Molecular phylogenetic analyses of Drakaeinae: Diurideae (Orchidaceae) based on DNA sequences of the internal transcribed spacer region
Joseph T. Miller A C and Mark A. Clements A BA Centre for Australian National Biodiversity Research, CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601, Australia.
B Australian National Botanic Gardens, GPO Box 1777, Canberra, ACT 2601, Australia.
C Corresponding author. Email: joe.miller@csiro.au
Australian Systematic Botany 27(1) 3-22 https://doi.org/10.1071/SB13036
Submitted: 14 August 2013 Accepted: 1 May 2014 Published: 30 June 2014
Abstract
Results of the analysis of rDNA sequences based on 55 collections representative of 32 Drakaeinae orchid species and outgroups supported the monophyly of the subtribe, with weak support for the inclusion of Spiculaea, and revealed six strongly supported monophyletic, well defined morphological groups. Caleana is monophyletic. Chiloglottis s.lat. is monophyletic when Simpliglottis and Myrmechila are included. Our results also suggested that the segregate genus Phoringopsis is better treated as part of Arthrochilus.There is sufficient molecular and morphological support for recognition of the leafless, mycroheterotrophic Thynninorchis to be maintained as a separate genus. A taxonomic summary is provided, including reassignment of taxa at generic ranks and new combinations for Caleana alcockii (Hopper & A.P.Br.) M.A.Clem., Caleana brockmanii (Hopper & A.P.Br.) M.A.Clem., Caleana disjuncta (D.L.Jones) M.A.Clem., Caleana dixonii (Hopper & A.P.Br.) M.A.Clem., Caleana gracilicordata (Hopper & A.P.Br.) M.A.Clem., Caleana granitica (Hopper & A.P.Br.) M.A.Clem., Caleana hortiorum (Hopper & A.P.Br.) M.A.Clem., Caleana lyonsii (Hopper & A.P.Br.) M.A.Clem., Caleana parvula (Hopper & A.P.Br.) M.A.Clem., Caleana terminalis (Hopper & A.P.Br.) M.A.Clem. and Caleana triens (Hopper & A.P.Br.) M.A.Clem.
Introduction
One of the most intriguing groups of Australian terrestrial orchids, with colloquial names to match, are the ant, bird, wasp, hammer, flying duck, elbow and truffle orchids (Jones 2006). Traditionally, this group of orchids has been classified as subtribe Drakaeinae Schltr. and had been interpreted as comprising Arthrochilus F.Muell., Caleana R.Br., Chiloglottis R.Br., Drakaea Lindl., Paracaleana Blaxell and Spiculaea Lindl., which is how they were treated in the most recent taxonomic treatment of the group (Jones and Clements 2001) or in separate revision of genera Paracaleana (Hopper and Brown 2006) and Drakaea (Hopper and Brown 2007). Drakaeinae are unusual because, although small in number (~66 species), they occur across mesic Australia, with outliers in New Zealand and New Guinea.
The 14 species of elbow and truffle orchids, Arthrochilus, inhabit woodlands, forests, heathlands and savanna woodlands of eastern and northern Australia, including the Torres Strait Islands, with outliers in the Kimberley in north-western Western Australia and Arnhem Land in the Northern Territory, as well as occurring in the savanna woodlands of southern New Guinea. The monospecific Caleana, flying duck orchid, is locally common and widespread throughout south-eastern Australia, where it favours sandy habitats. The 27 species of Chiloglottis, the ant, bird and wasp orchids, are also locally common and widespread throughout the forests and woodlands of south-eastern Australia, with two species also occurring in New Zealand, one as far south as the subantarctic Campbell Island. Drakaea, the hammer orchids, with 10 described species, are confined to south-western Western Australia, where they grow in sandy open-area habitats in woodlands. The 13 species of Paracaleana, also know as duck orchids, are distributed across southern and eastern Australia, with an outlier in the north island of New Zealand, where they favour sandy open forest habitats. Spiculaea, also an elbow orchid, is a monospecific genus endemic to south-western Western Australia, mainly inhabiting shallow moss-covered soils on granite tors.
Like so many other Australian taxa, the group has been in taxonomic turmoil, with no resolution because of conflicting alternative generic concepts coupled with the discovery of an earlier valid generic name for Paracaleana. The primary cause of these conflicts is differing interpretations of the level of significance placed on various morphological traits found in this unusual group of orchids. Although the initial taxonomic changes were by Blaxell (1972), when he described the segregate genus Paracaleana, the catalyst for a renewed wave of taxonomic activity was the splitting of Chiloglottis and recognition of the segregate genus, Simpliglottis (Szlachetko 2001), which was soon followed by the further division of that genus and Arthrochilus (Jones and Clements 2005; Jones et al. 2006). The resulting nomenclatural instability has inhibited conservation efforts of some of the rare taxa.
Taxonomic history and phylogeny of the tribes (Table 1)
Lindley (1840a) first treated Caleana, Chiloglottis, Drakaea and Spiculaea, along with other predominantly Australian genera, such as Thelymitra, Pterostylis, Lyperanthus, Microtis and Acianthus, in tribe Arethuseae Div. 2 Euarethuseae. Bentham and Hooker (1883) transferred these to tribe Diurideae. The German morphologist, Pfitzer (1887, 1889), proposed an alternative classification based on possession of the hinged labellum, which united Caleana and Spiculaea (including Arthrochilus) into the subtribe Pterostylidinae, a position followed by Dressler and Dodson (1960).

|
Subtribe Drakaeinae was created by Schlechter (1911) to encompass Arthrochilus, Caleana, Chiloglottis, Drakaea and Spiculaea, which all possess winged columns, as distinct from Pterostylidinae (now considered part of the Cranichideae). Rogers (1921) did not accept the Drakaeinae and linked the group to Caladeniinae. Dressler’s interpretation of the group changed in his various treatments of the family (Dressler 1974, 1979, 1981, 1983, 1986; Dressler and Dodson 1960), until he reinstated Schlechter’s concept, albeit with the proviso that it may prove merely a subclade of the Caladeniinae (Dressler 1993). Developmental embryological studies of the tribe Diurideae confirmed the alliance of Drakaeinae as part of tribe Diurideae, where they all shared a ‘diurid’ embryo developmental pattern (Clements 1995, 1996, 1999), although this character state is not restricted to this tribe (Kores et al. 2001). Molecular data from rbcL (Kores et al. 1997; Cameron et al. 1999), matK and trnL–F plastid genes (Kores et al. 2000, 2001), psaB (Cameron 2004) and internal transcribed spacer (ITS) nuclear rDNA (Clements et al. 2002; Górniak et al. 2010) confirmed this relationship of subtribe Drakaeinae within the tribe Diurideae, although there are still conflicting data on its position and monophyly. Other authors, such as Szlachetko (1991), have proposed alternative interpretations of these taxa, on the basis of morphological studies of the column, and created the separate subtribes Chiloglottidinae and Caleaninae. Chase et al. (2003) recognised fewer subtribes, amalgamating subtribe Drakaeinae with Thelymitrinae, within tribe Diurideae, on the basis of a combined analysis of plastid atpB, rbcL, matK, psaB, trnL–F, nuclear 26S rDNA and nad1 intron and mitochondrial (non-coding) DNA.
Generic relationships
The systematics of the group have also been the subject of change and controversy at the generic level. Initially, confusion existed about the limits of Drakaea, Spiculaea and Arthrochilus. Blaxell (1972) eventually clarified the situation, recognising each genus in its own right, and also splitting Caleana by creating Paracaleana for the two species with tuberculate labella. The concept of Paracaleana was initially accepted by many authors of popular and scientific literature (Erickson et al. 1973; Clements 1982; Hoffman and Brown 1984; Woolcock and Woolcock 1984; Green 1985; Weber and Bates 1986; Rye 1987; Jones 1988; Dixon et al. 1989). Paracaleana was rejected by Clements (1989) on the basis that none of the characters used was sufficiently different to maintain it as separate genus from Caleana. Despite this assessment, Paracaleana was accepted by most subsequent authors (Hoffman and Brown 1992, 2011; Backhouse and Jeanes 1995; Kores et al. 2001; Jones et al. 2002; Jeanes and Backhouse 2001; Hopper and Brown 2006; Brown et al. 2008). A preliminary analysis of the ITS nrDNA (Clements et al. 2002) failed to resolve this issue because of low taxon sampling. Additional controversy also surrounds this genus, with the recent discovery of an earlier published generic name, Sullivania, that was inadvertently, although legitimately, published for the rare apomitic form with fixed, vestigial labellum Caleana sullivanii (Jones and Clements 2005; Hopper and Brown 2006; St George 2010).
Additional names were added when Szlachetko (2001) created the genus Simpliglottis for a group of species related to Chiloglottis gunnii. Following detailed study of freshly collected material representative of all genera in the subtribe, further divisions were proposed (Jones et al. 2002). The following two segregate genera were split off from Arthrochilus: Phoringopsis, to account for those species with linear lanceolate leaves, and reverse position of the labellum callus, represented by P. byrnesi and P. dockrillii; and Thynninorchis for the semi-mycrohetrotrophic species, T. hunteriana, that inhabits the southern mountain regions of south-eastern Australia isolated from the remaining species. Jones and Clements (2005) later recognised and segregated a small group of species from Chiloglottis, as Myrmechila. This group of species can be distinguished by its winter to spring flowering, suberect to erect flowers, very short sepaline osmophores and suberect to erect labella.
Most of these proposed changes of traditional established genera have, in general, not been well received and adopted throughout the botanical community, both in Australia and overseas. A major objection to these proposed changes was their destabilising effect on traditional orchid nomenclature and classification (Hopper and Brown 2006, 2007; Hopper 2009). There were also objections about methods used, as almost all were exclusively morphologically based, without any cladistic or morphometric analyses of the data to support the proposed changes. Considering the high levels of current uncertainty surrounding the classification of taxa within Drakaeiinae and the lack of a published robust phylogeny of the group, one of the main aims of the present paper was to address this situation. A secondary aim was to test the monophyly of all traditional and recently proposed genera. We hypothesise that some of the characters used to define these genera were autapomorphies for subgroups within genera, rather than being synapomorphies for the entire genus. We discuss the clades and important taxonomic characters, test alterative classifications and propose a classification based on our results.
Materials and methods
Taxon sampling
DNA sequences for the nuclear rDNA ITS were obtained from 55 species, including representatives of all traditional or recently recognised higher taxa within Drakaeinae (Jones et al. 2002, 2006), and outgroups from Calochilus, Cryptostylis, Epiblema and Thelymitra. Successful analysis, based only on ITS data, has previously allowed establishment of viable, acceptable taxonomic outcomes, from which the results of further multigene analyses have deviated little. For example, Cox et al. (1997) on the phylogeny of the slipper orchids, and recent publications on Brassicaceae (Warwick et al. 2010), provided evidence of the utility of results from ITS sequence phylogenetic analysis. Details of source material and provenance, and GenBank accession numbers used are listed in Table 2. Reference vouchers for collections used in the study are housed either at CANB or CHR. Taxa were chosen on the basis of previous broader-level studies by Cameron et al. (1999), Clements et al. 2002), Cameron (2004), Salazar et al. (2003, 2009) and Górniak et al. (2010).
DNA isolation, amplification and sequencing
Genomic DNA was extracted from 10–100 mg of fresh or silica gel-dried leaf tissue, or from herbarium material, using the DNeasy Plant Mini Kit (Qiagen, Melbourne, Vic., Australia) either individually or in the 96-well plate format. The complete ITS region of the 18S–26S nrDNA was amplified by polymerase chain reaction (PCR), following methods outlined in Clements et al. (2002). The following primer pairs from Sun et al. (1994) were used: ITS4–ITS5, 17SE–26SE or 17SE–ITS4.
Phylogenetic analyses
Contiguous sequences were assembled and edited using Sequencher v.3.0 (Gene Codes Corp., AnnArbor, MI, USA) and manually aligned in BioEdit sequence alignment editor v.4.8.6 (Hall 1999). Sequence alignments and Nexus formatted files are available in TreeBase (accession number 15661) and all sequences are lodged in GenBank (see Table 2). Any uncertain base positions, generally located close to priming sites, and highly variable regions with equivocal sequence homology, were excluded from phylogenetic analysis. Individual base positions were coded as unordered multistates and potentially informative insertions/deletions (indels) were manually coded as additional binary characters.
Bayesian analyses were performed using MrBayes version 3.1.2. (Ronquist and Huelsenbeck 2003). The GTR+I+gamma model was applied to the ITS alignment. Indel characters were included as a separate partition and a standard (morphology) discrete state model with a gamma-shape parameter was applied to this partition. The Markov chain Monte Carlo search was run for 20 million generations with trees sampled every 50 000 generations. MrBayes performed two simultaneous analyses starting from different random trees (Nruns = 2), each with six Markov chains. In total 25% of the trees were discarded and the run ended with an average standard deviation of split frequencies of <0.005. A Bayesian consensus phylogram with posterior probability values plotted was calculated in MrBayes. Maximum parsimony analyses were performed with the heuristic search option (excluding uninformative characters) in PAUP* 4.02 (Swofford 2003). A two-step search method for multiple islands was performed with 10 000 random replicates with tree bisection reconnection (TBR) on, and saving only one of the shortest trees per replicate. The saved trees were then swapped to completion, saving all shortest-length trees. Support for internal branches was evaluated by the heuristic bootstrap method with 10 000 bootstrap replicates, each with 10 random-addition sequences with TBR and MULPARS activated (Felsenstein 1985).
Results
The aligned ITS dataset comprised 55 DNA sequences, with a matrix length of 762 nucleotide sites. There was a total of 396 shared polymorphisms. Six indels were scored. The parsimony analyses identified 143 equal-length trees of 1320 steps (consistency index (CI) = 0.60, rescaled consistency index (RCI) = 0.50).
The Bayesian analysis of the ITS region resolved a phylogenetic tree with moderate support for the monophyly of Drakaeinae (posterior probability, PP = 0.83), and Spiculaea cilata sister to the rest of the Drakineae. The parsimony analysis (not shown) was congruent with the Bayesian analysis, except that Spiculaea was not supported as part of the Drakineae, rather it was supported in a clade containing all the outgroups as sister to the Drakineae. A clade containing Thynninorchis and Arthrochilus (with Phoringopsis) (PP = 1.0, bootstrap values, BV = 80) was sister to a clade containing Caleana, Drakaea and Chiloglottis. Arthrochilus s.lat. was well supported, as were each of the internal clades, namely, Thynninorchis, (PP = 1.0, BV = 100) and a clade (PP = 1.0, BV = 97) comprising Arthrochilus (PP = 1.0, BV = 100) and Phoringopsis (Arthrochilus dockrillii, Fig. 1). Caleana s.lat. was sister to a clade containing Drakaea and Chiloglottis s.lat.
Caleana s.lat. was well supported (PP = 1.0, BV = 100) and showed support for monophyletic Caleana (PP = 0.97) and Paracaleana (PP = 98, BV = 69). Drakaea (PP = 1.0, BV = 100) was strongly supported but showed very little differentiation among species. Chiloglottis s.lat. (PP = 1.0, BV = 100) was also well supported, with strong support on short branches for Simpliglottis (PP = 1.00, BV = 99) and Myrmechila (PP = 1.00, BV = 100), whereas Chiloglottis s.s. was unresolved.
Discussion
Although it is recognised that the present study was based on the use of a single marker region, ITS, nevertheless it has provided an important analysis of the group. Our results, which are based on analysis of 32 species, increase the representation of all genera within Drakaeinae compared with previous studies (Fig. 1). The results are highly congruent with the previously published nuclear (Clements et al. 2002; Górniak et al. 2010) and plastid gene phylogenies (Kores et al. 2001; Cameron 2004), which were based on small samples of species in each genus. Our Bayesian results confirmed the monophyly of Drakaeinae consistent with outcomes of other broader based studies of the Diurideae (Weston et al., in press).
There are areas of incongruence between the previously published nuclear tree of Clements et al. (2002) and our current results. In particular, Arthrochilus s.lat. (including Thynninorchis) is sister to Drakaea and Chiloglottis, and the remainder of the Drakaeinae (Spiculaea, Caleana and Paracaleana), the latter forming a clade. However, there is little bootstrap support for phylogenetic relationships in this part of the backbone of the phylogeny. In the previous study, where the overall structure and phylogeny of the tribe was the focus, and no particular subtribe was investigated in detail (Clements et al. 2002), only 10 species were used as representatives of Drakaeinae. In the present study, we have expanded the range of collections, including several newly described species and additional material from disparate locations of some critical species, such as Caleana major and C. (Paracaleana) minor. The inclusion of sequence data from Caleana (Paracaleana) lyonsii, a species from south-western Western Australia, proved decisive in the assessment of that genus. C. (Paracaleana) lyonsii with a multiflowered inflorescence and erect, narrow lanceolate leaf is sister to the rest of Paracalena. C. (Paracaleana) nigrita, with its single flower and reptant, broadly ovate to ovate lanceolate leaf, is embedded within and not isolated from that clade.
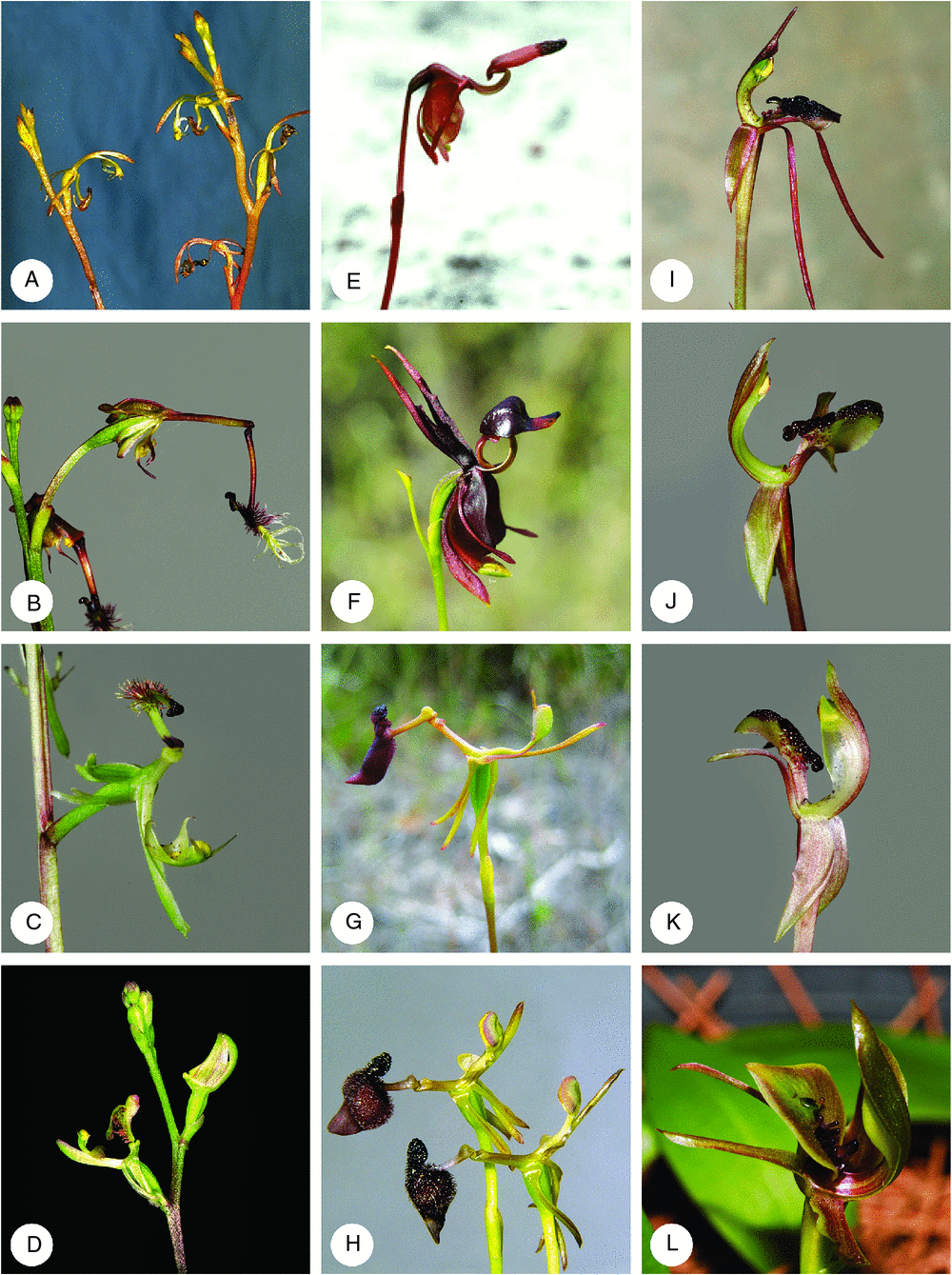
Spiculaea (Fig. 2A), as originally described (Lindley 1840b), is a monospecific genus, endemic to the inland south-west of Western Australia. Phylogenetically, it is sister to the rest of the Drakaeinae, but not well supported. Its inclusion in the Drakaeinae, therefore, is equivocal. Spiculaea is characterised by a single relatively broad, glabrous basal leaf, with a thin, wiry scape narrower at the base and fleshy, resupinate, yellowish-brown, sequentially developing flowers. The fleshy scape contains sufficient nutrients and water to enable flowers and capsules of pollinated flowers to develop to maturity, even when the basal stem dries during the extreme heat of late spring and early summer. Spiculaea is also characterised by several autapomorphies, including the prominent basal labellum stalk hinged to a very short column foot, a fleshy insectiform labellum callus, with strap-like apical appendage, and prominent, sickle-like column wings that are serrated along the inner margin.
Historically, Arthrochilus has been linked or confused with Drakaea and Spiculaea (Reichenbach 1871; von Mueller 1889; Schlechter 1926), and comprises three morphologically distinct groups. These groups were segregated by Jones and Clements (2005) into the following three genera, based primarily on differences in plant habit, but also on floral morphology: the dimorphic Arthrochilus (10 spp.), the monomorphic Phoringopsis (3 spp.) and mycroheterotrophic Thynninorchis (2 spp.).
Arthrochilus (Fig. 2C) predominantly inhabits the Australian tropical and temperate coastal woodlands and heathlands, extending along the eastern coast as far south as Sydney. Plants occur in colonies as rosettes or as multi-flowered racemes. Flowers are non- resupinate, greenish with inconspicuous sepals and petals and a prominent intricately insectiform, delicately hinged labellum that dangles like a fishing lure, and column with two unequal pairs of projecting column wings.
Phoringopsis (Fig. 2D) is distributed disjunctly in northern tropical Australia and south-eastern Papua New Guinea and was segregated from Arthrochilus (Jones et al. 2002) on the basis of possession of one or two elongate, distichous basal leaves, inflorescence emerging with the leaves, smooth, non-papillate column foot and sepal bases, labellum attached basally (not via a peltate stalk), and the callus fungiform and ornamented with penicillate calli.
Thynninorchis (Fig. 2B) comprises two leafless species that inhabit the montane eucalypt forests of south-eastern Australia, including Tasmania. These are the only fully mycroheterotrophic species of Drakaeinae and Jones and Clements (2005) erected the genus on account of this plant habit, but also because of possession of a hinged, insectiform labellum ornamented with long, multi-layered, barbed, caudiform cells, with the main head deeply divided into two lobes, each ending in a swollen, knob-like structure that hangs at the end of a long, narrow column foot, and column with two pairs of similar-sized, projecting, column wings that are reminiscent of those present in Spiculaea.
Sister to Thynninorchis is the clade containing Arthrochilus (10 species and the segregate genus Phoringopsis (3 species)). Phoringopsis is distinguished from Arthrochilus by possession of one or two narrow, erect, linear, lanceolate leaves and similarity of leaf morphology in flowering and sterile plants, flowers with smooth tepal bases, labellum hinge attached in the centre of the column foot, labellum lacking a peltately attached supporting stalk and the strongly swollen, fungi-like callus ornamented with long, thin, penicillate glands. Phoringopsis has a scattered disjunct distribution across northern Australia and one species, P. lavarackiana, also inhabits sites in the Melaleuca-dominated savanna woodlands of southern New Guinea.
Arthrochilus inhabits coastal, sandy heathlands and woodlands or mountain woodlands along the eastern coast of Australia from Sydney to north Cape York and some Torres Strait islands, with an isolated disjunct species, A. latipes, in Arnhem Land in the Northern Territory. Both Arthrochilus and Phoringopsis are colony-forming species, found in small clusters or in great numbers in some areas. Flowering occurs with or without leaves in Arthrochilus, depending on the species involved. Morphologically, these taxa have much in common despite these noted differences, particularly in plant habit.
The older (Blaxell 1972) and more recent treatment of Phoringopis as Arthrochilus s.lat. (Szlachetko 2003) is possibly more appropriate than the recognition of these taxa as separate genera. Thynninorchis is distinctive, supported by a long branch with the nrDNA ITS data, but the possession of several unique vegetative and floral characters makes it difficult to identify synapomorphies shared by it and Arthrochilus and Phoringopsis. Clearly, these DNA data can be interpreted to recognise alternative classifications. Features and characteristics that facilitate ready recognition of and isolation of Thynninorchis from Arthrochilus are the mycroheterotophic leafless plant habit, presence of protocorm or protocorm-like structures and absence of true tubers, labellum hinged to the end of a long narrow column foot and dangled like a fishing lure, labellum lamina simple, unlobed, with a long, peltately attached basal stalk, a callus intricately insectiform and dominating the labellum lamina, ornamented with long, multi-layered, barbed caudiform cells, with a main head that is deeply divided into two lobes, each lobe ending in a swollen knob-like structure, along with being distributed in tall wet forests throughout mountainous regions of south-eastern Australia. On this basis, we support a classification that recognises both Arthrochilus and Thynninorchis (Fig. 1).
A clade containing a monophyletic Caleana (Fig. 2F) and a monophyletic Paracaleana (Fig. 2E) is strongly supported. Caleana major (Fig. 2F), the type of that genus, is sister to representatives of Paracaleana (and Sullivania); however, both of these groups are on shorter branches than is the combined Caleana–Paracaleana clade. Caleana, the unmistakable flying duck orchid, is a south-eastern Australian species with disjunct outliers in the Mount Lofty Ranges in South Australia and Carnarvon Gorge–Blackdown Tableland region in central Queensland. P. minor often occurs sympatrically and has a distribution similar to that of C. major, although also occurring on the North island of New Zealand (St George 1999). However, the greatest number of Paracaleana species (11 spp.) inhabit areas in the south-west of Western Australia (Hopper and Brown 2006; Brown et al. 2008; Hoffman and Brown 2011). All are colony-forming species.
Caleana (Fig. 2F) is distinct from Paracaleana (Fig. 2E) on morphological grounds. This is reflective of the pollination syndrome and associated development of morphological features, such as tuberculate labella surface, first identified by Blaxell (1972) when describing the genus (Hopper and Brown 2006). Possession of a glabrous labellum or the apparent lack of glands on the labellum of C. major is attributable to the pollination syndrome, where flowers are visited by males of the long-tailed sawfly, Lophyrotoma leachii (Kirby), that land cross-wise on the smooth labellum surface (Cady 1965; Bates 1989; Bower 2001). All other species are apparently pollinated by male thynnine wasps (Erione spp.: Tiphiidae) that are attracted to the flower by allomones and psuedocopulate with the insectiform labellum (Hopper and Brown 2006). Possession of a hinged labellum sensitive to touch is characteristic for all species in this major clade and this is the only group in Drakaeinae with this character. Column wings are also highly developed, extending the whole length of that organ, forming a cup-like structure that temporarily holds the insect in place during pollination. From the results of our study, we conclude that Paracaleana (and Sullivania) are synonymous with Caleana, as originally proposed by Clements (1989).
The three sampled species representative of the genus Drakaea (Fig. 2G, H) form a strongly supported clade. These unique hammer orchids of south-western Western Australia possess several synapomorphies and the genus is now recognised as containing at least 10 species (Hopper and Brown 2006). Their separation from all other members of Drakaeinae belies past confusion surrounding their recognition as a distinct genus. Recent research (Phillips et al. 2011) has suggested that most species inhabit highly specialised microhabitats in open areas of sandy ground and that each species is associated with a single specific mycorrhizal fungus. All species are reported as being pollinated by thynnine wasps (Zaspilothynnus spp.) during attempted pseudocopulation with the highly specialised insectiform labellums (Hopper 2009). Column wings are more or less vestigial in all species and the anther is very prominent. In addition, Drakaea is characterised by possession of a single, short, reinform, spongy, glabrous leaf, a thin, wiry, glabrous scape that gradually widens towards the apex, and a one-flowered inflorescence, with a labellum stalk basally hinged and able to pivot on the hinge, returning to its original position.
Chiloglottis s.lat. comprises the final clade. Our results suggested that although species attributed to both Myrmechila (represented by C. truncata, C. platyptera, C. trapeziformis and C. formicifera) (Fig. 2J, K) and Simpliglottis (represented, for example, by C. cornuta, C. valida, C. chlorantha) (Fig. 2L) are monophyletic (PP = 1.00, BV = 100), on short branches, the rest of Chiloglottis (Chiloglottis s.s.) (Fig. 2I) is unresolved within Chiloglottis s.lat. Analysing a combined matrix of both ITS and trnL–trnF cpDNA intergenic spacer sequences, Mant et al. (2002) found strong support for the monophyly of two groups, where Chiloglottis s.str. was sister to Myrmechila, and these combined where sister to Simpliglottis, contrary to what was found here. These conflicting results are likely to be the result of ITS being a faster-evolving locus than trnL–F in these taxa. The arrangement of taxa where the Simpliglottis clade is sister to the remainer of Chiloglottis is also reflected in possession of a simpler, less elaborate floral morphology found in species of Simpliglottis. The nature of these conflicting results and the minimal nature of the morphological differences among taxa, in particular Myrmechila and Chiloglottis s.s., and the difficulty in applying this taxonomic segregation in practice, suggest that the original decision by Szlachetko (2001) and follow-up decisions by Jones and Clements (2005) to split Chiloglottis s.lat. were unnecessary. We, therefore, argue that Robert Brown’s original concept of a single Chiloglottis, supported by most subsequent authors, is the best taxonomic interpretation of these data.
Chiloglottis (Fig. 2I–L) is distributed throughout south-eastern Australia, including Tasmania, as well as occurring in New Zealand where it reaches as far south as the Auckland Island, where C. cornuta was first discovered and described by Hooker (1844–1847). Chiloglottis is characterised by possession of the following: two subequal, opposed leaves; a one (rarely two) flowered, fleshy, ephemeral inflorescence arising from the centre of the two leaves; flowers suberect to horizontal; sepals with cylindrical, apical osmophores; a prominent rhomboid-trapeziform, caudate, stiffly, hinged labellum with a wide lamina and prominent columnar; and stalked calli, weakly or strongly insectiform; and column wings extending the length of the column.
Conclusions
Although only based on analysis of ITS nuclear rDNA sequences, our results provided sufficient basis for a reinterpretation of generic cicumscription within subtribe Drakaeinae. These results are supported by those generated using both nuclear and chloroplast genes (Weston et al., in press), where the position and status of Drakaeinae, relative to the remainder of Diurideae, is the subject of further consideration. Given the very high levels of support and degree of genetic divergence, coupled with possession of readily definable morphological synapomorphies for each major clade, the Drakaeinae require significant taxonomic changes to conform to a monophyletic interpretation of the tribe and its genera. A reclassification of all the species involved, including some reallocation of taxa to appropriate genera, is provided (Appendix 1).
Acknowledgements
This research was primarily funded from a grant by the Gatsby Charitable Trust and, secondarily, for assistance with field work and collections, by the Australian Orchid Foundation, the Nell and Hermon Slade Trust, the Foundation for the Protection of Wild Orchids, Zürich, and the Foundation for Research, Science and Technology, New Zealand, and we are most grateful for their support. Plant material was collected with kind permission under Western Australian Department of Conservation and Land Management, Permit CE00418, New South Wales, Scientific Investigation Licence A1796, Tasmanian Department of Primary Industry, Water and Environment Permit FL 03121, and Nouvelle-Caledonie, Service de L’Environnement er de la Gestion des Parcs et Reserves, Authorisation 6024 765/ENV. We also thank our many colleagues who have aided in the collection or contributed plant material used in this study. DNA samples of some species were obtained from Mark Chase and Gerardo Salazar, Jodrell Laboratory, Royal Botanic Gardens, Kew, and for these we are most grateful. Technical assistance was provided by Kirsten Cowley, Kelli Gowland, Kristy Lam, Anne Mackenzie, Ish Sharma and Terri Weese.
References
Backhouse GH, Jeanes J (1995) ‘The Orchids of Victoria.’ (Miegynyah Press: Melbourne)Bates RJ (1989) Observations on the pollination of Caleana major R.Br. by male sawflies (Pterygophorus sp.). Orchadian 9, 208–210.
Bentham G (1873) ‘Flora Australiensis. Vol. 6.’ (L.Reeve & Co.: London)
Bentham G, Hooker JD (1883) ‘Genera Plantarum. Vol. 3 Part 2.’ (L. Reeve and Co.: London)
Blaxell DF (1972) Arthrochilus F.Muell. and related genera (Orchidaceae) in Australia. Contributions from the NSW National Herbarium 4, 275–283.
Bower CC (2001) Chiloglottis – pollination. In ‘Genera Orchidacearum, Vol. 2: Orchidoideae (Part 1)’. (Eds AM Pridgeon, PJ Cribb, MW Chase, FN Rasmussen) pp 146–148. (Oxford University Press: Oxford, UK)
Brown AP, Dundas P, Dixon K, Hopper SD (2008) ‘Orchids of Western Australia.’ (University of Western Australia Press: Perth)
Cady L (1965) Notes on the pollination of Caleana major R.Br. Orchadian 2, 34–35.
Cameron KM (2004) Utility of plastid psaB gene sequences for investigating intrafamilial relationships within Orchidaceae. Molecular Phylogenetics and Evolution 31, 1157–1180.
| Utility of plastid psaB gene sequences for investigating intrafamilial relationships within Orchidaceae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjs1alsbg%3D&md5=bc9d386e2fc1c47359ff7ad0262028b1CAS | 15120407PubMed |
Cameron KM, Chase MW, Whitten WM, Kores PJ, Jarrell DC, Albert VA, Yukawa T, Hills HG, Goldman DH (1999) A phylogenetic analysis of the Orchidaceae: evidence from rbcL nucleotide sequences. American Journal of Botany 86, 208–224.
| A phylogenetic analysis of the Orchidaceae: evidence from rbcL nucleotide sequences.Crossref | GoogleScholarGoogle Scholar | 21680360PubMed |
Chase MW, Cameron CM, Barrett RL, Freudenstein JV (2003) DNA data and Orchdiaceae systematics: a new phylogenetic classification. In ‘Orchid Conservation’. (Eds KW Dixon, SRKell, RL Barrett, PJ Cribb) pp. 69–89. (Natural History Publications (Borneo): Kota Kinabalu, Borneo)
Clements MA (1982) ‘Preliminary Checklist of Australian Orchidaceae.’ (National Botanic Gardens: Canberra)
Clements MA (1989) Catalogue of Australian Orchidaceae. Australian Orchid Research 1, 1–160.
Clements MA (1995) Reproductive biology in relation to phylogeny of the Orchidaceae, especially the tribe Diurideae. PhD Thesis, Australian National University, Canberra.
Clements MA (1996) Reproductive biology in relation to phylogeny of the Orchidaceae, especially the tribe Diurideae Journal and Proceedings of the Royal Society of New South Wales 129, 83 [Abstract]
Clements MA (1999) Embryology. In ‘Genera Orchidacearum. 1’. (Eds AM Pridgeon, PJ Cribb, MW Chase, FN Rasmussen) pp. 38–58. (Oxford University Press: Oxford, UK)
Clements MA, Jones DL, Sharma IK, Nightingale ME, Garratt MJ, FitzGerald KJ, Mackenzie AM, Molloy BPJ (2002) Phylogenetic systematics of the Diurideae (Orchidaceae) based on the ITS and 5.8S coding region of nuclear ribosomal DNA. Lindleyana 17, 135–171.
Cox AV, Pridgeon AM, Albert VA, Chase MW (1997) Phylogenetics of the slipper orchids (Cypripedioideae, Orchidaceae): nuclear rDNA ITS sequences. Plant Systematics and Evolution 208, 197–223.
| Phylogenetics of the slipper orchids (Cypripedioideae, Orchidaceae): nuclear rDNA ITS sequences.Crossref | GoogleScholarGoogle Scholar |
Dixon KW, Burchellet BJ, Collins MT (1989) ‘Orchids of Western Australia – Cultivation and Natural History’, 2nd edn. (Western Australian Native Orchid Study & Conservation Group: Perth)
Dressler RL (1974) Classification of the orchid family. In ‘Proceedings of 7th World Orchid Conference’, 24–27 April 1972, Medellin, Columbia. (Ed. H Mariano Ospina) pp. 259–279. (Conferencia Mundial de Orquidelogia: Medellin, Columbia)
Dressler RL (1979) The subfamilies of the Orchidaceae. Selbyana 5, 197–206.
Dressler RL (1981) ‘The Orchids: Natural History and Classification.’ (Harvard University Press: Cambridge, MA)
Dressler RL (1983) Classification of the Orchidaceae and their probable origin. Telopea 2, 413–424.
Dressler RL (1986) Recent advances in orchid phylogeny. Lindleyana 1, 5–20.
Dressler RL (1993) ‘Phylogeny and Classification of the Orchid Family.’ (Dioscorides Press: Portland, OR)
Dressler RL, Dodson CH (1960) Classification and phylogeny in the Orchidaceae. Annals of the Missouri Botanical Garden 47, 25–67.
| Classification and phylogeny in the Orchidaceae.Crossref | GoogleScholarGoogle Scholar |
Erickson R, George AS, Marchant NG, Morcombe MK (1973) ‘Flowers and Plants of Western Australia.’ (AH & AW Reed: Sydney)
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791.
| Confidence limits on phylogenies: an approach using the bootstrap.Crossref | GoogleScholarGoogle Scholar |
Górniak M, Paun O, Chase MW (2010) Phylogenetic relationships within Orchidaceae based on a low-copy nuclear coding gene, Xdh: congruence with organellar and nuclear ribosomal DNA results. Molecular Phylogenetics and Evolution 56, 784–795.
| Phylogenetic relationships within Orchidaceae based on a low-copy nuclear coding gene, Xdh: congruence with organellar and nuclear ribosomal DNA results.Crossref | GoogleScholarGoogle Scholar | 20211743PubMed |
Green JW (1985) ‘Census of the Vascular Plants of Western Australia.’ 2nd edn. (Department of Agriculture: Perth)
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41, 95–98.
Hoffman N, Brown AP (1984) ‘Orchids of South-west Australia’, 1st edn. (University of Western Australia Press: Perth)
Hoffman N, Brown AP (1992) ‘Orchids of South-west Australia’, 2nd edn. (University of Western Australia Press: Perth)
Hoffman N, Brown AP (2011) ‘Orchids of South-west Australia’, 3rd edn. (Scott Print: Perth)
Hooker JD (1844–1847) ‘The Botany of the Antarctic Voyage of H.M. Discovery Ships Erebus and Terror. I. Flora Antarctica.’ (Reeve: London)
Hopper SD (2009) Taxonomic turmoil down-under: recent developments in Australian orchid systematics. Annals of Botany 104, 447–455.
| Taxonomic turmoil down-under: recent developments in Australian orchid systematics.Crossref | GoogleScholarGoogle Scholar | 19398445PubMed |
Hopper SD, Brown AP (2006) Australia’s wasp-pollinated flying duck orchids revised (Paracaleana: Orchidaceae). Australian Systematic Botany 19, 211–244.
| Australia’s wasp-pollinated flying duck orchids revised (Paracaleana: Orchidaceae).Crossref | GoogleScholarGoogle Scholar |
Hopper SD, Brown AP (2007) A revision of Australia’s hammer orchids (Drakaea: Orchidaceae), with some field fata on species-specific sexually deceived wasp pollinations. Australian Systematic Botany 20, 252–285.
| A revision of Australia’s hammer orchids (Drakaea: Orchidaceae), with some field fata on species-specific sexually deceived wasp pollinations.Crossref | GoogleScholarGoogle Scholar |
Jeanes J, Backhouse G (2001) ‘Pictorial Guide to: Wild Orchids of Victoria, Australia.’ (Aquatic Photographics: Melbourne)
Jones DL (1988) ‘Native Oirchids of Australia.’ (Reed Books: Sydney)
Jones DL (1998) Contributions to Tasmanian orchidology 1–9: a taxonomic review of Chiloglottis in Tasmania. Australian Orchid Research 3, 61–71.
Jones DL (2006) ‘A complete Guide to Native Orchids of Australia Including the Island Territories.’ (Reed New Holland: Sydney)
Jones DL, Clements MA (2001) Subtribe Drakaeinae. In ‘Genera Orchidacearum, 2. Orchidoideae (Part 1)’. (Eds AM Pridgeon, PJ Cribb, MW Chase, FN Rasmussen) pp. 134–155. (Oxford University Press: Oxford, UK)
Jones DL, Clements MA (2005) Miscellaneous nomenclatural notes and changes in Australian, New Guinea and New Zealand Orchidaceae. Orchadian 15, 33–42.
Jones DL, Clements MA, Sharma IK, Mackenzie AM, Molloy BPJ (2002) Nomenclatural notes arising from studies into the tribe Diurideae (Orchidaceae). Orchadian 13, 437–468.
Jones DL, Hopley T, Duffy SM, Richards KJ, Clements MA, Zhang X (2006) ‘Australian Orchid Genera and Information and Identification System.’ (CSIRO Publishing: Melbourne)
Kores PJ, Cameron KM, Molvray M, Chase MW (1997) The phylogenetic relationships of Orchidoideae and Spiranthoideae (Orchidaceae) as inferred from rbcL plastid sequences. Lindleyana 12, 1–11.
Kores PJ, Weston PH, Molvray M, Chase MW (2000) Phylogenetic relationships within the Diurideae (Orchidaceae): inferences from plastid matK DNA sequences. In ‘Monocots: Systematics and Evolution’. (Eds KL Wilson, DA Morrison) pp. 449–456. (CSIRO Publishing: Melbourne)
Kores PJ, Molvray M, Weston PW, Hopper SD, Brown AP, Cameron KM, Chase MW (2001) A phylogenetic analysis of Diurideae (Orchidaceae) based on plastid DNA sequence data. American Journal of Botany 88, 1903–1914.
| A phylogenetic analysis of Diurideae (Orchidaceae) based on plastid DNA sequence data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXot1Knu7k%3D&md5=d59cc84fcdcf812ade6b8db985e3a7a6CAS | 21669623PubMed |
Lindley J (1840a) ‘The Genera and Species of Orchidaceous Plants.’ (Ridgways, Picadilly: London)
Lindley J (1840b) A sketch of the vegetation of the Swan River Colony. (James Ridgway: London)
Mant JG, Schiestl FP, Peakall R, Weston PH (2002) A phylogenetic study of pollinator conservatism among sexually deceptive orchids. Evolution 56, 888–898.
| A phylogenetic study of pollinator conservatism among sexually deceptive orchids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltFaku78%3D&md5=cc5b8943c297fa905d799a5423c04c5dCAS | 12093025PubMed |
Pfeiffer L (1874) Nomenclator botanicus, Vol. 1, part 2. p. 1134. (T. Fischeri: Kassel, Germany)
Pfitzer EHH (1887) ‘Entwurf einer natürlichen Anordnung der Orchideen.’ (Carl Winter’s Universitätsbuchhandlung: Heidelberg, Germany)
Pfitzer EHH (1889) Orchidaceae. In ‘Die Natürlichen Pflanzenfamilien’. (Eds A Engler, K Prantl) pp. 1–220. (Wilhelm Engelmann: Leipzig, Germany)
Phillips RD, Brown AP, Dixon KW, Hopper SD (2011) Orchid biogeography and the factors associated with rarity in a biodiversity hotspot: the South west Australia Floristic Region. Journal of Biogeography 38, 487–501.
| Orchid biogeography and the factors associated with rarity in a biodiversity hotspot: the South west Australia Floristic Region.Crossref | GoogleScholarGoogle Scholar |
Reichenbach HG (1871) Bemerkungen für eine Australische Orchideenkunde. In ‘Beiträge zur systematischen Pflanzenkunde’. pp. 53–70. (Druck von ThG Meissner: Hamburg, Germany)
Rogers RS (1921) Notes on the gynostemium in the genus Diuris, and on the polinary mechanism in Phajus. Transactions and Proceedings of the Royal Society of South Australia 45, 264–269.
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574.
| MrBayes 3: Bayesian phylogenetic inference under mixed models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXntlKms7k%3D&md5=d26483d123ccc51ff88c481fa85be749CAS | 12912839PubMed |
Rye BL (1987) Paracaleana. In ‘Flora of the Perth region’. (Eds NG Marchant, JR Wheeler, BL Rye, EM Bennett, NS Lander, TD Macfarlane) pp. 807–844. (Western Australian Herbarium, Department of Agriculture: Perth)
Salazar GA, Chase MW, Soto Arenas MA, Ingrouille M (2003) Phylogenetics of Cranichideae with emphasis on Spiranthinae (Orchidaceae, Orchidoideae): evidence from plastid and nuclear DNA Sequences. American Journal of Botany 90, 777–795.
| Phylogenetics of Cranichideae with emphasis on Spiranthinae (Orchidaceae, Orchidoideae): evidence from plastid and nuclear DNA Sequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkslGjtLo%3D&md5=53a304e64d0fcbd12e11b15e63d4873dCAS | 21659175PubMed |
Salazar GA, Cabrera LI, Madriñán S, Chase MW (2009) Phylogenetic relationships of Cranichidinae and Prescottiinae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences. Annals of Botany 104, 403–416.
| Phylogenetic relationships of Cranichidinae and Prescottiinae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFyhtLzJ&md5=829ad1f5ba0a9b960dd7c10336381dbcCAS | 19136493PubMed |
Schlechter R (1911) Die Polychondreae (Neottiinae Pfitz.) und ihre systematische Einteilung. Botanische Jahrbucher fur Systematik, Pflanzengeschichte und Pflanzengeographie 45, 375–410.
Schlechter R (1926) Das System der Orchidaceen Notizblatt des Botanischen Gartens und Museums zu Berlin-Dahlem 9, 563–591.
| Das System der OrchidaceenCrossref | GoogleScholarGoogle Scholar |
St George I (1999) ‘The Nature Guide to New Zealand Native Orchids’. (Godwit: Auckland)
St George I (2010) The NZ orchids: the editor’s annual list. New Zealand Native Orchid Journal 115, 31–40.
Sun T, Skinner DZ, Liang GH, Hulbert SH (1994) Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of the nuclear ribosomal DNA. Theoretical and Applied Genetics 89, 26–32.
| Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of the nuclear ribosomal DNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXivFWkt7k%3D&md5=8065b3fe2f7127b7608ca7919e5f5b63CAS |
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. (Sinauer Associates: Sunderland, MA, USA)
Szlachetko DL (1991) Thelymitroideae, a new subfamily within Orchidaceae. Fragmenta Floristica et Geobotanica 36, 33–49.
Szlachetko DL (2001) Genera et species Orchidalium. I. Polish Botanical Journal 46, 11–26.
Szlachetko DL (2003) Quelques notes additionnelles sur al sous-famille Thelymitroideae (Orchidaceae). Richardiana 3, 90–100.
Szlachetko DL, Rutkowski P (2002) Quelques notes additionnelles sur la sous-famille Thelymitroideae (Orchidaceae). Richardiana 3, 90–100.
von Mueller F (1889) Description of a new form of the orchid genus Drakaea, indigenous to New South Wales and Victoria. Victorian Naturalist 5, 174–175.
Warwick SI, Mummenhoff K, Sauder CA, Koch MA, Al-Shebaz IA (2010) Closing the gaps: phylogentic relationships in the Brassicaceae based on DNA sequence data of nuclear ribosomal ITS region. Plant Systematics and Evolution 285, 209–232.
| Closing the gaps: phylogentic relationships in the Brassicaceae based on DNA sequence data of nuclear ribosomal ITS region.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXls1Wmtb0%3D&md5=c46d52bd23401b36eb6f2d6dce8673b5CAS |
Weber JZ, Bates RJ (1986) Orchidaceae. In ‘Flora of South Australia. Part IV’. 4th edn. (Eds JP Jessop, HR Toelken) pp. 60–62. (The Flora and Fuana of South Australia Handbooks Committee: Adelaide)
Weston PH, Perkins AJ, Indsto JO, Clements MA (in press). Phylogeny of Orchidaceae tribe Diurideae and it implications for the evolution of pollination systems. In ‘Darwin’s Orchids: Then and Now’. (Eds P Bernhardt, R Edens-Meier) (University of Chicago Press: Chicago, IL)
Woolcock DT, Woolcock CE (1984) ‘Australian Terrestrial Orchids.’ (Thomas Nelson: Melbourne)
Appendix 1. Taxonomic enumeration of the subtribe Drakaeinae
!, type seen; Dist., distribution, codes follow Table 1
Drakaeinae Schltr., Bot. Jahrb. Syst. 45: 381 (1911). Type: Drakaea Lindl
Arthrochilus F.Muell., Fragm. 1: 42 (Jun. 1858). Type species: Arthrochilus irritabilis F.Muell.
Type species: Drakaea irritabilis F.Muell. and D. hunterianan F.Muell.
Subgen. Arthrochilus
Arthrochilus apectus D.L.Jones, Orchadian 14(8): Suppl. i, ii (June 2004)
Type: Queensland: Cook District; old slaughterhouse area, Heathlands Reserve, Cape York Peninsula, 25 Jan. 1992, D.L.Jones 8940 and C.H.Broers (CBG 9220228) (holo CANB!).
Dist: Qco.
Arthrochilus aquilus D.L.Jones, Orchadian 14(8): Suppl. ii, iii (June 2004)
Type: Queensland: Cook District; old slaughterhouse area, Heathlands Reserve, Cape York Peninsula, 25 Jan. 1992, D.L.Jones 8941 and C.H.Broers (CBG 9220229) (holo CANB!).
Dist: Qco.
Arthrochilus corinnae D.L.Jones, Orchadian 14(8): Suppl. iii, iv (June 2004)
Type: Queensland: Cook District; swamp on south bank of Dulhunty River, Old Telegraph Road crossing, Cape York Peninsula, 23 Jan. 1992, D.L.Jones 8888 and C.H.Broers (CBG 9220175) (holo CANB!; iso BRI!, MEL!).
Dist: Qco.
Arthrochilus irritabilis F.Muell., Fragm. 1: 43 (1858)
Type: ‘Moreton Bay’, W.Hill and F.Mueller s.n. (holo MEL!; iso K-LINDL!).
Dist: Ncc, Nnc, Qmo, Qwb, Qkn, Ddg.
Arthrochilus latipes D.L.Jones, Austral. Orch. Res. 2: 8, 9, f. 7 (1991)
Type: Northern Territory: Radon Gorge, Mount Brockman, 12°45′S, 132°53′E, 7 Dec. 1978, C.R.Dunlop 5044 (holo DNA, iso DNA).
Dist. Ddg.
Arthrochilus oreophilus D.L.Jones, Austral. Orch. Res. 2: 9, 10, f. 8 (1991)
Type: Queensland; Cook District; Herberton Range, 17°24′S, 142°20′E, 22 Jan 1988, L.Lawler 24 (holo CANB!; iso BRI!, CANB!).
Dist. Qco.
Arthrochilus prolixus D.L.Jones, Austral. Orch. Res. 2: 10, f. 9 (1991)
Type: New South Wales; Bellangry, NW of Wauchope, 31°21′S, 152°37′E, 9 Dec. 1985, D.L.Jones 2228, L.Barton and T.D.Jones (holo CANB!; iso BRI!, CANB!, NSW!).
Dist: Ncc, Nnc,Qmo.
Arthrochilus rosulatus D.L.Jones, Austral. Orch. Res. 2: 10, 11, f. 10 (1991)
Type: Queensland; Cook District; Double Barrel Pinch, Shipton’s Flat Road, ~8 km from Big Forks junction, S of Cooktown, 15°45′S, 148°15′E, 6 June 1990, L.Lawler 83 (holo CANB!; iso BRI!, CANB!).
Dist: Qco.
Arthrochilus sabulosus D.L.Jones, Austral. Orch. Res. 2: 11, 12, f. 11 (1991)
Type: Queensland; Horn Island, ~4.5 km W of airstrip towards jetty, 10°35′S, 142°15′E, 9 Feb. 1989, D.L.Jones 3558, B.Gray, P.S.Lavarack and J.R.Clarkson (holo CANB!; iso BRI!, CANB!, MEL!, NSW!).
Dist: Qco.
Arthrochilus stenophyllus D.L.Jones, Austral. Orch. Res. 2: 12, 13, f. 12 (1991)
Type: Queensland; Sunday Creek, south of Cardwell, 18°30′S, 146°10′E, 12 Dec. 1988, P.S.Lavarack 3624 (holo CANB!; iso CANB!).
Dist: Qkn.
Subgen. Phoringopsis (D.L.Jones & M.A.Clem.) Szlach., Richardiana 3(2): 97
Basionym: Phoringopsis D.L.Jones et M.A.Clem., Orchadian 13(10): 457 (Feb. 2002).
Type species: Arthrochilus byrnesii Blaxell.
Arthrochilus byrnesii Blaxell, Contr. New South Wales Natl. Herb. 4: 278 (1972)
Type: ‘Waterfall Creek, South Alligator River, Northern Territory’, 2 Apr.1969, N. Byrnes 1530 (holo NSW!; iso DNA!).
Dist: Ddg, Wga.
Arthrochilus dockrillii Lavarack, Proc. Roy. Soc. Queensland 86(25): 155, f. 1 (1975)
Type: ‘Kurrimine, near Innisfail, North Queensland’, 17 July1972, P. Lavarack N.P. [National Park number series] 1691 (holo BRI!; iso NSW!).
Dist: Qkn, Qco.
Arthrochilus lavarackiana (D.L.Jones) Lavarack, Austrobaileya 7(20); 385 (2006)
Type: Queensland. Cultivated Australian National Botanic Gardens, 16 May 1990, ex ‘Moa Island, ~1 km NE of airport, Kubin’, D.L.Jones 6006 (CBG 9010305) (holo CANB).
Dist: Qco; PG.
Caleana R.Br., Prod. 329 (1810)
Type species: Caleana major R.Br., fide Blaxell (1972).
Type species: Caleya sullivanii F.Muell., fide Jones and Clements (2005).
Type species: Caleana minor R.Br.
Basionym: Paracaleana Blaxell subgen. Tanychila D.L.Jones et M.A.Clem., Orchadian 13(10): 458 (2002); Sullivania subgen. Tanychila (D.L.Jones et M.A.Clem.) D.L.Jones et M.A.Clem., Orchadian 15(1): 36 (2005).
Type species: Paracaleana nigrita (J.Drummond ex Lindl.) Blaxell.
Type species: Caleana minor R.Br. (Paracaleana minor (R.Br.) Blaxell).
Caleana alcockii (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana alcockii Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 222, 223, f.2, 6, 7 (map) (30 June 2006).
Type: NW Coastal Highway, 14 km N of Galena Bridge (over the Murchison River), 27°42′39″S, 114°40′35″E, 9 Sep. 2005, s.d.Hopper 8660, A.Brown, G.Brockman and R.Phillips (holo PERTH; iso: MEL).
Dist: Wir.
Caleana brockmanii (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana brockmanii Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 222, 223, f. 223–225, figs 2, 8, 9 (map) (30 June 2006).
Type: Extension of Douglas Road into Victoria Dam catchment MARTIN, 450 m N of locked Water Corp. gate, E side of track, 32°04′16″S, 116°04′04″E, 23 Nov. 2004, G.Brockman 1471 (holo PERTH 06945643).
Dist: Wda.
Caleana disjuncta (D.L.Jones) M.A.Clem., comb. nov.
Basionym: Paracaleana disjuncta D.L.Jones, Orchadian 14(5): 226–228, f. (2003); Sullivania disjuncta (D.L.Jones) D.L.Jones et M.A.Clem., Orchadian 15(1): 36 (2005).
Type: South Australia; Coxs Scrub Conservation Park, 29 Oct.1995, D.E.Murfet 2292B and R.L.Taplin (holo CANB!; iso AD).
Dist: Wda, Wey, Wro, Ski, Sls, Vwh.
Caleana dixonii (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana dixonii Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 228, 229, f. 2, 12, 13 (map) (2006).
Type: Leeman Road on both the N and S sides, 17 Nov. 1987, A.Brown 781 (holo PERTH 1256912).
Dist: Wda.
Caleana gracilicordata (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana gracilicordata Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 229, 230, f.2, 14, 15 (map) (2006).
Type: Granite rock 1.5 km SE of Blue Rock, Jarrahdale–Albany Highway road, 32°20′01″S, 116°07′58″E, 21 Oct. 2003, G.Brockman 1117 (holo PERTH 06734693).
Dist: Wda.
Caleana granitica (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana granitica Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 230–232, f.2, 16, 17 (map) (2006).
Type: Sullivan Rock on Albany Highway, 32°23′S, 116°15′E, 26 Nov. 1986, s.d.Hopper 5835 (holo PERTH 904309).
Dist: Wda.
Caleana hortiorum (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana hortiorum Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 232–234, f. 2, 18, 19 (map) (2006).
Type: Flynn State Forest, Deefor Road, York. 350 m W of Surrey Road, 32°1′58″S, 116°28′42″E, 11 Oct. 2003, F.Hort & J. Hort 2053 (holo: PERTH 06639674).
Dist: Wda.
Caleana lyonsii (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana lyonsii Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 219–221, f. 4, 5 (map) (2006).
Type: 15.7 km E of the North West Coastal Highway along fenceline, ENE of Nerren Nerren Station, 27°07′17″S, 114°46′44″E, 28 Sep. 1994, A.P.Brown 1131 (holo PERTH 03926044; iso AD, CBG[CANB]!).
Dist: Wir, Wau, Wco.
Caleana major R.Br., Prod. 329 (1810)
Type: ‘(J) v.v.’ [Port Jackson; Bennelong Point’, Sep. 1803, R.Brown s.n.] (lectotype specimen (a) BM!; isolectotype E!, FI!, K!, K-LINDL!, P!), fide Clements (1989).
Dist: Sls, Ski, Sse, Vwp, Vwh, Vgi, Veh, Tas, Nsc, Nst, Ncc, Nct, Nnc, Qmo, Qdd, Qle.
Caleana minor R.Br., Prod. 329 (1810)
Type: ‘(J.) v.v.’ [Port Jackson; Sandy ground between brickfields and Barclay Lagoon, Oct.–Nov. 1803, R.Brown s.n.] (lectotype specimen (a) BM!; isolectotype E!, G!, K!, K-LINDL!, P!), fide Clements (1989).
Type: ‘Near Mt. Zero, Grampians in Victoria’, Jan. 1882, D.Sullivan s.n. (holo MEL!).
Type: ‘New South Wales, Bell, Blue Mountains, 27 Dec.1930, E.Nubling s.n. (holo AD!).
Dist: Sls, Sse, Vwp, Vwh, Veh, Vgi, Tas, Nsc, Nst, Can, Ncc, Nct, Nnc, Ncs, Qmo, Qdd, Qwb, Qle; NZ.
Caleana nigrita J.Drummond ex Lindl. in Edwards’s, Bot. Reg. 1–23: Swan Riv. Append. liv (1840)
Type: ‘Swan River’, 1839, J.Drummond s.n. (holo K-LINDL!; iso E!, G!, K!).
Dist: Wda, Wey.
Caleana parvula (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana parvula Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 237, 238, f. 2, 23, 24 (map) (2006).
Type: Mount Ragged track, 4.2 km N of Fisheries Road, E of Esperance, 33°43′06″S, 123°07′13″E, 30 Oct. 2004, G.Brockman 1449 (holo PERTH 06945686).
Dist: Wey.
Caleana terminalis (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana terminalis Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 239–240, f. 2, 25, 26 (map) (2006).
Type: Z Bend Gorge, Kalbarri National Park, 27°39′S, 114°28′E, 21 Aug. 1984, A.Brown s.n. (holo PERTH 929158).
Dist: Wir.
Caleana triens (Hopper & A.P.Br.) M.A.Clem., comb. nov.
Basionym: Paracaleana triens Hopper & A.P.Br., Aust. Syst. Bot. 19(3): 240–242, f. 2, 27, 28 (map) (2006)
Type: Cut Hill, W of York, 31°54′23″S, 116°43′11″E, 15 Sep. 1907, O.H.Sargent s.n. (holo PERTH 00337307).
Dist: Wda, Wav, Wro.
Chiloglottis R.Br., Prod. 322, 323 (1810)
Type species: Chiloglotis diphylla R.Br.
Chiloglottis anaticeps D.L.Jones, Austral. Orch. Res. 2: 37, f. 44 (1991)
Type: New South Wales; ~2 km SE of Forbes River crossing, Hastings Forest Way,W of Wauchope, 31°09′S, 152°22′E, 9 Dec. 1985, D.L.Jones 2229, L Barton and T.D.Jones (holo CANB!; iso CANB!, NSW!).
Dist: Nnt.
Chiloglottis diphylla R.Br., Prod. 323 (1810)
Types: ‘(J) v.v.’ [Port Jackson; Sydney and Parramatta, Mar. 1805, R.Brown s.n.] (lectotype specimen (a) BM!; isolectotype E!, K!, K-LINDL!, P!), fide Clements (1989); Syntype: ‘(J) v.v.’ [Port Jackson, 1804, R.Brown s.n.] (AD!).
Dist: Nsc, Ncc, Nct, Nnc, Ncs, Nnt, Qmo, Qdd, Qwb, Qbn, Qle.
Chiloglottis longiclavata D.L.Jones, Austral. Orch. Res. 2: 38–39, f. 46 (1991)
Type: Queensland; Cook District; SFR 194, Parish of Western, Herberton Range, 17°20′S, 145°25′E, 100 m, 26 April 1987, B.Gray 4455 (holo CANB!; iso CANB!).
Dist: Qle, Qks, Qkn, Qco.
Chiloglottis palachila D.L.Jones, Austral. Orch. Res. 2: 39, f. 47 (1991)
Type: ‘New South Wales; Barrington Tops, 31°59′S, 151°58′E, 8 Dec. 1985, D.L.Jones 208, L Barton and T.D.Jones (holo CANB!; iso BRI!, CANB!, MEL!, NSW!).
Dist: Ncs, Nnt.
Chiloglottis reflexa (Labill.) Druce, Rep. Bot. Exch. Cl. Brit. Isles Suppl. 2: 614 (1917)
Basionym: Epipactis reflexa Labill., Nov. Holl. pl. 2: 60, t. 211 (1806).
Type: Van Diemen, J. Labillardiere s.n. (lecto FI!; illustration only!), fide Clements (1989).
Type: Van Diemen, J. Labillardiere s.n. (lecto FI!; illustration only).
Dist: Vwp, Vwh, Vgi, Veh, Tas, Nsc, Ncc, Nnt.
Chiloglottis seminuda D.L.Jones, Austral. Orch. Res. 2: 41, f. 50 (1991)
Type: ‘New South Wales; Penrose State Forest, 34°40′S, 150°13′E, 14 Mar. 1990, D.L.Jones 5745 and C.H.Broers (holo CANB, iso CANB, NSW, MEL, AD, BRI).
Dist: Ncc, Nst, Nct.
Chiloglottis sphaerula D.L.Jones, Austral. Orchid. Res. 5: 73, 74, f.3.7 (21 Dec. 2006)
Type: New South Wales. Barrington Tops, 5 Feb. 1993, R.Tunstall, D.Herd. G.Hillman and J.Riley (D.L.Jones 11282) (holo CANB 677939!).
Dist: Nnt.
Chiloglottis sphyrnoides D.L.Jones, Austral. Orch. Res. 2: 41, 42, f. 51 (1991)
Type: Queensland; Pat’s Bluff, Lamington National Park, 28°16′S, 153°07′E, 1 Apr. 1988, C.Harman 3 (holo CANB!; iso BRI!, CANB!, NSW!).
Dist: Nnc, Qmo.
Chiloglottis sylvestris D.L.Jones et M.A.Clem., Proc. Roy. Soc. Queensland 98: 123–4, f. 1 (1987)
Type: ‘Queensland Springbrook’, 12 Jan. 1986, D.L.Jones 2231 (holo BRI!; iso AMES!, BRI!, CANB!, K!, MO!, NSW!, PERTH!, US!).
Dist: Ncc, Nnc, Qmo.
Chiloglottis trilabra Fitzg., J. Bot. 21: 204 (1883)
Type: ‘Mount York, Blue Mountains, N.S.W.’, R.D.Fitzgerald s.n. (holo BM!).
Type: ‘Barrington Tops, New South Wales’, Feb. 1953, A.W.Dockrill s.n. (holo NSW!; iso MEL!), fide Clements (1989).
Dist: Tas, Vgi, Veh, Nst, Can, Nct, Nnt.
Sect. Myrmechila (D.L.Jones et M.A.Clem.) M.A.Clem., comb. et stat. nov.
Basionym: Myrmechila D.L.Jones et M.A.Clem., Orchadian 15(1): 36, 37 (2005).
Type species: Chiloglottis formicifera Fitzg.
Chiloglottis formicifera Fitzg., Austral. Orch. 1(3): [t. 9] (1877)
Type: ‘Liverpool’, Sep.–Oct., C. King s.n. (holo not found; lectotype Fitzgerald’s plate!), fide Clements (1989).
Dist: Nsc, Ncc, Nct, Nnc, Nnt, Qdd; NZ.
Chiloglottis platyptera D.L.Jones, Austral. Orch. Res. 2: 39, 40, f. 48 (1991)
Type: New South Wales; N of Dingo Gate, Barrington Tops, 31°55′S, 151°21′E, 26 Sep. 1989, C.Bower s.n. (D.L.Jones 5093) (holo CANB!; iso CANB!, NSW!).
Dist: Nnt., Nnc.
Chiloglottis trapeziformis Fitzg., Austral. Orch. 1(3): [t. 9] (1877)
Basionym: Type: ‘New South Wales, Liverpool’, C. King s.n. (holo BM!).
Dist: Sse, Vgi, Veh, Tas, Nst, Can, Nss, Ncc, Nct, Ncs.
Chiloglottis trullata D.L.Jones, Austral. Orch. Res. 2: 42, 43, f. 52 (1991)
Type: Queensland; Rainbow Falls, Blackdown Tableland, 23°39′S, 149°04′E, 18 July 1989, E.Pederson s.n. (D.L.Jones 4552) (holo CANB, iso BRI).
Dist: Qle.
Chiloglottis truncata D.L.Jones et M.A.Clem., Proc. Roy. Soc. Queensland 98: 124, f. 2 (1987)
Type: ‘Queensland, Anduramba, W of Toogoolawah’, Aug. 1984, D.L.Jones s.n. (holo BRI!; iso AMES!, CANB!, K!, MO!, NSW!).
Dist: Qdd, Qle.
sect. Simpliglottis (Szlach.) M.A.Clem., sect. et stat. nov.
Basionym: Simpliglottis Szlach., Polish Bot. J. 46(1): 13 (2001).
Type species: Simpliglottis valida (D.L.Jones) Szlach. (Chiloglottis valida D.L.Jones).
Chiloglottis chlorantha D.L.Jones, Austral. Orch. Res. 2: 37–38, f. 45 (1991)
Type: New South Wales; Jamberoo Mountain SW of Wollongong, 34°38′S, 150°44′E, Oct. 1988, R.G.Tunstall s.n. (holo CANB!; iso CANB!, MEL!, NSW!).
Dist: Ncc.
Chiloglottis cornuta Hook.f., Fl. \Antarct. 1: 69 (1844)
Type: ‘Campbell Islands’, Lyall s.n. (holo K!).
Type: ‘Loddon, Victoria’, Nov., C.French ex F.Mueller s.n. (holo not found; lectotype Fitzgerald’s plate!), fide Clements (1989).
Dist: Sse, Veh, Tas, Nst; NZ.
Chiloglottis grammata G.W.Carr, Indig. Fl. & Fauna Assoc. Misc. Paper 1: 20, 21 (1991)
Type: Tasmania, Jackeys Marsh, 20 Nov. 1982, G.W. Carr s.n. (holo MEL 223596!; iso CANB, HO, K, NSW).
Dist: Tas.
Chiloglottis gunnii Lindl., Gen. sp. orchid. pl. 387 (1840)
Type: ‘Tasmania, Circular Head’, Nov. 1837, R.Gunn 913 (holo K-LINDL!; iso K!, P!).
Type: Tasmania, western city limits of Hobart at foot of Mt Wellington, 20 Dec. 1984, G.W.Carr 10055 (holo MEL!; iso AD, CANB, HO, K, NSW), fide Jones (1998).
Dist: Tas.
Chiloglottis jeanesii D.L.Jones, Muelleria 10: 63–67, f.1 (1997)
Type: Victoria, Toorongo, 14 Jan. 1995, C. Bower (D.L.Jones 13809) (holo CANB!; iso AD!, BRI!, MEL!, NSW!).
Dist: Veh.
Chiloglottis pluricallata D.L.Jones, Austral. Orch. Res. 2: 40, f. 49 (1991)
Type: New South Wales; Point Lookout, New England National Park, 30°29′S, 152°25′E, 7 Dec. 1989, D.L.Jones 5538 and C. Broers (holo CANB!; iso BRI!, CANB!, MEL!, NSW!).
Dist: Nn, Nnc, Ncc, Nct.
Chiloglottis triceratops D.L.Jones, Aust. Orch. Res. 3: 66, 67, f. 3.3 (1998)
Type: Tasmania; Coquette Ck., Scottsdale Road, 7 Nov. 1990, D.L.Jones 7060, C.H.Broers and J. Campbell (holo CANB!; iso AD, CANB, HO, MEL, NSW).
Dist: Tas.
Chiloglottis turfosa D.L.Jones, Austral. Orch. Res. 2: 43, f. 53 (1991)
Type: New South Wales; ~7 km along Tantangara Dam Road, Kosciusko National Park, 35°52′S, 148°38′E, ~1000 m, 16 Dec. 1989, D.L.Jones 5571, B.E.Jones and T.D.Jones (holo CANB, iso CANB).
Dist: Nst.
Chiloglottis valida D.L.Jones, Austral. Orch. Res. 2: 43, 44, f. 54 (1991)
Type: Australian Capital Territory; track from Ginini Flats to Stockyard Arboretum, 35°32′S, 148°47′E’, 3 Dec. 1989, D.L.Jones 5453 and B.E.Jones (holo CANB!; iso CANB!, NSW!, MEL!).
Dist: Vwh, Vwp, Vgi, Veh, Tas, Nst, Can.
Chiloglottis × pescottiana R.S.Rogers, Proc. Roy. Soc. Victoria (new ser.), 30: 139, t. 25 (1918)
Type: ‘Cravensville near Tallangatta’, 8 Oct. 1917, A.B.Braine s.n. (holo AD!; iso MEL!).
Notes: a natural hybrid between Chiloglottis gunnii and C. trapeziformis.
Dist: Veh, Nst.
Drakaea Lindl. in Edwards’s, Bot. Reg. 1–23: Swan Riv. Append. lv (1840)
Type species: Drakaea elastica Lindl., fide Pfeiffer (1874).
Drakaea andrewsiae Hopper & A.P.Br., Austral. Syst. Bot. 20: 261, 262, f., 3, 7, 8 (2007).
Type: ‘Gnowangerup’, 7 Sep. 1930, P.Andrews s.n. (holo AD97034193).
Dist: Wda.
Drakaea concolor Hopper & A.P.Br., Austral. Syst. Bot. 20: 262–264, f., 3, 9, 10 (2007)
Type: ‘Kalbarri National Park’, 21 Aug. 1984, A.P.Brown s.n. (holo PERTH 0929190).
Dist: Wir.
Drakaea confluens Hopper & A.P.Br., Austral. Syst. Bot. 20: 264–266, f., 3, 11, 12 (2007)
Type: ‘Boyup Brook area’, 6 Oct. 1983, s.d.Hopper 3461 (holo PERTH 00228893; iso AD, CANB!, K, MEL).
Dist: Wda.
Drakaea elastica Lindl. in Edwards’s, Bot. Reg. 1–23: Swan Riv. Append. lvi (1840)
Type: ‘Swan River’, J.Drummond s.n. (lectotype one flower in a packet (b) and the illustation on the sheet in Lindl.’s herbarium (c) K-LINDL!; isolectotype K!), fide Clements (1989).
Type: ‘Swan River’, J.Drummond s.n. (lectotype specimen (a) K-LINDL!; isolectotypes E!, FI!, G!, K!, K-LINDL!), fide Clements (1989).
Type: ‘Found in sandy soil at Ravenswood, near Pinjarra, Western Australia’, 1 Oct. 1919, J.S.Rogers s.n. (holo AD!).
Dist: Wda.
Drakaea glyptodon Fitzg., Gard. Chron. (new ser.), 17: 494 (1882)
Type: ‘Dardanup and Albany’ [Bunbury], Sep., R.D.Fitzgerald s.n. (holo BM!; iso BM!, NSW!).
Dist: Wir, Wda, Wav, Wro, Wey.
Drakaea gracilis Hopper & A.P.Br., Austral. Syst. Bot. 20: 271–273, f., 3, 17, 18 (2007)
Type: ‘Stirling Range National Park, 3 km ENE of Mondurup Peak, 34°24′S, 117°51′E, 7 Oct. 1983, s.d.Hopper 3508 (holo PERTH 00232270!; iso AD, CANB!, MEL).
Dist: Wda.
Drakaea isolata Hopper & A.P.Br., Austral. Syst. Bot. 20: 273–275, f., 3, 19, 20 (2007)
Type: ‘near Pingrup’, 12 Sept. 1985, s.d.Hopper 4548 (holo PERTH 00902217; iso CANB!).
Dist: Wav.
Drakaea livida J.Drummond in Hooker’s, London J. Bot. 1: 628 (1842)
Type: ‘Swan River’, J.Drummond s.n. (lectotype K!; isolectotype K!).
Type: ‘Perth, Western Australia’, Sep., R.D.Fitzgerald s.n. (holo BM!; icon Fitzgerald’s plate).
Dist: Wda, Wav.
Drakaea micrantha Hopper & A.P.Br., Austral. Syst. Bot. 20: 278–280, f., 3, 23, 24 (2007)
Type: ‘Nannup area’, 10 Oct. 1983, s.d.Hopper 3566 (holo PERTH 00231762; iso AD, CANB!).
Dist: Wda
Drakaea thynniphila A.S.George, Nuytsia 5(1): 60, f. 3, F–K (1984)
Type: ‘Gull Rock Road, E of Oyster Harbour, Western Australia’, 1 Oct. 1971, A.S.George 11099 (holo PERTH!; iso CANB!).
Dist: Wda.
Spiculaea Lindl. in Edwards’s, Bot. Reg. 1–23: Swan Riv. Append. lvi (1840)
Type species: Spiculaea ciliata Lindl.
Spiculaea ciliata Lindl. in Edwards’s, Bot. Reg. 1–23: Swan Riv. Append. lvi (1840)
Type: ‘Swan River ‘, J.Drummond 325 (holo K-LINDL!; iso BM!, FI!, G!, K!).
Dist: Wda, Wav, Wro, Wey, Wco.
Thynninorchis D.L.Jones et M.A.Clem., Orchadian 13(10): 457 (Feb. 2002)
Type species: Drakaea huntiana F.Muell. (Arthrochilus huntianus (F.Meull.) Blaxell).
Thynninorchis huntiana (F.Muell.) D.L.Jones et M.A.Clem., Orchadian 13(10): 457 (Feb. 2002)
Basionym: Drakaea huntiana F.Muell., Victorian Naturalist 5: 174 (1889); Spiculaea huntiana (F.Muell.) Schltr., Repert. Spec. Nov. Regni Veg. Beih. 17: 81 (1921); Arthrochilus huntianus (F.Muell.) Blaxell, Contr. New South Wales Natl. Herb. 4: 277 (1972).
Type: Tingerinji Mountain, [New South Wales], 2 Mar. 1889, W.Baeuerlen 175 (holo MEL!; iso NSW!).
Dist: Vwp, Vwh, Vgi, Tas (Flinders Island), Nst, Can, Ncc, Nct.
Thynninorchis nothofagicola (D.L.Jones) D.L.Jones et M.A.Clem., Orchadian 13(10): 457 (Feb. 2002)
Basionym: Arthrochilus huntianus (F.Muell.) Blaxell subsp. nothofagicola D.L.Jones, Austral. Orch. Res. 3: 4, f. 1.1, t.2 (1998).
Type: Tasmania, behind Needles Picnic area, Gordon River Road, 24 Feb.1994, A.Garner and D.Ziegler (Jones 12812A) (holo CANB!).
Dist: Tas.