zhang and Wake 2009; Pyron 2014). In other words, the various over-water dispersal events are inferred within a time frame that generally supports a plausible history of vicariance, in line with much geological evidence. This result will come as no surprise or affront to modern ‘dispersalists,’ who are all perfectly accepting of vicariance as an important process. However, Heads must reject the molecular-dating results and rely on unsupported scenarios of prior land to preserve the notion that LDD is insignificant for amphibians (and in general).
Heads also presented lists of disjunctions that he believes argue strongly for vicariant origins for the biotas of São Tomé and Príncipe. These involve cases where the apparent closest relatives of São Tomé and Príncipe taxa are found in areas very distant from these islands, including eastern Africa, the Indian Ocean region, areas well north and west of São Tomé and Príncipe (e.g. Sierra Leone and Liberia), and the Americas. However, this argument is fraught with problems in the data themselves and, even more so, in their interpretation.
First, these lists of disjunctions are made up primarily of cases based only on taxonomy rather than phylogenetic analyses, so their reliability can be questioned on that basis. Also, deciphering the relationships of São Tomé and Príncipe taxa to those in other areas is compromised by the fact that many African taxa have been poorly studied. Both Heads and I noted the connection of some São Tomé and Príncipe taxa to eastern African groups, but I now wonder whether this link is, at least partly, an artefact of limited sampling for some taxa, as suggested by Bell et al. (2015) for amphibians. An obvious potential bias is that, for political reasons, eastern African species are more likely to have been collected and described than those from central and western Africa.
Even assuming that the connections that Heads listed are real, they do not make a strong case for vicariant origins. First, these lists do not suggest that any of the distant geographic connections he mentions make up the dominant pattern for São Tomé and Príncipe taxa. If it could be shown, for instance, that the eastern Africa connection is the most common one among the taxa of these islands, that would be significant and would warrant re-evaluating the origins of the biotas of the islands (although it would not necessarily require a vicariant explanation). However, no such compilation has been made.
On the other side of the coin, the fact that São Tomé and Príncipe taxa show connections to various different areas, some of them distant from the islands, does not argue against origins by LDD. Although one would expect that many São Tomé and Príncipe taxa originated from the nearby African mainland, chance dispersal from other areas should not be ruled out, especially given the relatively great age of these two islands compared to most volcanic islands. For instance, as I described in the book (following Measey et al. 2007), an eastern Africa to Gulf of Guinea route is plausible by rafting via the Congo River, and would have been more likely when the climate was wetter. Also, extinction on the mainland is expected to produce greater geographic separation of some São Tomé and Príncipe taxa and their closest relatives than was true at the time of colonisation.
By analogy, native Hawaiian taxa show connections to many different areas, all of which are very distant from those islands, but this is not a reason to invoke vicariant origins for the Hawaiian biota (Cowie and Holland 2008; Gillespie et al. 2012). (Of course, Heads did exactly that, but his inferences for Hawaiian taxa require, among other things, rejecting the evidence that these taxa are young, see below.) Heads seems to think the fact that different, unrelated taxa show the same pattern of disjunction indicates an ecosystem-wide vicariance event, but such patterns for Hawaii (and other oceanic islands) indicate that this is not a logical conclusion.
Heads’ discussion of São Tomé and Príncipe also highlights, once again, how far he is willing to strain the evolutionary timeline to avoid explaining distributions by LDD. This is especially evident in his suggestion that disjunctions with the Americas, involving two marine fish species, a beetle, and two flowering plants, reflect the opening of the Atlantic, more than 100 million years ago. These examples have generally been interpreted as cases of trans-Atlantic dispersal, because the taxa are thought to be far too young to have been affected by the opening of the Atlantic (Wirtz et al. 2007; Michalak et al. 2010; Frolov 2013). For the two plant species, that age assumption has been validated by molecular dating. The Lentibulariaceae, the family that includes the disjunct Utricularia, is estimated to have split from its nearest relatives tens of millions of years after the separation of Africa and South America (Schäferhoff et al. 2010), and the divergence of interest within Utricularia must be far younger still. Similarly, the divergence of the Gulf of Guinea Hernandia from their New World relatives is estimated to have occurred within the past 15 million years (Michalak et al. 2010).
In summary, Heads’ vicariance-only view of the origins of the biota of São Tomé and Príncipe relies on assumptions about land connections or close stepping-stone islands that lack any clear geological support, the erroneous notion that repeated connections to distant areas imply vicariance, and rejection of estimates for the ages of taxa. The idea that chance, over-water colonisation is significant for islands that have existed for at least 31 million years (Príncipe) and 13 million years (São Tomé) within several hundred kilometres of a continent, and are in the path of prevailing ocean currents from that continent, hardly seems surprising, yet Heads seems to view such colonisation events as next to impossible.
The Chatham Islands
The Chatham Islands, which lie some 850 km east of New Zealand, are composed partly of Gondwanan continental rocks, and contain Late Cretaceous fossils of typical Gondwanan groups such as theropod dinosaurs, Nothofagus, and podocarp conifers (Stilwell et al. 2006; Campbell and Hutching 2007). Clearly, the Chathams have a geological connection to Gondwana. However, the current islands are generally believed to have emerged through volcanic activity within the past several million years (Campbell and Hutching 2007; Stilwell and Consoli 2012). The flora and fauna of the Chathams are in keeping with the hypothesised young age of the current islands (as subaerial land); in particular, molecular-dating studies of various plant and animal groups (see below), and the observation that there are few endemic genera (Holdaway et al. 2001; Emberson 1998; de Lange et al. 2011) argue against ancient origins of the biota. In fact, most Chathams populations are classified as conspecific with ones found elsewhere (Emberson 1998; Holdaway et al. 2001; Heenan et al. 2010; de Lange et al. 2011).
Not surprisingly, Heads argued against origins of Chathams groups by LDD, and instead raised supposed evidence for vicariant origins of the biota. However, his arguments suffer from the same kinds of flaws as those he made regarding São Tomé and Príncipe. Specifically, he relied on geological reconstructions that are poorly supported or irrelevant in terms of refuting LDD, and he rejected all young ages of taxa estimated using molecular data or other evidence. In addition, his discussion of molecular-dating studies for the Chathams suffers from multiple errors and misinterpretations.
With respect to possible prior land in the region that could have contained the ancestors of current Chathams taxa, Heads mentioned numerous seamounts on the Chatham Rise and the Hikurangi Plateau, and islands associated with the Mernoo and Veryan Banks. However, none of these possible former islands indicate vicariant origins for the Chathams biota. Regarding the seamounts on the Chatham Rise, if one assumes, incautiously, that all of the known seamounts were subaerial at some point, and that they overlapped in time to provide stepping-stone paths to the Chatham Islands, the positions of these structures still indicate significant ocean gaps that, for many land organisms, would likely have required chance crossings (see Rowden et al. 2005, fig. 1). Within the Hikurangi Plateau, the volcanic activity that formed the current guyots is thought to have occurred some 89–99 million years ago or even earlier (Davy et al. 2008), and is thus irrelevant to the origins of the much younger biota of the Chathams. Finally, parts of the Mernoo and Veryan Banks did become emergent land during recent glacial periods (Heenan et al. 2010), but these islands were much closer to the main islands of New Zealand than to the Chathams; thus, if Chathams ancestors lived on these intermittent islands, LDD would still have been required for them to colonise the Chathams. In short, the geological ‘evidence’ cited by Heads for the Chathams suggests few if any routes of normal dispersal, which is the type of dispersal required by vicariance scenarios.
Heads’ portrayal of the molecular evidence for the Chathams is also strongly misleading. A general problem is that he wrongly interpreted divergence ages between Chathams lineages and those elsewhere as minimum ages for the existence of these groups in the area of the Chathams. However, as pointed out above, minimum ages are given by divergences within the area in question. The lineage ages that Heads referred to as being older than 3 million years all refer to divergences between Chathams taxa and those elsewhere. Furthermore, the oldest divergence age cited by Heads, namely 7–14 million years ago for the borage Myosotidium hortense, was described by the cited authors (Heenan et al. 2010, p. 107) as being possibly ‘a significant overestimation due to incomplete taxonomic sampling and/or extinctions’, and they raised the possibility that such problems might apply to other cases as well. (In fact, overestimation of the relevant age because of incomplete sampling is often a possible problem.) It should also be pointed out that, even if these divergence ages were taken as minimum ages for occurrences of taxa in the area, they would suggest only that former islands were colonised, not that LDD was unimportant in the colonisation process.
Heads (p. 294) also claimed that ‘Several Chatham Islands groups are basal to (not nested in) groups that are diverse and widespread on the New Zealand plateau, and so deriving the Chathams Islands forms from the mainland requires extra, ad hoc hypotheses that are not needed in a simple vicariance model.’ However, the examples of Cyanoramphus parakeets and Anas ducks that he cited, far from suggesting vicariance, again indicate long-distance colonisation. It is true that one of the endemic Chathams Cyanoramphus parakeets, C. forbesi, is estimated to be sister to a clade including lineages occurring on the North and South Islands of New Zealand and various smaller islands in the area, but the other Chathams endemic, C. novaezelandiae chathamensis, is deeply nested within that larger clade, a pattern that does not suggest a deep split between the Chathams and other areas (Chambers et al. 2001). Furthermore, C. forbesi is estimated to have diverged from its sister group less than 0.5 million years ago, and these together are estimated to have split from New Caledonian relatives less than 0.6 million years ago. Chambers et al. (2001) rightly interpreted these results as indicating recent over-water colonisation of the Chathams.
Similarly, although the extinct Chatham duck (Anas [formerly Pachyanas] chathamica) is estimated to be the sister of a clade of three species in the New Zealand area, the estimated age of this split is 0.69–1.80 million years (95% HPD interval; Mitchell et al. 2014b), which is much too recent to be accounted for by any plausible vicariance event. Furthermore, these four are inferred to have diverged from the Madagascar teal (Anas bernieri) only 1.78–3.97 million years ago (95% HPD interval; Mitchell et al. 2014b), which implies a dispersal event (or events) over the Indian Ocean.
For both the parakeets and the ducks, it is worth emphasising that the ‘extra, ad hoc hypotheses’ required by LDD explanations amount to several over-water colonisation events by highly mobile birds. The phylogenetic relationships within parrots and dabbling ducks, and their occurrence on many volcanic islands indicate numerous oceanic dispersal events in both groups, a conclusion reached by investigators even without reference to molecular timetrees (Johnson and Sorenson 1999; Schweizer et al. 2010). Given a choice between accepting the reality of such dispersal events v. wholesale rejection of timetree results and a reliance on ad hoc and implausible hypotheses of prior routes for normal dispersal, namely, routes that would have to encompass not only the Chathams, but several other islands distant from the main islands of New Zealand where the parakeets or ducks are or were found, I see no problem in choosing the dispersal explanation.
In summary, none of the geological or molecular evidence that Heads brought up refutes the notion that the biota of the Chathams has been derived by recent long-distance, over-water colonisation. Geological ‘evidence’ for prior land that can plausibly explain the biota of the islands by normal dispersal and vicariance amounts to wishful thinking, and molecular phylogenetic results, far from countering origins by LDD, collectively provide strong support for that explanation (Trewick 2000; Chambers et al. 2001; Heenan et al. 2010; Mitchell et al. 2014b).
Other islands
Heads’ discussions of other islands likewise suffer from wholesale rejection of evidence for the ages of taxa and reliance on unsupported or irrelevant geologic reconstructions of prior land, among other problems. Detailed deconstructions of Heads’ views on all these islands are possible, but here I will simply describe ‘highlights’ for some of these areas, to give a sense of the pervasive weakness of his arguments.
Madagascar
Heads’ primary argument against LDD origins is that the timetree results indicating origins of Malagasy lineages after the separation of this island from other Gondwanan landmasses could be incorrect. However, for most of these taxa, the point estimates for divergence ages between Malagasy lineages and those elsewhere (which, as noted above, bias origins towards older dates) would have to be more than doubled to be consistent with a Gondwanan fragmentation hypothesis (Yoder and Nowak 2006). Given my arguments above regarding bias in divergence-age estimations, such strong, pervasive errors seem unlikely. Furthermore, although Gondwanan vicariance predicts that Malagasy taxa should have closest relatives in India, the Seychelles or the Mascarenes, the sister groups of Malagasy lineages are much more commonly African, which is expected if most of them arrived by over-water dispersal (Yoder and Nowak 2006).
The granitic Seychelles
I argued that the fact that most Seychellian lineages are congeneric or conspecific with taxa found elsewhere indicates that most are not ancient hold-overs, but instead arrived recently by over-water dispersal. Heads discounted this argument because it assumes that taxonomic rank is proportional to age. However, the assumption in this case is not a very stringent or controversial one; I was merely positing that most species and genera do not extend back to the time of the most recent separation of the Seychelles from other landmasses (i.e. from India, some 65 million years ago). Furthermore, several molecular-dating analyses cited in the book give divergence ages for Seychellian taxa that are far too young to be explained by Gondwanan breakup (Vences et al. 2003; Austin et al. 2004; Daniels 2011; Guo et al. 2012).
The Hawaiian Islands
Heads’ key claim is that the native Hawaiian biota in general could be derived from ancestral metapopulations that inhabited former land in the area. Such prior land could have existed or, in some cases, definitely did exist as parts of various volcanic formations, including the Hawaiian chain itself, which, as is well known, long predates the modern islands. In Heads’ view, colonisation of the current Hawaiian Islands did not require chance dispersal, but could have occurred by normal, ‘garden variety’ dispersal from these areas.
As I noted in The Monkey’s Voyage, divergences within some endemic Hawaiian clades predate the emergence of the current islands (e.g. Megalagrion damselflies, Jordan et al. 2003; Mecaphesa crab spiders, Garb and Gillespie 2009; lobeliads, Givnish et al. 2009; drosophilid flies, Russo et al. 2013; Hyposmocoma moths, Haines et al. 2014), suggesting that these groups colonised islands that are now submerged. These findings represent an important contribution of molecular timetree studies to knowledge of the history of the Hawaiian biota. However, they do not support the scenario envisioned by Heads. First, most of the areas of possible prior land in the region, i.e. the bulk of the Mid-Pacific Mountains, the Necker Ridge, the Hess Rise and the Musicians Seamounts, are estimated to have formed in the Cretaceous and are likely to have been submerged by the end of that period or in the early Tertiary (Thiede et al. 1981; Vallier et al. 1983; Kalnins and Watts 2009; Gardner et al. 2013). Thus, if ancestors of Hawaiian lineages occurred in these old areas, the divergence ages of current Hawaiian taxa from continental relatives (which should be more distantly related than those hypothetical mid-Pacific ancestors) should, minimally, fall in that same time frame. However, the old Hawaiian groups just mentioned are estimated to have separated from continental relatives between 10 million years ago and 30 million years ago (Garb and Gillespie 2009; Givnish et al. 2009; Russo et al. 2013; Haines et al. 2014), thus refuting the involvement of Mesozoic or Early Tertiary islands. Heads (2011, 2012a) also invoked terranes accreted to or subducted beneath North America, but these areas are substantially older than even those just mentioned, and, thus, are even less plausibly tied to the current Hawaiian biota.
A key point is that the nearby former lands that are the appropriate age to account for the origins of these various old Hawaiian lineages are much fewer and farther between than Heads (2011, 2012a, 2014c) would have people believe. These possible source areas include the older islands in the Hawaiian chain, the Line Islands, and hypothetical islands within the formations mentioned above that formed after the main volcanic events had created the bulk of those formations.
In addition, and critically, these potential source areas all were volcanic islands, and, thus, one has to wonder how they were colonised by normal dispersal in the first place. Heads’ scenarios require, at some point, colonisation from areas other than oceanic islands, which would necessitate a sequence through deep time of emerging land areas, with each subsequent one close enough to prior land to allow normal dispersal by a diversity of organisms. However, as just noted, the ages of the geologic formations in the region suggest that such a set of closely spaced islands relevant to the origins of the Hawaiian biota is unlikely.
Furthermore, most Hawaiian native lineages probably colonised the area within the past 5 million years, as indicated by timetree analyses (Price and Clague 2002; Bennett and O’Grady 2013; Roy et al. 2013) or by very limited anatomical divergence from non-Hawaiian relatives (e.g. the owl Asio flammeus sandwichensis, the stilt Himantopus mexicanus knudseni, and the convolvulaceous vine Jacquemontia ovalifolia, among others, are all classified as conspecific with populations elsewhere). These findings suggest that most Hawaiian lineages colonised the current high islands, not former land in the area, and that they must have crossed wide expanses of ocean to reach the archipelago.
Heads is correct in pointing out that the mapping of seamounts is far from complete (Kim and Wessel 2011; Sandwell et al. 2014), and many researchers studying the history of island biotas would no doubt be excited at the prospect of obtaining accurate maps of seamounts, and, especially, knowledge of when some of these were islands. But it is a major leap from the reasonable assumption that many such islands existed to the extreme conclusion that such former lands housed metapopulations from which all or nearly all native lineages of the Hawaiian archipelago are derived by normal dispersal, especially given the apparently young ages of most taxa on these islands. Interestingly, Zimmerman (1947) long ago suggested that the Hawaiian biota might have been partly derived from former islands in the region, but, nonetheless, he found no reason to reject origins of the biota by LDD. New geological information has changed the details regarding former land in the area, but Zimmerman’s basic conclusion is still sound.
Fernando de Noronha
In my book, I described the case of the skink Trachylepis (=Mabuya) atlantica, and I followed Carranza and Arnold (2003) in concluding that the ancestors of this species had arrived from Africa by crossing the Atlantic. Heads noted that Carranza and Arnold’s timetree analysis used a potentially highly erroneous calibration that assumed that the ages of several lizard clades endemic to El Hierro in the Canary Islands must be no older than the age of that island. He claimed that this calibration is likely to have inflated the age estimate, and that the Fernando de Noronha skink could ‘be derived from ancestral generalised forms of Mabuya that were already in the region before the opening of the Atlantic’ (Heads, p. 297). However, other estimates of skink divergence ages, using different sets of calibrations, all strongly refute a Mesozoic origin for T. atlantica (Pyron 2010; Gamble et al. 2011, their supplementary fig. 3; Mulcahy et al. 2012; Hedges et al. 2015, with data accessed from http://www.timetree.org/; except for the estimate from Hedges et al. (2015), these estimates are highly conservative because they involve deeper divergences that subsume all those within Trachylepis). Also, Carranza and Arnold (2003) noted that the rate of substitution in Trachylepis would have to have been more than 10 times slower than the rate calculated for other scleroglossan lizards to be accounted for by the opening of the Atlantic. In short, Heads has misleadingly focused on one possibly faulty calibration point and has, therefore, missed seeing that the conclusions regarding T. atlantica’s origins do not depend on that calibration.
Ratites and primates
In The Monkey’s Voyage, I followed most recent researchers in explaining the distributions of ratite birds and primates partly by oceanic dispersal (e.g. Poux et al. 2006; Mitchell et al. 2014a), including the inference that monkeys colonised the New World from Africa by rafting across the Atlantic. In contrast, Heads argued that vicariance alone, involving only normal dispersal, explains the distributions of these groups.
For the ratites, Heads (p. 298) suggested that the striking allopatry within the group ‘is consistent with an origin of the clades by vicariance of a widespread, global ancestor’. However, it is, in the first place, unclear why this case represents ‘striking’ allopatry because, as Heads himself admitted, the group shows considerable sympatry; specifically, tinamous are broadly sympatric with rheas in South America and kiwis and moas were broadly sympatric in New Zealand. Furthermore, it is also unclear why the degree of allopatry seen in ratites cannot be explained at least partly by LDD. In particular, rare chance dispersal events should often produce allopatric distributions, especially if some form of competitive exclusion is operating, such that early colonists decrease the likelihood of establishment by later ones (Mitchell et al. 2014a).
In the case of primates, Heads (p. 299) wondered ‘Why are haplorhine primates in America but not Madagascar, while members of the sister-group, strepsirrhines, are in Madagascar but not America?’ and also ‘Why have no primates at all crossed Salue Timpaus Strait (20 km across) from Sulawesi to Australasia (although monkeys introduced in New Guinea have thrived)?’ He suggested that these distributional facts are inexplicable under LDD explanations, but, again, his logic is faulty. In fact, Heads perhaps provided part of a dispersal answer to the first question himself; he noted that haplorhines are more diverse in western Africa, whereas strepsirrhines are more diverse in eastern Africa, which suggests that the potential source pools could explain why haplorhines successfully dispersed across the Atlantic and strepsirrhines crossed the Mozambique Strait. In any case, given that primate dispersal across any significant body of water is expected to be rare, a more relevant question would be ‘Why, under LDD, would one expect both haplorhines and strepsirrhines to have successfully colonized both the New World and Madagascar?’ The answer is ‘You wouldn’t.’
Similarly, the fact that primates have not been able to colonise Australasia from Sulawesi should not come as a huge surprise given that (1) Sulawesi has not always been so close to Australasian land (Stelbrink et al. 2012), and (2) the primates (macaques and tarsiers) of the island might have arrived there only within the past 3 million years (Ziegler et al. 2007; Driller et al. 2015, their fig. 1e). In short, Heads seems to assume that, under the view that LDD is important, it is inexplicable that monkeys have not dispersed the short distance between Sulawesi and islands to the east; however, that is an overly simplistic view of the probabilities involved. This case, rather than illustrating the implausibility of LDD, emphasises the need for modelling dispersal probabilities, taking into account variables beyond the present distance between areas.
However, the greatest weakness in Heads’ arguments regarding ratites and primates is that they rely on vicariance tied to geological events that are far too ancient to be relevant for these groups. For example, to explain the divergence of ostriches from other ratites, Heads invoked the opening of the Mozambique Channel 160 million years ago, which is some 65 million years earlier than even a high-end estimate for the evolutionary split in question (i.e. the upper limit of the credibility interval given in Jarvis et al. 2014), and also predates estimates for the earliest crown-group birds, whether estimated from molecular data (Jarvis et al. 2014; Ksepka and Phillips 2015; Prum et al. 2015) or purely from fossils (Lee et al. 2014). On the more recent end of the spectrum, Heads explained the emu–cassowary divergence by volcanic activity that occurred c. 100 million years ago, an age that has been strongly refuted by recent molecular studies, which have placed that divergence some 70–90 million years later (Mitchell et al. 2014a; Prum et al. 2015).
Similarly, Heads’ (2010) vicariance-only scenario for primates requires the existence of crown-group primates in the early Jurassic, c. 180 million years ago, and invokes the opening of the South Atlantic c. 120–130 million years ago to explain the distribution of monkeys. These ages are far older than even the high end of recent, data-rich estimates for the corresponding nodes, being c. 80 million years and 60 million years too old respectively (Fig. 1). Also, under Heads’ scenario, monkeys have been in South America for at least 120 million years, yet the oldest known monkey fossil from that continent is no more than c. 36 million years old and appears to be closely related to similarly young fossil anthropoids from Africa (Bond et al. 2015). Furthermore, Heads’ suppositions require that primates not only existed but also were widespread by the early Jurassic, making their absence from some 120 million years of the fossil record even more mystifying (Goswami and Upchurch 2010).
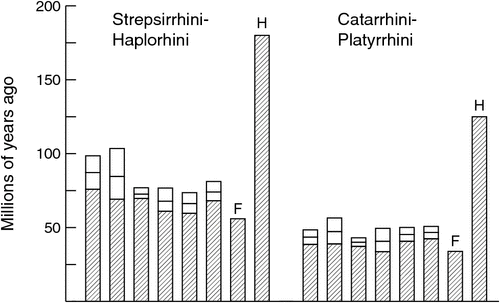
|
Summary of Heads’ area and taxa critiques
Heads’ critiques of my discussions of island biotas, and of ratites and primates show his persistent use of unsound arguments in an attempt to deny the significance of long-distance dispersal. The most obvious manifestation of this predilection is his straining of the timelines for groups, such that, although some events might be too young to explain the divergence between two lineages, they can never be too old. This results in a series of implausible inferences, including, among many others, the opening of the Atlantic, more than 100 million years ago, causing the divergence of the Fernando de Noronha skink from African relatives (estimated by molecular data to have occurred in the Oligocene or later), the relevance of Mesozoic volcanic islands in the central Pacific to the origins of the Hawaiian biota (despite the widely accepted inference that Hawaiian lineages mostly separated from relatives elsewhere within the past 5 million years), and the separation of Africa from Madagascar at c. 160 million years ago producing the divergence of ostriches from other ratites (although molecular timetree and fossil data indicate that ratites did not exist until at least 65 million years later). This lack of constraint on the maximum age of geological or other events that might be relevant to specific lineage divergences seriously compromises the validity of Heads’ approach. As illustrated by the examples of ratites and primates, in particular, inferences about biogeographical history devolve, under this approach, into a game of finding a series of fragmentation events in the right order and in roughly the right areas, regardless of absolute age.
Heads’ bias is also shown in other kinds of unjustified conclusions drawn from divergence-age, distributional, and phylogenetic data. He repeatedly equated the divergence age between an island lineage and relatives elsewhere as the minimum age for existence of the lineage on the archipelago in question, but it is actually the divergence within the archipelago that provides that minimum age. He erroneously assumed that allopatry within a clade indicates vicariance, despite the fact that rare, chance dispersal is also expected to produce allopatric distributions. He assumed that phylogenetic connections between areas that are repeated in many different taxa preclude origins by chance dispersal, a belief that reflects a misunderstanding of dispersal processes (see section above on a trio of specious arguments).
In short, Heads’ discussions of specific areas and taxa strongly reinforce the notion that his critique of The Monkey’s Voyage, and his views on historical biogeography in general, are based, not on a measured evaluation of new information, but on adherence to prior beliefs regardless of evidence.
Concluding remarks
At the core of The Monkey’s Voyage is the recent explosion of molecular timetree results, and their use in tests of historical biogeographic hypotheses. This development is widely seen as a crucial one. Thus, for example, Donoghue and Moore (2003) described the incorporation of timetree results as a logical step in the evolution of historical biogeography as an integrative science, similar to the earlier assimilation of phylogenetic branching-order evidence. Likewise, Crisp et al. (2011) viewed timetrees as a key aspect of a more hypothesis-driven, as opposed to narrative, historical biogeography, and Sanmartín (2012) described the critical role of such information in the elaboration of parametric biogeographic methods (also see Ree and Smith 2008; Ronquist and Sanmartín 2011). A general outcome of this integration of timetree evidence, also widely recognised as a crucial development, is the validation of LDD as a frequent cause of disjunctions, including many cases that had previously been attributed to vicariance (de Queiroz 2005; McGlone 2005; Gibbs 2006; Sanmartín 2012; Wen et al. 2013).
Obviously, Heads, holding to the belief that vicariance is the dominant cause of disjunctions, does not accept any of these positive views of timetrees, and he does not believe that such timing information indicates a multitude of LDD events. His general views, and the more specific arguments attached to them, are shared to a significant extent by some others. For example, his practice of pushing lineage divergences back to unrealistically deep ages, making these splits consistent with ancient vicariance, has been used by several other authors as well (McCarthy 2005; Grehan 2006; Cavalcanti and Gallo 2008; Costa 2013). Similarly, Heads’ criticisms of molecular dating, especially the idea that clocks calibrated with fossils can provide only minimum ages for branching points, have been echoed by other authors (Grehan 2006; Nelson and Ladiges 2009; Parenti and Ebach 2013). Perhaps most strikingly, his habit of invoking unsupported scenarios of geologic history is also seen in the work of others who dismiss timetree results and the importance of LDD, as with McCarthy’s (2003) promotion of an expanding-Earth hypothesis (critiqued in Briggs 2004), and Nelson’s (2006) suggestion of former land connections between Kauai and Oahu, and between Maui Nui and Hawaii (critiqued in Holland and Cowie 2006). These extreme views expressed by Heads and others are increasingly seen as insupportable (e.g. Briggs 2007; Goswami and Upchurch 2010; Renner 2010; O’Grady et al. 2012; Swenson et al. 2012; Waters et al. 2013; Matzke 2015; McGlone 2015), and, optimistically, one can anticipate that they will eventually fade away. My hope is that the present reply will, through its extensive detailing of flaws in Heads’ criticisms of my book, make people further question the legitimacy of such views.
Having dwelled at great length on the negative, I would like to end on a more positive note, by emphasising that these are exciting times for historical biogeography, and that much of the progress being made revolves around incorporating the results of molecular-dating analyses. In particular, I highlight here the following six important and diverse conclusions that have emerged from timetree studies, with respect to distributions of land taxa broken up by oceans:
-
The biotas of ancient continental islands, such as New Zealand and Madagascar, typically include some lineages that are continental hold-overs, reflecting ancient vicariance, but these biotas seem to be dominated by lineages that arrived by over-water dispersal (Goldberg et al. 2008; Grandcolas et al. 2008; Samonds et al. 2012; de Queiroz 2014). This conclusion emphasises the biotic turnover on such islands, and debunks the popular notion that they are ‘lands that time forgot.’
-
A substantial number of oceanic island radiations predate the current islands, indicating colonisation of prior land in the area (e.g. Rassmann 1997; Torres-Carvajal et al. 2014; Bradler et al. 2015; see Hawaiian section above). These findings indicate the potential complexity of island-colonisation histories, especially where many former islands existed. They also highlight two points on which Heads and I can agree, namely, the need for more complete and reliable reconstructions of the distribution of past oceanic islands, and the problematic nature of calibrating molecular clocks using the ages of current islands (Heads 2011).
-
Emerging taxonomic patterns of LDD, although often in agreement with intuitive expectations, include some surprises. For instance, current evidence indicates that birds as a whole are more constrained by ocean barriers than one might expect and, thus, disjunctions within this group are surprisingly often a result of vicariance (Claramunt and Cracraft 2015). In contrast, amphibians have been unexpectedly effective at surmounting modest sea barriers (see above), and burrowing reptiles have apparently crossed oceans on several occasions (Vidal et al. 2010; Longrich et al. 2015). Furthermore, some groups, such as mabuyine skinks (Carranza and Arnold 2003; Hedges and Conn 2012; Lima et al. 2013) and cucurbit plants (Schaefer et al. 2009), have colonised areas by long-distance over-water dispersal an astonishing number of times. None of these examples is so inexplicable that it calls into question biogeographic methods, but they do suggest a need to rethink how particular organismal features influence dispersal probabilities.
-
It often is assumed, reasonably, that abiotic factors, such as the directions of wind and ocean currents, and the freshening of sea-surface waters by the output from large rivers, influence probabilities of successful long-distance colonisation. Tests of possible relationships between such factors and patterns of LDD through time are few, but show great promise. For instance, colonisations of Madagascar by animals that probably required rafting for over-water dispersal occurred less frequently after the Mid-Miocene, when paleocurrent models indicate that the prevailing current shifted from an Africa-to-Madagascar pattern to the reverse (Ali and Huber 2010; Samonds et al. 2012).
-
Overseas colonisations have had a deeper effect on continental biotas than is generally assumed. A case in point is the vertebrate fauna of South America, which contains hundreds of species that are likely to have been derived from such events, including large groups such as platyrrhine monkeys, caviomorph and sigmodontine rodents, Rhinella toads and xenodontine snakes (de Queiroz 2014 and references therein). Another indication of this effect is that many continental species used by humans owe their existence to overseas dispersal by their ancestors (de Queiroz 2014).
-
Plausible explanations for the distributions of widespread groups that show multiple disjunctions often involve a combination of vicariance and LDD (e.g. Renner et al. 2010; Gamble et al. 2011; Krosch et al. 2011; Simonsen et al. 2011; Springer et al. 2012; Thomas et al. 2014; Pyron 2014; Thornhill et al. 2015). In fact, such results are so common that the default expectation for disjunctions in such taxa might be that they are not fully explained by either pure vicariance or pure LDD scenarios.
This list highlights the importance of long-distance colonisation, but it also shows that the practitioners of molecular dating are not simply using this approach to support hypotheses of recent dispersal. In many cases that has been the outcome, but, in numerous others, ancient vicariance events have been supported (Points 1, 3 and 6). I raise that point here to emphasise that neither The Monkey’s Voyage nor the present paper should be interpreted as an argument that timetrees show the dominance of relatively recent LDD as an explanation for disjunctions. It seems clear that long-distance over-water colonisation has been highly significant in shaping the history of life, and that claim is only strengthened by the unrealistic assumptions that are required to reject it. However, I view the relative importance of dispersal v. vicariance as secondary to a more fundamental message that hopefully comes through in the book, namely that biogeographers, and scientists in general, should evaluate the evidence with open minds rather than accepting a cherished hypothesis a priori and then bending (or discarding) contrary evidence to preserve their views. In fact, although it is something of an inside joke, the title The Monkey’s Voyage was meant to be a double entendre, referring to the literal dispersal of platyrrhine ancestors across the Atlantic and to the intellectual path taken by scientists, some of whom had to overcome their initial prejudice against such seemingly improbable journeys.
Acknowledgments
I thank Daniel Murphy for the invitation to reply to Michael Heads’ review, and Nick Matzke, John Gatesy, Malte Ebach, and several anonymous reviewers for helpful comments on the manuscript.
References
Ali JR, Huber M (2010) Mammalian biodiversity on Madagascar controlled by ocean currents. Nature 463, 653–656.| Mammalian biodiversity on Madagascar controlled by ocean currents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsFehtg%3D%3D&md5=3433ddfd4606cce6d1394ffb8eb94cd5CAS |
Allwood J, Gleeson D, Mayer G, Daniels S, Beggs JR, Buckley TR (2010) Support for vicariant origins of the New Zealand Onychophora. Journal of Biogeography 37, 669–681.
| Support for vicariant origins of the New Zealand Onychophora.Crossref | GoogleScholarGoogle Scholar |
Aoyama Y, Kawakami K, Chiba S (2012) Seabirds as adhesive seed dispersers of alien and native plants in the oceanic Ogasawara Islands, Japan. Biodiversity and Conservation 21, 2787–2801.
| Seabirds as adhesive seed dispersers of alien and native plants in the oceanic Ogasawara Islands, Japan.Crossref | GoogleScholarGoogle Scholar |
Austin JJ, Arnold EN, Jones CG (2004) Reconstructing an island radiation using ancient and recent DNA: the extinct and living day geckos (Phelsuma) of the Mascarene islands. Molecular Phylogenetics and Evolution 31, 109–122.
| Reconstructing an island radiation using ancient and recent DNA: the extinct and living day geckos (Phelsuma) of the Mascarene islands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhvFSks70%3D&md5=75b47c6657e6f6ae4f8bb1b6bfebff56CAS |
Beaulieu JM, O’Meara BC, Crane P, Donoghue MJ (2015) Heterogeneous rates of molecular evolution and diversification could explain the Triassic age estimate for angiosperms. Systematic Biology 64, 869–878.
| Heterogeneous rates of molecular evolution and diversification could explain the Triassic age estimate for angiosperms.Crossref | GoogleScholarGoogle Scholar |
Bell CD, Soltis DE, Soltis PS (2010) The age and diversification of the angiosperms re-revisited. American Journal of Botany 97, 1296–1303.
| The age and diversification of the angiosperms re-revisited.Crossref | GoogleScholarGoogle Scholar |
Bell RC, Drewes RC, Channing A, Gvoždík V, Kielgast J, Lötters S, Stuart BL, Zamudio KR (2015) Overseas dispersal of Hyperolius reed frogs from Central Africa to the oceanic islands of São Tomé and Príncipe. Journal of Biogeography 42, 65–75.
| Overseas dispersal of Hyperolius reed frogs from Central Africa to the oceanic islands of São Tomé and Príncipe.Crossref | GoogleScholarGoogle Scholar |
Bellamy DJ, Springett B, Hayden P (1990) ‘Moa’s Ark: the Voyage of New Zealand.’ (Viking: New York, NY, USA)
Bennett GM, O’Grady PM (2013) Historical biogeography and ecological opportunity in the adaptive radiation of native Hawaiian leafhoppers (Cicadellidae: Nesophrosyne). Journal of Biogeography 40, 1512–1523.
| Historical biogeography and ecological opportunity in the adaptive radiation of native Hawaiian leafhoppers (Cicadellidae: Nesophrosyne).Crossref | GoogleScholarGoogle Scholar |
Benton MJ, Wills MA, Hitchin R (2000) Quality of the fossil record through time. Nature 403, 534–537.
| Quality of the fossil record through time.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXht1Shurs%3D&md5=78cae91d6d79736ddf78788895a866d4CAS |
Benton MJ, Donoghue PCJ, Asher RJ, Friedman M, Near TJ, Vinther J (2015) Constraints on the timescale of animal evolutionary history. Palaeontologia Electronica 18, 1FC
Berg RY (1983) Plant distribution as seen from plant dispersal: general principles and basic modes of plant dispersal. In ‘Dispersal and Distribution’. (Ed. K Kubitzki) pp. 13–36. (Paul Parey: Hamburg, Germany)
Biffin E, Hill RS, Lowe AJ (2010) Did kauri (Agathis: Araucariaceae) really survive the Oligocene drowning of New Zealand? Systematic Biology 59, 594–602.
| Did kauri (Agathis: Araucariaceae) really survive the Oligocene drowning of New Zealand?Crossref | GoogleScholarGoogle Scholar |
Bond M, Tejedor MF, Campbell KE, Chornogubsky L, Novo N, Goin F (2015) Eocene primates of South America and the African origins of New World monkeys. Nature 520, 538–541.
| Eocene primates of South America and the African origins of New World monkeys.Crossref | GoogleScholarGoogle Scholar |
Bossuyt F, Brown RM, Hillis DM, Cannatella DC, Milinkovitch MC (2006) Phylogeny and biogeography of a cosmopolitan frog radiation: Late Cretaceous diversification resulted in continent-scale endemism in the family Ranidae. Systematic Biology 55, 579–594.
| Phylogeny and biogeography of a cosmopolitan frog radiation: Late Cretaceous diversification resulted in continent-scale endemism in the family Ranidae.Crossref | GoogleScholarGoogle Scholar |
Bradler S, Cliquennois N, Buckley TR (2015) Single origin of the Mascarene stick insects: ancient radiation on sunken islands? BMC Evolutionary Biology 15, 196
| Single origin of the Mascarene stick insects: ancient radiation on sunken islands?Crossref | GoogleScholarGoogle Scholar |
Briggs JC (2004) The ultimate expanding earth hypothesis. Journal of Biogeography 31, 855–857.
| The ultimate expanding earth hypothesis.Crossref | GoogleScholarGoogle Scholar |
Briggs JC (2007) Panbiogeography: its origin, metamorphosis and decline. Russian Journal of Marine Biology 33, 273–277.
| Panbiogeography: its origin, metamorphosis and decline.Crossref | GoogleScholarGoogle Scholar |
Brown JH, Gibson AC (1983) ‘Biogeography’, 2nd edn. (C. V. Mosby: St Louis, MO, USA)
Brown RM, Siler CD, Richards SJ, Diesmos AC, Cannatella DC (2015) Multilocus phylogeny and a new classification for Southeast Asian and Melanesian forest frogs (family Ceratobatrachidae). Zoological Journal of the Linnean Society 174, 130–168.
| Multilocus phylogeny and a new classification for Southeast Asian and Melanesian forest frogs (family Ceratobatrachidae).Crossref | GoogleScholarGoogle Scholar |
Brundin L (1966) Transantarctic relationships and their significance as evidenced by chironomid midges with a monograph of the subfamilies Podonominae and Aphroteniinae and the austral Heptagyiae. Kungliga Svenska Vetenskapsakademiens Handlingar, Series 4 11, 1–472.
Burkhardt F, Smith S, Eds. (1989) ‘The Correspondence of Charles Darwin. Vol. 5.’ (Cambridge University Press: Cambridge, UK)
Butlin R, Debelle A, Kerth C, Snook RR, Beukeboom LW, Cajas RFC, Diao W, Maan ME, Paolucci S, Weissing FJ, van de Zande L, Hoikkala A, Geuverink E, Jennings J, Kankare M, Knott KE, Tyukmaeva VI, Zoumadakis C, Ritchie MG, Barker D, Immonen E, Kirkpatrick M, Noor M, Garcia CM, Schmitt T, Schilthuizen M (2012) What do we need to know about speciation? Trends in Ecology & Evolution 27, 27–39.
| What do we need to know about speciation?Crossref | GoogleScholarGoogle Scholar |
Campbell HJ, Hutching GD (2007) ‘In Search of Ancient New Zealand.’ (Penguin: Auckland, New Zealand)
Carlquist S (1974) ‘Island Biology.’ (Columbia University Press: New York, NY, USA)
Carranza S, Arnold EN (2003) Investigating the origin of transoceanic distributions: mtDNA shows Mabuya lizards (Reptilia, Scincidae) crossed the Atlantic twice. Systematics and Biodiversity 1, 275–282.
| Investigating the origin of transoceanic distributions: mtDNA shows Mabuya lizards (Reptilia, Scincidae) crossed the Atlantic twice.Crossref | GoogleScholarGoogle Scholar |
Cavalcanti MJ, Gallo V (2008) Panbiogeographical analysis of distribution patterns in hagfishes (Craniata: Myxinidae). Journal of Biogeography 35, 1258–1268.
| Panbiogeographical analysis of distribution patterns in hagfishes (Craniata: Myxinidae).Crossref | GoogleScholarGoogle Scholar |
Censky EJ, Hodge K, Dudley J (1998) Over-water dispersal of lizards due to hurricanes. Nature 395, 556
| Over-water dispersal of lizards due to hurricanes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXms1alu7c%3D&md5=1d816a41e15671d5143fb123d732e31dCAS |
Chambers GK, Boon WM, Buckley TR, Hitchmough RA (2001) Using molecular methods to understand the Gondwanan affinities of the New Zealand biota: three case studies. Australian Journal of Botany 49, 377–387.
| Using molecular methods to understand the Gondwanan affinities of the New Zealand biota: three case studies.Crossref | GoogleScholarGoogle Scholar |
Claramunt S, Cracraft J (2015) A new time tree reveals Earth history’s imprint on the evolution of modern birds. Science Advances 1, e1501005
| A new time tree reveals Earth history’s imprint on the evolution of modern birds.Crossref | GoogleScholarGoogle Scholar |
Clarke JT, Warnock RCM, Donoghue PCJ (2011) Establishing a time-scale for plant evolution. New Phytologist 192, 266–301.
| Establishing a time-scale for plant evolution.Crossref | GoogleScholarGoogle Scholar |
Cooper RA, Millener PR (1993) The New Zealand biota: historical background and new research. Trends in Ecology & Evolution 8, 429–433.
| The New Zealand biota: historical background and new research.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itV2msw%3D%3D&md5=e4060f11680257b33ee3f2f5c55e52e5CAS |
Costa WJEM (2013) Historical biogeography of aplocheiloid killifishes (Teleostei: Cyprinidontiformes). Vertebrate Zoology 63, 139–154.
Cowie RH, Holland BS (2008) Molecular biogeography and diversification of the endemic terrestrial fauna of the Hawaiian Islands. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 363, 3363–3376.
| Molecular biogeography and diversification of the endemic terrestrial fauna of the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar |
Cox CB (1998) From generalized tracks to ocean basins: how useful is Panbiogeography? Journal of Biogeography 25, 813–828.
| From generalized tracks to ocean basins: how useful is Panbiogeography?Crossref | GoogleScholarGoogle Scholar |
Coyne JA (2009) ‘Why Evolution is True.’ (Viking: New York, NY, USA)
Coyne JA, Orr HA (2004) ‘Speciation.’ (Sinauer: Sunderland, MA, USA)
Cracraft J (1974) Continental drift and vertebrate distribution. Annual Review of Ecology and Systematics 5, 215–261.
| Continental drift and vertebrate distribution.Crossref | GoogleScholarGoogle Scholar |
Craw RC (1979) Generalized tracks and dispersal in biogeography: a response to R. M. McDowall. Systematic Zoology 28, 99–107.
| Generalized tracks and dispersal in biogeography: a response to R. M. McDowall.Crossref | GoogleScholarGoogle Scholar |
Crisp MD, Trewick SA, Cook LG (2011) Hypothesis testing in biogeography. Trends in Ecology & Evolution 26, 66–72.
| Hypothesis testing in biogeography.Crossref | GoogleScholarGoogle Scholar |
Croizat L (1958) ‘Panbiogeography or an Introductory Synthesis of Zoogeography, Phytogeography, and Geology. Vol. I. The New World.’ (Published by the author: Caracas, Venezuela)
Croizat L (1962) ‘Space, Time, Form: the Biological Synthesis.’ (Published by the author: Caracas, Venezuela)
Daniels SR (2011) Reconstructing the colonisation and diversification history of the endemic freshwater crab (Seychellum alluaudi) in the granitic and volcanic Seychelles Archipelago. Molecular Phylogenetics and Evolution 61, 534–542.
| Reconstructing the colonisation and diversification history of the endemic freshwater crab (Seychellum alluaudi) in the granitic and volcanic Seychelles Archipelago.Crossref | GoogleScholarGoogle Scholar |
Darwin C (1859) ‘On the Origin of Species by Means of Natural Selection.’ (John Murray: London, UK)
Daugherty CH, Gibbs GW, Hitchmough RA (1993) Mega-island or micro-continent? New Zealand and its fauna. Trends in Ecology & Evolution 8, 437–442.
| Mega-island or micro-continent? New Zealand and its fauna.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itV2msQ%3D%3D&md5=9654634a0a41417baf1c32754c3f01dbCAS |
Davy B, Hoernle K, Werner R (2008) Hikurangi plateau: crustal structure, rifted formation, and Gondwana subduction history. Geochemistry Geophysics Geosystems 9, Q07004
| Hikurangi plateau: crustal structure, rifted formation, and Gondwana subduction history.Crossref | GoogleScholarGoogle Scholar |
de Lange PJ, Heenan PB, Rolfe JR (2011) Checklist of vascular plants recorded from Chatham Islands. Report prepared for the Department of Conservation, Wellington Hawke’s Bay Conservancy. New Zealand Department of Conservation, Wellington, New Zealand.
de Queiroz A (2005) The resurrection of oceanic dispersal in historical biogeography. Trends in Ecology & Evolution 20, 68–73.
| The resurrection of oceanic dispersal in historical biogeography.Crossref | GoogleScholarGoogle Scholar |
de Queiroz A (2014) ‘The Monkey’s Voyage: How Improbable Journeys Shaped the History of Life.’ (Basic Books: New York, NY, USA)
de Queiroz A, Lawson R (2008) A peninsula as an island: multiple forms of evidence for overwater colonization of Baja California by the gartersnake Thamnophis validus. Biological Journal of the Linnean Society. Linnean Society of London 95, 409–424.
| A peninsula as an island: multiple forms of evidence for overwater colonization of Baja California by the gartersnake Thamnophis validus.Crossref | GoogleScholarGoogle Scholar |
Didham RK (2005) New Zealand: ‘fly-paper’ of the Pacific? The Weta 29, 1–5.
Donoghue MJ, Moore BR (2003) Toward an integrative historical biogeography. Integrative and Comparative Biology 43, 261–270.
| Toward an integrative historical biogeography.Crossref | GoogleScholarGoogle Scholar |
dos Reis M, Inoue J, Hasegawa M, Asher RJ, Donoghue PCJ, Yang Z (2012) Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proceedings of the Royal Society of London – B. Biological Sciences 279, 3491–3500.
| Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny.Crossref | GoogleScholarGoogle Scholar |
Driller C, Merker S, Perwitasari-Farajallah D, Sinaga W, Anggraeni N, Zischler H (2015) Stop and go: waves of tarsier dispersal mirror the genesis of Sulawesi Island. PLoS One 10, e0141212
| Stop and go: waves of tarsier dispersal mirror the genesis of Sulawesi Island.Crossref | GoogleScholarGoogle Scholar |
Emberson RM (1998) The beetle (Coleoptera) fauna of the Chatham Islands. New Zealand Entomologist 21, 25–64.
| The beetle (Coleoptera) fauna of the Chatham Islands.Crossref | GoogleScholarGoogle Scholar |
Enting B, Molloy L (1982) ‘The Ancient Islands: New Zealand’s Natural Environments.’ (Port Nicholson Press: Wellington, New Zealand)
Ericson PGP, Christidis L, Cooper A, Irestedt M, Jackson J, Johansson US, Norman JA (2002) A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proceedings of the Royal Society of London – B. Biological Sciences 269, 235–241.
| A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjvFCiurk%3D&md5=406620c441174260c025450253f44078CAS |
Finstermeier K, Zinner D, Brameier M, Meyer M, Kreuz E, Hofreiter M, Roos C (2013) A mitogenomic phylogeny of living primates. PLoS One 8, e69504
| A mitogenomic phylogeny of living primates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1Wms7vN&md5=9359875bbfc28be4d7346333b9d71077CAS |
Flannery T (1994) ‘The Future Eaters: an Ecological History of the Australasian Lands and People.’ (Reed New Holland: Sydney, NSW, Australia)
Fleming CA (1975) The geological history of New Zealand and its biota. In ‘Biogeography and Ecology in New Zealand’. (Ed. G Kuschel) pp. 1–86. (Junk: The Hague, Netherlands)
Friis EM, Pedersen KR, Crane PR (2010) Diversity in obscurity: fossil flowers and the early history of angiosperms. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 365, 369–382.
| Diversity in obscurity: fossil flowers and the early history of angiosperms.Crossref | GoogleScholarGoogle Scholar |
Frolov A (2013) Stenosternus Karsch, a possible link between Neotropical and Afrotropical Orphninae (Coleoptera, Scarabaeidae). ZooKeys 335, 33–46.
| Stenosternus Karsch, a possible link between Neotropical and Afrotropical Orphninae (Coleoptera, Scarabaeidae).Crossref | GoogleScholarGoogle Scholar |
Gamble T, Bauer AM, Colli GR, Greenbaum E, Jackman TR, Vitt LJ, Simons AM (2011) Coming to America: multiple origins of New World geckos. Journal of Evolutionary Biology 24, 231–244.
| Coming to America: multiple origins of New World geckos.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7lt1egsA%3D%3D&md5=6b8079821a5bcdf372645590a0bd9ab3CAS |
Garb JE, Gillespie RG (2009) Diversity despite dispersal: colonization history and phylogeography of Hawaiian crab spiders inferred from multilocus genetic data. Molecular Ecology 18, 1746–1764.
| Diversity despite dispersal: colonization history and phylogeography of Hawaiian crab spiders inferred from multilocus genetic data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsFOntro%3D&md5=aaaf41b642bc6e17403c245946ebfb62CAS |
Gardner JV, Calder BR, Malik M (2013) Geomorphometry and processes that built Necker Ridge, central North Pacific Ocean. Marine Geology 346, 310–325.
| Geomorphometry and processes that built Necker Ridge, central North Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Garzón-Orduña IJ, Silva-Brandão KL, Willmott KR, Freitas AVL, Brower AVZ (2015) Incompatible ages for clearwing butterflies based on alternative secondary calibrations. Systematic Biology 64, 752–767.
| Incompatible ages for clearwing butterflies based on alternative secondary calibrations.Crossref | GoogleScholarGoogle Scholar |
Gibbs G (2006) ‘Ghosts of Gondwana: the History of Life in New Zealand.’ (Craig Potton Publishing: Nelson, New Zealand)
Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK (2012) Long-distance dispersal: a framework for hypothesis testing. Trends in Ecology & Evolution 27, 47–56.
| Long-distance dispersal: a framework for hypothesis testing.Crossref | GoogleScholarGoogle Scholar |
Giribet G, Boyer SL (2010) ‘Moa’s ark’ or ‘goodbye Gondwana’: is the origin of New Zealand’s terrestrial invertebrate fauna ancient, recent or both? Invertebrate Systematics 24, 1–8.
| ‘Moa’s ark’ or ‘goodbye Gondwana’: is the origin of New Zealand’s terrestrial invertebrate fauna ancient, recent or both?Crossref | GoogleScholarGoogle Scholar |
Givnish TJ, Millam KC, Mast AR, Paterson TB, Theim TJ, Hipp AL, Henss JM, Smith JF, Wood KR, Sytsma KJ (2009) Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae). Proceedings of the Royal Society of London – B. Biological Sciences 276, 407–416.
| Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae).Crossref | GoogleScholarGoogle Scholar |
Goldberg J, Trewick SA, Paterson AM (2008) Evolution of New Zealand’s terrestrial fauna: a review of molecular evidence. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 363, 3319–3334.
| Evolution of New Zealand’s terrestrial fauna: a review of molecular evidence.Crossref | GoogleScholarGoogle Scholar |
Goldblatt P (Ed.) (1993) ‘Biological Relationships between Africa and South America.’ (Yale University Press: New Haven, CT, USA)
Goswami A, Upchurch P (2010) The dating game: a reply to Heads (2010). Zoologica Scripta 39, 406–409.
| The dating game: a reply to Heads (2010).Crossref | GoogleScholarGoogle Scholar |
Grandcolas P, Murienne J, Robillard T, Desutter-Grandcolas L, Jourdan H, Guilbert E, Deharveng L (2008) New Caledonia: a very old Darwinian island? Philosophical Transactions of the Royal Society of London – B. Biological Sciences 363, 3309–3317.
| New Caledonia: a very old Darwinian island?Crossref | GoogleScholarGoogle Scholar |
Grehan JR (2006) A brief look at Pacific biogeography: the trans-oceanic travels of Microseris (Angiosperms: Asteraceae). In ‘Biogeography in a Changing World’. (Eds MC Ebach, RS Tangney) pp. 83–94. (CRC Press: New York, NY, USA)
Grehan JR, Schwartz JF (2009) Evolution of the second orangutan: phylogeny and biogeography of hominid origins. Journal of Biogeography 36, 1823–1844.
| Evolution of the second orangutan: phylogeny and biogeography of hominid origins.Crossref | GoogleScholarGoogle Scholar |
Guja LK, Merritt DJ, Dixon KW (2010) Buoyancy, salt tolerance and germination of coastal seeds: implications for oceanic hydrochorous dispersal. Functional Plant Biology 37, 1175–1186.
| Buoyancy, salt tolerance and germination of coastal seeds: implications for oceanic hydrochorous dispersal.Crossref | GoogleScholarGoogle Scholar |
Guo P, Liu Q, Xu Y, Jiang K, Hou M, Ding L, Pyron RA, Burbrink FT (2012) Out of Asia: natricine snakes support the Cenozoic Beringian dispersal hypothesis. Molecular Phylogenetics and Evolution 63, 825–833.
| Out of Asia: natricine snakes support the Cenozoic Beringian dispersal hypothesis.Crossref | GoogleScholarGoogle Scholar |
Haines WP, Schmitz P, Rubinoff D (2014) Ancient diversification of Hyposmocoma moths in Hawaii. Nature Communications 5, 3502
| Ancient diversification of Hyposmocoma moths in Hawaii.Crossref | GoogleScholarGoogle Scholar |
Heads M (1985) Biogeographic analysis of Nothofagus (Fagaceae). Taxon 34, 474–480.
| Biogeographic analysis of Nothofagus (Fagaceae).Crossref | GoogleScholarGoogle Scholar |
Heads M (2005) Dating nodes on molecular phylogenies: a critique of molecular biogeography. Cladistics 21, 62–78.
| Dating nodes on molecular phylogenies: a critique of molecular biogeography.Crossref | GoogleScholarGoogle Scholar |
Heads M (2009) Inferring biogeographic history from molecular phylogenies. Biological Journal of the Linnean Society. Linnean Society of London 98, 757–774.
| Inferring biogeographic history from molecular phylogenies.Crossref | GoogleScholarGoogle Scholar |
Heads M (2010) Evolution and biogeography of primates: a new model based on plate tectonics, molecular phylogenetics and vicariance. Zoologica Scripta 39, 107–127.
| Evolution and biogeography of primates: a new model based on plate tectonics, molecular phylogenetics and vicariance.Crossref | GoogleScholarGoogle Scholar |
Heads M (2011) Old taxa on young islands: a critique of the use of island age to date island-endemic clades and calibrate phylogenies. Systematic Biology 60, 204–218.
| Old taxa on young islands: a critique of the use of island age to date island-endemic clades and calibrate phylogenies.Crossref | GoogleScholarGoogle Scholar |
Heads M (2012a) ‘Molecular Panbiogeography of the Tropics.’ (University of California Press: Berkeley, CA, USA)
Heads M (2012b) Bayesian transmogrification of clade divergence dates: a critique. Journal of Biogeography 39, 1749–1756.
| Bayesian transmogrification of clade divergence dates: a critique.Crossref | GoogleScholarGoogle Scholar |
Heads M (2012c) South Pacific biogeography, tectonic calibration, and pre-drift tectonics: cladogenesis in Abrotanella (Asteraceae). Biological Journal of the Linnean Society. Linnean Society of London 107, 938–952.
| South Pacific biogeography, tectonic calibration, and pre-drift tectonics: cladogenesis in Abrotanella (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Heads M (2014a) Biogeography by revelation: investigating a world shaped by miracles. Australian Systematic Botany 27, 282–304.
| Biogeography by revelation: investigating a world shaped by miracles.Crossref | GoogleScholarGoogle Scholar |
Heads M (2014b) ‘Biogeography of Australasia: a Molecular Analysis.’ (Cambridge University Press: Cambridge, UK)
Heads M (2014c) Panbiogeography, its critics, and the case of the ratite birds. Australian Systematic Botany 27, 241–256.
| Panbiogeography, its critics, and the case of the ratite birds.Crossref | GoogleScholarGoogle Scholar |
Heath TA, Huelsenbeck JP, Stadler T (2014) The fossilized birth-death process for coherent calibration of divergence-time estimates. Proceedings of the National Academy of Sciences of the United States of America 111, E2957–E2966.
| The fossilized birth-death process for coherent calibration of divergence-time estimates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFyqtbvL&md5=1c52f42bd9ce550f50dec9c64ee169e7CAS |
Hedges SB, Conn CE (2012) A new skink fauna from Caribbean islands (Squamata, Mabuyidae, Mabuyinae). Zootaxa 3288, 1–244.
Hedges SB, Marin J, Suleski M, Paymer M, Kumar S (2015) Tree of Life reveals clock-like speciation and diversification. Molecular Biology and Evolution 32, 835–845.
| Tree of Life reveals clock-like speciation and diversification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtlWnu7%2FM&md5=20093cf3b9eb9293f3971d0769f6f4bcCAS |
Heenan P, Mitchell A, de Lange P, Keeling J, Paterson A (2010) Late-Cenozoic origin and diversification of Chatham Islands endemic plant species revealed by analyses of DNA sequence data. New Zealand Journal of Botany 48, 83–136.
| Late-Cenozoic origin and diversification of Chatham Islands endemic plant species revealed by analyses of DNA sequence data.Crossref | GoogleScholarGoogle Scholar |
Holdaway RN, Worthy TH, Tennyson AJD (2001) A working list of breeding bird species of the New Zealand region at first human contact. New Zealand Journal of Zoology 28, 119–187.
| A working list of breeding bird species of the New Zealand region at first human contact.Crossref | GoogleScholarGoogle Scholar |
Holland BS, Cowie RH (2006) Dispersal and vicariance in Hawaii: submarine slumping does not create deep inter-island channels. Journal of Biogeography 33, 2155
| Dispersal and vicariance in Hawaii: submarine slumping does not create deep inter-island channels.Crossref | GoogleScholarGoogle Scholar |
Humphries CJ (1981) Biogeographical methods and the southern beeches (Fagaceae: Nothofagus). In ‘Advances in Cladistics’. (Eds VA Funk, DR Brooks) pp. 177–207. (The New York Botanical Garden: New York, NY, USA)
Jameson NM, Hou ZC, Sterner KN, Weckle A, Goodman M, Steiper ME, Wildman DE (2011) Genomic data reject the hypothesis of a prosimian primate clade. Journal of Human Evolution 61, 295–305.
| Genomic data reject the hypothesis of a prosimian primate clade.Crossref | GoogleScholarGoogle Scholar |
Jarvis ED, Mirarab S, Aberer AJ, Li B, Houde P, Li C, Ho SYW, Faircloth BC, Nabholz B, Howard JT, Suh A, Weber CC, Fonseca RRD, Li J, Zhang F, Li H, Zhou L, Narula N, Liu L, Ganapathy G, Boussau B, Bayzid MS, Zavidovych V, Subramanian S, Gabaldón T, Capella-Gutiérrez S, Huerta-Cepas J, Rekepalli B, Munch K, Schierup M, Lindow B, Warren WC, Ray D, Green RE, Bruford MW, Zhan X, Dixon A, Li S, Li N, Huang Y, Derryberry EP, Bertelsen MF, Sheldon FH, Brumfield RT, Mello CV, Lovell PV, Wirthlin M, Schneider MPC, Prosdocimi F, Samaniego JA, Velazquez AMV, Alfaro-Nuñez A, Campos PF, Petersen B, Sicheritz-Ponten T, Pas A, Bailey T, Scofield P, Bunce M, Lambert DM, Zhou Q, Perelman P, Driskell AC, Shapiro B, Xiong Z, Zeng Y, Liu S, Li Z, Liu B, Wu K, Xiao J, Yinqi X, Zheng Q, Zhang Y, Yang H, Wang J, Smeds L, Rheindt FE, Braun M, Fjeldsa J, Orlando L, Barker FK, Jønsson KA, Johnson W, Koepfli K-P, O’brien S, Haussler D, Ryder OA, Rahbek C, Willerslev E, Graves GR, Glenn TC, Mccormack J, Burt D, Ellegren H, Alström P, Edwards SV, Stamatakis A, Mindell DP, Cracraft J, Braun EL, Warnow T, Jun W, Gilbert MTP, Zhang G (2014) Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346, 1320–1331.
| Whole-genome analyses resolve early branches in the tree of life of modern birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitVCgs7%2FK&md5=f5fa74b92ac589eafcd283144a516e64CAS |
Jesus J, Harris DJ, Brehm A (2007) Relationships of Afroablepharus Greer, 1974 skinks from the Gulf of Guinea islands based on mitochondrial and nuclear DNA: patterns of colonization and comments on taxonomy. Molecular Phylogenetics and Evolution 45, 904–914.
| Relationships of Afroablepharus Greer, 1974 skinks from the Gulf of Guinea islands based on mitochondrial and nuclear DNA: patterns of colonization and comments on taxonomy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlajsr%2FJ&md5=86d3f30a2ee7a5a7795651ae83a81496CAS |
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 491, 444–448.
| The global diversity of birds in space and time.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1ajtrjJ&md5=9bc8fc3ed22a23a2eba90c59b7a9025fCAS |
Johnson KP, Sorenson MD (1999) Phylogeny and biogeography of dabbling ducks (genus: Anas): a comparison of molecular and morphological evidence. The Auk 116, 792–805.
| Phylogeny and biogeography of dabbling ducks (genus: Anas): a comparison of molecular and morphological evidence.Crossref | GoogleScholarGoogle Scholar |
Jordan S, Simon C, Polhemus D (2003) Molecular systematics and adaptive radiation of Hawaii’s endemic damselfly genus Megalagrion (Odonata: Coenagrionidae). Systematic Biology 52, 89–109.
| Molecular systematics and adaptive radiation of Hawaii’s endemic damselfly genus Megalagrion (Odonata: Coenagrionidae).Crossref | GoogleScholarGoogle Scholar |
Joyce WG, Parham JF, Lyson TR, Warnock RCM, Donoghue PCJ (2013) A divergence dating analysis of turtles using fossil calibrations: an example of best practices. Journal of Paleontology 87, 612–634.
| A divergence dating analysis of turtles using fossil calibrations: an example of best practices.Crossref | GoogleScholarGoogle Scholar |
Kalnins LM, Watts AB (2009) Spatial variations in effective elastic thickness in the western Pacific Ocean and their implications for Mesozoic volcanism. Earth and Planetary Science Letters 286, 89–100.
| Spatial variations in effective elastic thickness in the western Pacific Ocean and their implications for Mesozoic volcanism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtV2ntLzL&md5=dcadc8d6c1cb126c831c8b6e1cf5604dCAS |
Kim SS, Wessel P (2011) New global seamount census from altimetry-derived gravity data. Geophysical Journal International 186, 615–631.
| New global seamount census from altimetry-derived gravity data.Crossref | GoogleScholarGoogle Scholar |
Knapp M, Mudaliar R, Havell D, Wagstaff SJ, Lockhart PJ (2007) The drowning of New Zealand and the problem of Agathis. Systematic Biology 56, 862–870.
| The drowning of New Zealand and the problem of Agathis.Crossref | GoogleScholarGoogle Scholar |
Krosch MN, Baker AM, Mather PB, Cranston PS (2011) Systematics and biogeography of the Gondwanan Orthocladiinae (Diptera: Chironomidae). Molecular Phylogenetics and Evolution 59, 458–468.
| Systematics and biogeography of the Gondwanan Orthocladiinae (Diptera: Chironomidae).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mvms1CltA%3D%3D&md5=31c9236caee825469137ff7709ba8e57CAS |
Ksepka DT, Phillips MJ (2015) Avian diversification patterns across the K–Pg boundary: influence of calibrations, datasets, and model misspecification. Annals of the Missouri Botanical Garden 100, 300–328.
| Avian diversification patterns across the K–Pg boundary: influence of calibrations, datasets, and model misspecification.Crossref | GoogleScholarGoogle Scholar |
Ksepka DT, Parham JF, Allman JF, Benton MJ, Carrano MT, Cranston KA, Donoghue PCJ, Head JJ, Hermsen EJ, Irmis RB, Joyce WG, Kohli M, Lamm KD, Leehr D, Patané JL, Polly PD, Phillips MJ, Smith NA, Smith ND, van Tuinen M, Ware JL, Warnock RCM (2015) The Fossil Calibration Database: a new resource for divergence dating. Systematic Biology 64, 853–859.
| The Fossil Calibration Database: a new resource for divergence dating.Crossref | GoogleScholarGoogle Scholar |
Lee DE, Bannister JM, Lindqvist JK (2007) Late Oligocene–Early Miocene leaf macrofossils confirm a long history of Agathis in New Zealand. New Zealand Journal of Botany 45, 565–578.
| Late Oligocene–Early Miocene leaf macrofossils confirm a long history of Agathis in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1Grs7rE&md5=4335203cb9e58ec592dd4b9f4138a290CAS |
Lee MSY, Cau A, Naish D, Dyke GJ (2014) Morphological clocks in paleontology, and a Mid-Cretaceous origin of crown Aves. Systematic Biology 63, 442–449.
| Morphological clocks in paleontology, and a Mid-Cretaceous origin of crown Aves.Crossref | GoogleScholarGoogle Scholar |
Lima A, Harris DJ, Rocha S, Miralles A, Glaw F, Vences M (2013) Phylogenetic relationships of Trachylepis skink species from Madagascar and the Seychelles (Squamata: Scincidae). Molecular Phylogenetics and Evolution 67, 615–620.
| Phylogenetic relationships of Trachylepis skink species from Madagascar and the Seychelles (Squamata: Scincidae).Crossref | GoogleScholarGoogle Scholar |
Lohman DJ, Tsang SM (2014) A manifesto of panbiogeography, Australasian edition. Frontiers of Biogeography 6, 191–193.
Longrich NR, Vinther J, Pyron RA, Pisani D, Gauthier JA (2015) Biogeography of worm lizards (Amphisbaenia) driven by end-Cretaceous mass extinction. Proceedings of the Royal Society of London – B. Biological Sciences 282, 20143034
| Biogeography of worm lizards (Amphisbaenia) driven by end-Cretaceous mass extinction.Crossref | GoogleScholarGoogle Scholar |
Magallón S (2014) A review of the effect of relaxed clock method, long branches, genes, and calibrations in the estimation of angiosperm age. Botanical Sciences 92, 1–22.
| A review of the effect of relaxed clock method, long branches, genes, and calibrations in the estimation of angiosperm age.Crossref | GoogleScholarGoogle Scholar |
Marjanović D, Laurin M (2007) Fossils, molecules, divergence times, and the origin of lissamphibians. Systematic Biology 56, 369–388.
| Fossils, molecules, divergence times, and the origin of lissamphibians.Crossref | GoogleScholarGoogle Scholar |
Matute DR (2013) The role of founder effects on the evolution of reproductive isolation. Journal of Evolutionary Biology 26, 2299–2311.
| The role of founder effects on the evolution of reproductive isolation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c%2Fktlehtg%3D%3D&md5=5980a158cdc3016d3fd7b2f4c3654b8aCAS |
Matzke NJ (2014) Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Systematic Biology 63, 951–970.
| Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades.Crossref | GoogleScholarGoogle Scholar |
Matzke NJ (2015) Review of ‘Biogeography of Australasia: a molecular analysis’ by Michael Heads. The Quarterly Review of Biology 90, 327–328.
| Review of ‘Biogeography of Australasia: a molecular analysis’ by Michael Heads.Crossref | GoogleScholarGoogle Scholar |
Mayr E (1982) ‘The Growth of Biological Thought: Diversity, Evolution, and Inheritance.’ (Belknap Press: Cambridge, MA, USA)
Mayr G (2013) The age of the crown group of passerine birds and its evolutionary significance: molecular calibrations versus the fossil record. Systematics and Biodiversity 11, 7–13.
| The age of the crown group of passerine birds and its evolutionary significance: molecular calibrations versus the fossil record.Crossref | GoogleScholarGoogle Scholar |
Mazza P (2014) Pushing your luck. Review of ‘The monkey’s voyage,’ by A de Queiroz. Bioscience 64, 458–459.
| Pushing your luck. Review of ‘The monkey’s voyage,’ by A de Queiroz.Crossref | GoogleScholarGoogle Scholar |
McAtee WL (1914) Birds transporting food supplies. The Auk 31, 404–405.
| Birds transporting food supplies.Crossref | GoogleScholarGoogle Scholar |
McCarthy D (2003) The trans-Pacific zipper effect: disjunct sister taxa and matching geological outlines that link the Pacific margins. Journal of Biogeography 30, 1545–1561.
| The trans-Pacific zipper effect: disjunct sister taxa and matching geological outlines that link the Pacific margins.Crossref | GoogleScholarGoogle Scholar |
McCarthy D (2005) Biogeography and scientific revolutions. The Systematist 25, 3–12.
McGlone MS (2005) Goodbye Gondwana. Journal of Biogeography 32, 739–740.
| Goodbye Gondwana.Crossref | GoogleScholarGoogle Scholar |
McGlone MS (2015) Once more into the wilderness of panbiogeography: a reply to Heads (2014). Australian Systematic Botany 28, 388–393.
| Once more into the wilderness of panbiogeography: a reply to Heads (2014).Crossref | GoogleScholarGoogle Scholar |
Measey GJ, Vences M, Drewes RC, Chiari Y, Melo M, Bourles B (2007) Freshwater paths across the ocean: molecular phylogeny of the frog Ptychadena newtoni gives insights into amphibian colonization of oceanic islands. Journal of Biogeography 34, 7–20.
| Freshwater paths across the ocean: molecular phylogeny of the frog Ptychadena newtoni gives insights into amphibian colonization of oceanic islands.Crossref | GoogleScholarGoogle Scholar |
Melville R (1981) Vicariance plant distributions and paleogeography of the Pacific region. In ‘Vicariance Biogeography: a Critique’. (Eds G Nelson, DE Rosen) pp. 238–274. (Columbia University Press: New York, NY, USA)
Meredith RW, Janečka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC, Goodbla A, Eizirik E, Simão TLL, Stadler T, Rabosky DL, Honeycutt RL, Flynn JJ, Ingram CM, Steiner C, Williams TL, Robinson TJ, Burk-Herrick A, Westerman M, Ayoub NA, Springer MS, Murphy WJ (2011) Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524.
| Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlKjtrnF&md5=5171dae81e4c5ef6fb9d17e04f8c7fbcCAS |
Michalak I, Zhang L-B, Renner SS (2010) Trans-Atlantic, trans-Pacific and trans-Indian Ocean dispersal in the small Gondwanan Laurales family Hernandiaceae. Journal of Biogeography 37, 1214–1226.
| Trans-Atlantic, trans-Pacific and trans-Indian Ocean dispersal in the small Gondwanan Laurales family Hernandiaceae.Crossref | GoogleScholarGoogle Scholar |
Mitchell KJ, Llamas B, Soubrier J, Rawlence NJ, Worthy TH, Wood J, Lee MSY, Cooper A (2014a) Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution. Science 344, 898–900.
| Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXotlWht7c%3D&md5=27b3c4055b45d67d4666538aaf7ce7c2CAS |
Mitchell KJ, Wood JR, Scofield RP, Llamas B, Cooper A (2014b) Ancient mitochondrial genome reveals unsuspected taxonomic affinity of the extinct Chatham duck (Pachyanas chathamica) and resolves divergence times for New Zealand and sub-Antarctic brown teals. Molecular Phylogenetics and Evolution 70, 420–428.
| Ancient mitochondrial genome reveals unsuspected taxonomic affinity of the extinct Chatham duck (Pachyanas chathamica) and resolves divergence times for New Zealand and sub-Antarctic brown teals.Crossref | GoogleScholarGoogle Scholar |
Morrone JJ (2015) Track analysis beyond panbiogeography. Journal of Biogeography 42, 413–425.
| Track analysis beyond panbiogeography.Crossref | GoogleScholarGoogle Scholar |
Mulcahy DG, Noonan BP, Moss T, Townsend TM, Reeder TW, Sites JW, Wiens JJ (2012) Estimating divergence dates and evaluating dating methods using phylogenomic and mitochondrial data in squamate reptiles. Molecular Phylogenetics and Evolution 65, 974–991.
| Estimating divergence dates and evaluating dating methods using phylogenomic and mitochondrial data in squamate reptiles.Crossref | GoogleScholarGoogle Scholar |
Nagy ZT, Joger U, Wink M, Glaw F, Vences M (2003) Multiple colonization of Madagascar and Socotra by colubrid snakes: evidence from nuclear and mitochondrial gene phylogenies. Proceedings of the Royal Society of London – B. Biological Sciences 270, 2613–2621.
| Multiple colonization of Madagascar and Socotra by colubrid snakes: evidence from nuclear and mitochondrial gene phylogenies.Crossref | GoogleScholarGoogle Scholar |
Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A (2008) Mechanisms of long-distance seed dispersal. Trends in Ecology & Evolution 23, 638–647.
| Mechanisms of long-distance seed dispersal.Crossref | GoogleScholarGoogle Scholar |
Near TJ, Sanderson MJ (2004) Assessing the quality of molecular divergence time estimates by fossil calibrations and fossil-based model selection. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 359, 1477–1483.
| Assessing the quality of molecular divergence time estimates by fossil calibrations and fossil-based model selection.Crossref | GoogleScholarGoogle Scholar |
Nelson G (1975) Review of ‘Biogeography and ecology of New Zealand’ ed. by G. Kuschel. Systematic Zoology 24, 494–495.
Nelson G (2006) Hawaiian vicariance. Journal of Biogeography 33, 2154–2155.
| Hawaiian vicariance.Crossref | GoogleScholarGoogle Scholar |
Nelson G, Ladiges PY (2009) Biogeography and the molecular dating game: a futile revival of phenetics? Bulletin de la Société Géologique de France 180, 39–43.
| Biogeography and the molecular dating game: a futile revival of phenetics?Crossref | GoogleScholarGoogle Scholar |
Njome MS, de Wit MJ (2014) The Cameroon line: analysis of an intraplate magmatic province transecting both oceanic and continental lithospheres: constraints, controversies and models. Earth-Science Reviews 139, 168–194.
| The Cameroon line: analysis of an intraplate magmatic province transecting both oceanic and continental lithospheres: constraints, controversies and models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1Ors7jE&md5=56c0976bccbc0a6ae5b601053b63c090CAS |
Nogales M, Heleno R, Traveset A, Vargas P (2012) Evidence for overlooked mechanisms of long-distance seed dispersal to and between oceanic islands. New Phytologist 194, 313–317.
| Evidence for overlooked mechanisms of long-distance seed dispersal to and between oceanic islands.Crossref | GoogleScholarGoogle Scholar |
Norell MA, Novacek MJ (1992) The fossil record and evolution: comparing cladistic and paleontologic evidence for vertebrate history. Science 255, 1690–1693.
| The fossil record and evolution: comparing cladistic and paleontologic evidence for vertebrate history.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvitlersg%3D%3D&md5=210694b7fc2e87c416018299ab410cb0CAS |
O’Grady PM, Bennett GM, Funk VA, Altheide TK (2012) Retrograde biogeography: a review of Heads, M. 2012, Molecular panbiogeography of the tropics. Taxon 61, 702–705.
O’Leary M, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo Z-X, Meng J, Ni X, Novacek MJ, Perini FA, Randall ZS, Rougier GW, Sargis EJ, Silcox MT, Simmons NB, Spaulding M, Velazco PM, Weksler M, Wible JR, Cirranello AL (2013) The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339, 662–667.
| The placental mammal ancestor and the post-K-Pg radiation of placentals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFOktrg%3D&md5=0e4764ec905deecd1775e6e0e674394aCAS |
Page RDM, Lydeard C (1994) Towards a cladistic interpretation of the Caribbean. Cladistics 10, 21–41.
| Towards a cladistic interpretation of the Caribbean.Crossref | GoogleScholarGoogle Scholar |
Parenti LR (2006) Common cause and historical biogeography. In ‘Biogeography in a Changing World’. (Eds MC Ebach, RS Tangney) pp. 61–82. (CRC Press: New York, NY, USA)
Parenti LR, Ebach MC (2013) Evidence and hypothesis in biogeography. Journal of Biogeography 40, 813–820.
| Evidence and hypothesis in biogeography.Crossref | GoogleScholarGoogle Scholar |
Parham JF, Donoghue PCJ, Bell CJ, Calway TD, Head JJ, Holroyd PA, Inoue JG, Irmis RB, Joyce WG, Ksepka DT, Patané JSL, Smith ND, Tarver JE, van Tuinen M, Yang Z, Angielczyk KD, Greenwood JM, Hipsley CA, Jacobs L, Makovicky PJ, Müller J, Smith KT, Theodor JM, Warnock RCM, Benton MJ (2012) Best practices for justifying fossil calibrations. Systematic Biology 61, 346–359.
| Best practices for justifying fossil calibrations.Crossref | GoogleScholarGoogle Scholar |
Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MAM, Kessing B, Pontius J, Roelke M, Rumpler Y, Schneider MPC, Silva A, O’Brien SJ, Pecon-Slattery J (2011) A molecular phylogeny of living primates. PLOS Genetics 7, e1001342
| A molecular phylogeny of living primates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXktVWgsrY%3D&md5=7ce80bb65a88cf6383ffb1a2c689593dCAS |
Pole M (2008) The record of Araucariaceae macrofossils in New Zealand. Alcheringa: An Australasian Journal of Palaeontology 32, 405–426.
| The record of Araucariaceae macrofossils in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Poux C, Chevret P, Huchon D, de Jong WW, Douzery EJP (2006) Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Systematic Biology 55, 228–244.
| Arrival and diversification of caviomorph rodents and platyrrhine primates in South America.Crossref | GoogleScholarGoogle Scholar |
Pozzi L, Hodgson JA, Burrell AS, Sterner KN, Raaum RL, Disotell TR (2014) Primate phylogenetic relationships and divergence dates inferred from complete mitochondrial genomes. Molecular Phylogenetics and Evolution 75, 165–183.
| Primate phylogenetic relationships and divergence dates inferred from complete mitochondrial genomes.Crossref | GoogleScholarGoogle Scholar |
Pramuk JB, Robertson T, Sites JW, Noonan BP (2008) Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae). Global Ecology and Biogeography 17, 72–83.
| Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae).Crossref | GoogleScholarGoogle Scholar |
Price JP, Clague DA (2002) How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence. Proceedings of the Royal Society of London – B. Biological Sciences 269, 2429–2435.
| How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence.Crossref | GoogleScholarGoogle Scholar |
Proctor VW (1968) Long-distance dispersal of seeds by retention in digestive tract of birds. Science 160, 321–322.
| Long-distance dispersal of seeds by retention in digestive tract of birds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1c7mt1ahug%3D%3D&md5=10eb5a1c56a1fb3fc9818e4f1e3e1d72CAS |
Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR (2015) A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569–573.
| A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhslWitbzO&md5=569092b14343e8ce9fb6842d94324a6eCAS |
Pufal G, Garnock-Jones P (2010) Hygrochastic capsule dehiscence supports safe site strategies in New Zealand alpine Veronica (Plantaginaceae). Annals of Botany 106, 405–412.
| Hygrochastic capsule dehiscence supports safe site strategies in New Zealand alpine Veronica (Plantaginaceae).Crossref | GoogleScholarGoogle Scholar |
Pyron RA (2010) A likelihood method for assessing molecular divergence time estimates and the placement of fossil calibrations. Systematic Biology 59, 185–194.
| A likelihood method for assessing molecular divergence time estimates and the placement of fossil calibrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1aksrs%3D&md5=e776a574716ea0ebd125bf5d55cadc54CAS |
Pyron RA (2014) Biogeographic analysis reveals ancient continental vicariance and recent oceanic dispersal in amphibians. Systematic Biology 63, 779–797.
| Biogeographic analysis reveals ancient continental vicariance and recent oceanic dispersal in amphibians.Crossref | GoogleScholarGoogle Scholar |
Rassmann K (1997) Evolutionary age of the Galápagos iguanas predates the age of the present Galápagos islands. Molecular Phylogenetics and Evolution 7, 158–172.
| Evolutionary age of the Galápagos iguanas predates the age of the present Galápagos islands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXivFentrc%3D&md5=f8198af9f04821a687421b0496992e5eCAS |
Raven PH, Axelrod DI (1972) Plate tectonics and Australasian paleobiogeography. Science 176, 1379–1386.
| Plate tectonics and Australasian paleobiogeography.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvls1Cnsg%3D%3D&md5=adff3c5d3230c81edabfdfb792ce5628CAS |
Ree RH, Smith S (2008) Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57, 4–14.
| Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis.Crossref | GoogleScholarGoogle Scholar |
Renner SS (2004) Multiple Miocene Melastomataceae dispersal between Madagascar, Africa and India. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 359, 1485–1494.
| Multiple Miocene Melastomataceae dispersal between Madagascar, Africa and India.Crossref | GoogleScholarGoogle Scholar |
Renner SS (2010) Review of ‘Comparative biogeography: discovering and classifying biogeographical patterns of a dynamic earth,’ by LR Parenti and MC Ebach. The Quarterly Review of Biology 85, 224
| Review of ‘Comparative biogeography: discovering and classifying biogeographical patterns of a dynamic earth,’ by LR Parenti and MC Ebach.Crossref | GoogleScholarGoogle Scholar |
Renner SS, Strijk JS, Strasberg D, Thébaud C (2010) Biogeography of the Monimiaceae (Laurales): a role for East Gondwana and long-distance dispersal, but not West Gondwana. Journal of Biogeography 37, 1227–1238.
| Biogeography of the Monimiaceae (Laurales): a role for East Gondwana and long-distance dispersal, but not West Gondwana.Crossref | GoogleScholarGoogle Scholar |
Ronquist F (1997) Dispersal–vicariance analysis: a new approach to the quantification of historical biogeography. Systematic Biology 46, 195–203.
| Dispersal–vicariance analysis: a new approach to the quantification of historical biogeography.Crossref | GoogleScholarGoogle Scholar |
Ronquist F, Sanmartín I (2011) Phylogenetic methods in biogeography. Annual Review of Ecology, Evolution and Systematics 42, 441–464.
| Phylogenetic methods in biogeography.Crossref | GoogleScholarGoogle Scholar |
Ronquist F, Klopfstein S, Vilhelmsen L, Schulmeister S, Murray DL, Rasnitsyn AP (2012) A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Systematic Biology 61, 973–999.
| A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera.Crossref | GoogleScholarGoogle Scholar |
Rota-Stabelli O, Daley AC, Pisani D (2013) Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Current Biology 23, 392–398.
| Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFWjuro%3D&md5=cffd13216406733fe3fa51d5b91e533bCAS |
Rowden AA, Clark MR, Wright IC (2005) Physical characterization and a biologically focused classification of ‘seamounts’ in the New Zealand region. New Zealand Journal of Marine and Freshwater Research 39, 1039–1059.
| Physical characterization and a biologically focused classification of ‘seamounts’ in the New Zealand region.Crossref | GoogleScholarGoogle Scholar |
Roy T, Chang T-H, Lan T, Lindqvist C (2013) Phylogeny and biogeography of New World Stachydeae (Lamiaceae) with emphasis on the origin and diversification of Hawaiian and South American taxa. Molecular Phylogenetics and Evolution 69, 218–238.
| Phylogeny and biogeography of New World Stachydeae (Lamiaceae) with emphasis on the origin and diversification of Hawaiian and South American taxa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVOjsLjI&md5=7cbc6eb0ef6c1bb424d527fedc976e5eCAS |
Russo CAM, Mello B, Frazão A, Voloch CM (2013) Phylogenetic analysis and a time tree for a large drosophilid data set (Diptera: Drosophilidae). Zoological Journal of the Linnean Society 169, 765–775.
| Phylogenetic analysis and a time tree for a large drosophilid data set (Diptera: Drosophilidae).Crossref | GoogleScholarGoogle Scholar |
Samonds KE, Godfrey LR, Ali JR, Goodman SM, Vences M, Sutherland MR, Irwin MT, Krause DW (2012) Spatial and temporal arrival patterns of Madagascar’s vertebrate fauna explained by distance, ocean currents, and ancestor type. Proceedings of the National Academy of Sciences of the United States of America 109, 5352–5357.
| Spatial and temporal arrival patterns of Madagascar’s vertebrate fauna explained by distance, ocean currents, and ancestor type.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlslOjs7Y%3D&md5=8d7798ef064f9c0c906719b8093d41d3CAS |
Sandwell DT, Müller RD, Smith WHF, Garcia E, Francis R (2014) New global marine gravity model from CryoSat-2 and Jason-1 reveals buried tectonic structure. Science 346, 65–67.
| New global marine gravity model from CryoSat-2 and Jason-1 reveals buried tectonic structure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1CitL3L&md5=ebbd17ab482383447a94618b5847803cCAS |
Sanmartín I (2012) Historical biogeography: evolution in time and space. Evolution: Education and Outreach 5, 555–568.
| Historical biogeography: evolution in time and space.Crossref | GoogleScholarGoogle Scholar |
Schaefer H, Heibl C, Renner SS (2009) Gourds afloat: a dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Proceedings of the Royal Society of London – B. Biological Sciences 276, 843–851.
| Gourds afloat: a dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events.Crossref | GoogleScholarGoogle Scholar |
Schäferhoff B, Fleischmann A, Fischer E, Albach DC, Borsch T, Heubl G, Müller KF (2010) Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences. BMC Evolutionary Biology 10, 352
| Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences.Crossref | GoogleScholarGoogle Scholar |
Schweizer M, Seehausen O, Güntert M, Hertwig ST (2010) The evolutionary diversification of parrots supports a taxon pulse model with multiple trans-oceanic dispersal events and local radiations. Molecular Phylogenetics and Evolution 54, 984–994.
| The evolutionary diversification of parrots supports a taxon pulse model with multiple trans-oceanic dispersal events and local radiations.Crossref | GoogleScholarGoogle Scholar |
Silvestro D, Cascales-Miñana B, Bacon CD, Antonelli A (2015) Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytologist 207, 425–436.
| Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record.Crossref | GoogleScholarGoogle Scholar |
Simonsen TJ, Zakharov EV, Djernaes M, Cotton AM, Vane-Wright R, Sperling FA (2011) Phylogenetics and divergence times of Papilioninae (Lepidoptera) with special reference to the enigmatic genera Teinopalpus and Meandrusa. Cladistics 27, 113–137.
| Phylogenetics and divergence times of Papilioninae (Lepidoptera) with special reference to the enigmatic genera Teinopalpus and Meandrusa.Crossref | GoogleScholarGoogle Scholar |
Skipworth JP (1974) Continental drift and the New Zealand biota. New Zealand Journal of Geography 57, 1–13.
| Continental drift and the New Zealand biota.Crossref | GoogleScholarGoogle Scholar |
Smith AB, Pisani D, Mackenzie-Dodds JA, Stockley B, Webster BL, Littlewood DTJ (2006) Testing the molecular clock: molecular and paleontological estimates of divergence times in the Echinoidea (Echinodermata). Molecular Biology and Evolution 23, 1832–1851.
| Testing the molecular clock: molecular and paleontological estimates of divergence times in the Echinoidea (Echinodermata).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVWgs73I&md5=ce9c92563098650100a17d411d32713fCAS |
Sohn J-C, Labandeira CC, Davis DR (2015) The fossil record and taphonomy of butterflies and moths (Insecta, Lepidoptera): implications for evolutionary diversity and divergence-time estimates. BMC Evolutionary Biology 15, 12
| The fossil record and taphonomy of butterflies and moths (Insecta, Lepidoptera): implications for evolutionary diversity and divergence-time estimates.Crossref | GoogleScholarGoogle Scholar |
Sousa WP (1993) Size-dependent predation on the salt-marsh snail Cerithidea californica Haldeman. Journal of Experimental Marine Biology and Ecology 166, 19–37.
| Size-dependent predation on the salt-marsh snail Cerithidea californica Haldeman.Crossref | GoogleScholarGoogle Scholar |
Springer MS, Meredith RW, Gatesy J, Emerling CA, Park J, Rabosky DL, Stadler T, Steiner C, Ryder OA, Janečka JE, Fisher CA, Murphy WJ (2012) Macroevolutionary dynamics and historical biogeography of primate diversification inferred from a species supermatrix. PLoS One 7, e49521
| Macroevolutionary dynamics and historical biogeography of primate diversification inferred from a species supermatrix.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVShsrjF&md5=97d179eb68b8841f01bcd381b27692e4CAS |
Stelbrink B, Albrecht C, Hall R, von Rintelen T (2012) The biogeography of Sulawesi revisited: is there evidence for a vicariant origin of taxa on Wallace’s ‘anomalous island’? Evolution 66, 2252–2271.
| The biogeography of Sulawesi revisited: is there evidence for a vicariant origin of taxa on Wallace’s ‘anomalous island’?Crossref | GoogleScholarGoogle Scholar |
Stilwell JD, Consoli CP (2012) Tectono-stratigraphic history of the Chatham Islands, SW Pacific: the emergence, flooding and reappearance of eastern ‘Zealandia’. Proceedings of the Geologists’ Association 123, 170–181.
| Tectono-stratigraphic history of the Chatham Islands, SW Pacific: the emergence, flooding and reappearance of eastern ‘Zealandia’.Crossref | GoogleScholarGoogle Scholar |
Stilwell JD, Consoli CP, Sutherland R, Salisbury S, Rich TH, Vickers-Rich PA, Currie PJ, Wilson GJ (2006) Dinosaur sanctuary on the Chatham Islands, Southwest Pacific: first record of theropods from the K-T boundary Takatika Grit. Palaeogeography, Palaeoclimatology, Palaeoecology 230, 243–250.
| Dinosaur sanctuary on the Chatham Islands, Southwest Pacific: first record of theropods from the K-T boundary Takatika Grit.Crossref | GoogleScholarGoogle Scholar |
Swenson U, Nylinder S, Wagstaff SJ (2012) Are Asteraceae 1.5 billion years old? A reply to Heads. Systematic Biology 61, 522–532.
| Are Asteraceae 1.5 billion years old? A reply to Heads.Crossref | GoogleScholarGoogle Scholar |
Templeton AR (2008) The reality and importance of founder speciation in evolution. BioEssays 30, 470–479.
| The reality and importance of founder speciation in evolution.Crossref | GoogleScholarGoogle Scholar |
Thiede J, Dean WE, Rea DK, Vallier TL, Adelseck CG (1981) The geologic history of the Mid-Pacific Mountains in the central North Pacific Ocean: a synthesis of deep-sea drilling studies. In ‘Initial Reports of the Deep Sea Drilling Project, Vol. 62’. (Eds J Thiede, TL Vallier) pp. 1073–1120. (United States Government Printing Office: Washington, DC, USA)
Thomas N, Bruhl JJ, Ford A, Weston PH (2014) Molecular dating of Winteraceae reveals a complex biogeographical history involving both ancient Gondwanan vicariance and long-distance dispersal. Journal of Biogeography 41, 894–904.
| Molecular dating of Winteraceae reveals a complex biogeographical history involving both ancient Gondwanan vicariance and long-distance dispersal.Crossref | GoogleScholarGoogle Scholar |
Thornhill AH, Ho SYW, Külheim C, Crisp MD (2015) Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Molecular Phylogenetics and Evolution 93, 29–43.
| Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny.Crossref | GoogleScholarGoogle Scholar |
Tolley KA, Townsend TM, Vences M (2013) Large-scale phylogeny of chameleons suggests African origins and Eocene diversification. Proceedings of the Royal Society of London – B. Biological Sciences 280, 20130184
| Large-scale phylogeny of chameleons suggests African origins and Eocene diversification.Crossref | GoogleScholarGoogle Scholar |
Torres-Carvajal O, Barnes CW, Pozo-Andrade MJ, Tapia W, Nicholls G (2014) Older than the islands: origin and diversification of Galápagos leaf-toed geckos (Phyllodactylidae: Phyllodactylus) by multiple colonizations. Journal of Biogeography 41, 1883–1894.
| Older than the islands: origin and diversification of Galápagos leaf-toed geckos (Phyllodactylidae: Phyllodactylus) by multiple colonizations.Crossref | GoogleScholarGoogle Scholar |
Trewick SA (2000) Molecular evidence for dispersal rather than vicariance as the origin of flightless insect species on the Chatham Islands, New Zealand. Journal of Biogeography 27, 1189–1200.
| Molecular evidence for dispersal rather than vicariance as the origin of flightless insect species on the Chatham Islands, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Trewick SA, Gibb GC (2010) Vicars, tramps and assembly of the New Zealand avifauna: a review of molecular phylogenetic evidence. The Ibis 152, 226–253.
| Vicars, tramps and assembly of the New Zealand avifauna: a review of molecular phylogenetic evidence.Crossref | GoogleScholarGoogle Scholar |
Trewick SA, Wallis GP, Morgan-Richards M (2000) Phylogeographical pattern correlates with Pliocene mountain building in the alpine scree weta (Orthoptera, Anostostomatidae). Molecular Ecology 9, 657–666.
| Phylogeographical pattern correlates with Pliocene mountain building in the alpine scree weta (Orthoptera, Anostostomatidae).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3czgslGjtQ%3D%3D&md5=6304f19df2097624f57f392c7c3dbc38CAS |
Vallier TL, Dean WE, Rea DK, Thiede J (1983) Geologic evolution of Hess Rise, central North Pacific Ocean. Geological Society of America Bulletin 94, 1289–1307.
| Geologic evolution of Hess Rise, central North Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXls1Wkt78%3D&md5=11676f69b85d4e2afa2ed790ae50ba5aCAS |
Van Duzer C (2004) ‘Floating Islands: a Global Bibliography.’ (Cantor: Los Altos Hills, CA, USA)
van Leeuwen CHA, van der Velde G, van Lith B, Klaassen M (2012) Experimental quantification of long distance dispersal potential of aquatic snails in the gut of migratory birds. PLoS One 7, e32292
| Experimental quantification of long distance dispersal potential of aquatic snails in the gut of migratory birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XktVOms78%3D&md5=13b2384582fae5643b49982c44d8facfCAS |
Vences M, Vieites DR, Glaw F, Brinkmann H, Kosuch J, Veith M, Meyer A (2003) Multiple overseas dispersal in amphibians. Proceedings of the Royal Society of London – B. Biological Sciences 270, 2435–2442.
| Multiple overseas dispersal in amphibians.Crossref | GoogleScholarGoogle Scholar |
Viana DS, Santamaría L, Michot TC, Figuerola J (2013) Allometric scaling of long-distance seed dispersal by migratory birds. American Naturalist 181, 649–662.
| Allometric scaling of long-distance seed dispersal by migratory birds.Crossref | GoogleScholarGoogle Scholar |
Viana DS, Gangoso L, Bouten W, Figuerola J (2016) Overseas seed dispersal by migratory birds. Proceedings of the Royal Society of London – B. Biological Sciences 283, 20152406
| Overseas seed dispersal by migratory birds.Crossref | GoogleScholarGoogle Scholar |
Vidal N, Marin J, Morini M, Donnellan S, Branch WR, Thomas R, Vences M, Wynn A, Cruaud C, Hedges SB (2010) Blindsnake evolutionary tree reveals long history on Gondwana. Biology Letters 6, 558–561.
| Blindsnake evolutionary tree reveals long history on Gondwana.Crossref | GoogleScholarGoogle Scholar |
Warnock RCM, Parham JF, Joyce WG, Lyson TR, Donoghue PCJ (2014) Calibration uncertainty in molecular dating analyses: there is no substitute for the prior evaluation of time priors. Proceedings of the Royal Society of London – B. Biological Sciences 282, 20141013
| Calibration uncertainty in molecular dating analyses: there is no substitute for the prior evaluation of time priors.Crossref | GoogleScholarGoogle Scholar |
Waters JM, Craw D (2006) Goodbye Gondwana? New Zealand biogeography, geology, and the problem of circularity. Systematic Biology 55, 351–356.
| Goodbye Gondwana? New Zealand biogeography, geology, and the problem of circularity.Crossref | GoogleScholarGoogle Scholar |
Waters JM, Trewick SA, Paterson AM, Spencer HG, Kennedy M, Craw D, Burridge CP, Wallis GP (2013) Biogeography off the tracks. Systematic Biology 62, 494–498.
| Biogeography off the tracks.Crossref | GoogleScholarGoogle Scholar |
Wen J, Ree RH, Ickert-Bond SM, Nie Z, Funk V (2013) Biogeography: where do we go from here? Taxon 62, 912–927.
| Biogeography: where do we go from here?Crossref | GoogleScholarGoogle Scholar |
Wilf P, Escapa IH (2015) Green Web or megabiased clock? Plant fossils from Gondwanan Patagonia speak on evolutionary radiations. New Phytologist 207, 283–290.
| Green Web or megabiased clock? Plant fossils from Gondwanan Patagonia speak on evolutionary radiations.Crossref | GoogleScholarGoogle Scholar |
Wilkinson RD, Steiper ME, Soligo C, Martin RD, Yang Z, Tavaré S (2011) Dating primate divergences through an integrated analysis of palaeontological and molecular data. Systematic Biology 60, 16–31.
| Dating primate divergences through an integrated analysis of palaeontological and molecular data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFGru7%2FN&md5=6b4e6050cd5de5a04d4c7f2d514b2b34CAS |
Wills MA (2002) The tree of life and the rock of ages: are we getting better at estimating phylogeny? BioEssays 24, 203–207.
| The tree of life and the rock of ages: are we getting better at estimating phylogeny?Crossref | GoogleScholarGoogle Scholar |
Winkworth RC, Wagstaff SJ, Glenny D, Lockhart PJ (2002) Plant dispersal NEWS from New Zealand. Trends in Ecology & Evolution 17, 514–520.
| Plant dispersal NEWS from New Zealand.Crossref | GoogleScholarGoogle Scholar |
Wirtz P, Ferreira CEL, Floeter SR, Fricke R, Gasparini JL, Iwamoto T, Rocha L, Sampaio CLS, Schleiwen UK (2007) Coastal fishes of São Tomé and Príncipe islands, Gulf of Guinea (eastern Atlantic Ocean): an update. Zootaxa 1523, 1–48.
Yang Z, Rannala B (2006) Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Molecular Biology and Evolution 23, 212–226.
| Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtleqtLnI&md5=43db2d0c577f1695187483721d587940CAS |
Yoder AD, Nowak MD (2006) Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell. Annual Review of Ecology, Evolution and Systematics 37, 405–431.
| Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell.Crossref | GoogleScholarGoogle Scholar |
Zeng L, Zhang Q, Sun R, Kong H, Zhang N, Ma H (2014) Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times. Nature Communications 5, 4956
| Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitVShsb%2FP&md5=09a3efd1d81ab60831ac5e2d65055882CAS |
Zhang P, Wake MH (2009) A mitogenomic perspective on the phylogeny and biogeography of living caecilians (Amphibia: Gymnophiona). Molecular Phylogenetics and Evolution 53, 479–491.
| A mitogenomic perspective on the phylogeny and biogeography of living caecilians (Amphibia: Gymnophiona).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVKls7bP&md5=3dbce18a933ad6d3661c12e31f2e64f6CAS |
Ziegler T, Abegg C, Meijaard E, Perwitasari-Farajallah D, Walter L, Hodges JK, Roos C (2007) Molecular phylogeny and evolutionary history of Southeast Asian macaques forming the M. silenus group. Molecular Phylogenetics and Evolution 42, 807–816.
| Molecular phylogeny and evolutionary history of Southeast Asian macaques forming the M. silenus group.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVCnsLg%3D&md5=becaeb589b5a7439fefb594528cd91aeCAS |
Zimkus BM, Rödel M-O, Hillers A (2010) Complex patterns of continental speciation: molecular phylogenetics and biogeography of sub-Saharan puddle frogs (Phrynobatrachus). Molecular Phylogenetics and Evolution 55, 883–900.
| Complex patterns of continental speciation: molecular phylogenetics and biogeography of sub-Saharan puddle frogs (Phrynobatrachus).Crossref | GoogleScholarGoogle Scholar |
Zimmerman EC (1947) ‘Insects of Hawaii. Vol. 1. Introduction.’ (University of Hawaii Press: Honolulu, HI, USA)


