Managing rain-filled wetlands for carbon sequestration: a synthesis
Susanne C. Watkins A E , Darren S. Baldwin B C , Helen P. Waudby C D and Sarah E. M. A. Ning AA Murray Darling Wetlands Working Group Ltd, PO Box 7016, East Albury, NSW 2640, Australia.
B CSIRO Land and Water and Murray–Darling Freshwater Research Centre, La Trobe University, PO Box 821, Wodonga, Vic. 3689, Australia.
C Present address: Institute for Land, Water and Society, Charles Sturt University, Thurgoona, NSW 2640, Australia.
D Murray Local Land Services, PO Box 797, North Albury, NSW 2640, Australia.
E Corresponding author. Email: s.watkins7@bigpond.com
The Rangeland Journal 39(2) 145-152 https://doi.org/10.1071/RJ16077
Submitted: 8 August 2016 Accepted: 20 January 2017 Published: 23 February 2017
Journal Compilation © Australian Rangeland Society 2017 Open Access CC BY-NC-ND
Abstract
Global acknowledgement of climate change and its predicted environmental consequences has created a need for practical management techniques that increase a landscape’s ability to capture and store atmospheric carbon (C). Globally, wetlands sequester disproportionally more C per unit surface area than many other components of the landscape. However, wetlands vary in their capacity to store C and regulate greenhouse gas emissions. Hydrology, in particular, is a critical driver of wetland C capture and storage. Rain-filled wetlands offer a challenge for the management of C sequestration and storage because the hydrology of these systems is almost entirely driven by rainfall. We present a conceptual model of how management options, including weed and pest control, grazing and crop management and revegetation, will affect C sequestration and storage in rain-filled wetlands. Given the intensive nature of agricultural activities in areas where rain-filled wetlands are common, further work is needed to increase our understanding of the effects of these activities on wetland C capture and storage. Key knowledge gaps relating to the effect of management actions on wetland C sequestration include: (a) the benefits of integrated wetland management; (b) the appropriateness of different grazing regimes and the effect of total grazing pressure; (c) the effects of fire; and (d) the extent to which wetland function (C storage) can be restored following agricultural activities, such as cropping.
Additional keywords: cropping, fire, grazing, pests, weeds, wetland rehabilitation.
Introduction
Global acknowledgement of climate change and its predicted environmental consequences has created a need for practical management techniques that increase the landscape’s ability to capture and store atmospheric carbon (C). Wetlands store the most C of any ecosystem by unit surface area (constituting ~35% of the global terrestrial C store) and twice as much C as the next most efficient ecosystem, temperate forests (Department of Sustainability, Environment, Water, Population and Communities 2012). Consequently, the role of wetlands in the global C cycle is receiving increasing recognition (Danone Fund for Nature 2010; Lawrence et al. 2012; UNEP and Center for International Forestry Research 2014). However, a scientific rationale to underpin wetland management actions that support the capture and storing of C is often lacking (Mcleod et al. 2011). Wetland managers, therefore, are tasked with making decisions about how best to manage wetlands for C sequestration without clear evidence of the mechanisms by which management actions will improve wetland C storage.
Wetlands globally vary in their capacity to store C and regulate greenhouse gas (GHG) emissions (Bernal and Mitsch 2012). Differences in hydrology, topography, morphology, vegetation type, climate, soil moisture and soil pH affect wetland capacity to store C. Hydrology is a critical driver of wetland ecosystem function, particularly C capture and storage (Bunn and Arthington 2002; Adhikari et al. 2009; Baldwin et al. 2013). Wetland plant communities are shaped by the duration, timing and frequency of inundation (Casanova and Brock 2000), whereas soil dynamics and associated biogeochemical processes, such as decomposition, change with flooding (Wilson et al. 2011). Although managed inundation (environmental flows) could be used to manage wetland C dynamics (e.g. Baldwin et al. 2016), this management tool is not necessarily feasible for rain-filled wetlands, which are typically isolated from river systems and floodplains.
Rain-filled wetlands and associated water management infrastructure are broadly defined as shallow water bodies occurring in depressions in the landscape, which dry periodically (Keeley and Zedler 1998). These wetlands depend entirely on rainfall and fill when rainfall exceeds evapotranspiration. Rain-filled wetlands differ from other seasonal wetlands in that they are isolated from large water bodies, are surrounded by a small catchment, and are over-represented in semi-arid and arid environments. For example, in semi-arid inland New South Wales (NSW), rain-filled wetlands are common (Bowen 2010) covering an area of ~300 000 ha, largely on private properties in agricultural areas (Kingsford et al. 2003).
As rain-filled wetlands are moist environments in typically dry arid and semi-arid zones, they could be important sites for regulating C dynamics, including C sequestration, in these zones. In this synthesis, we review management actions for improving the C sequestration role of Australian rain-filled wetlands and identify key knowledge gaps.
Threats to rain-filled wetlands and their effects on carbon storage
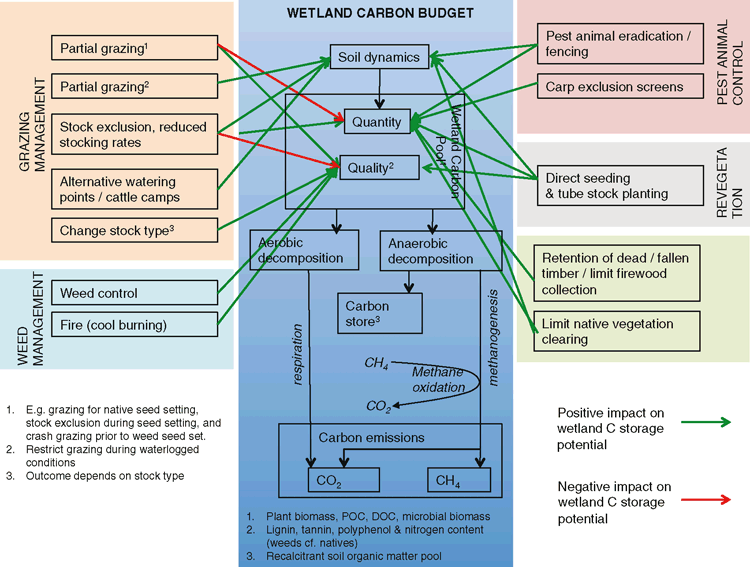
Worldwide, the condition of rain-filled wetlands is threatened by livestock grazing, vegetation clearing, cropping, and associated anthropogenic disturbances, such as pests and weeds (Kingsford et al. 2003). These threats could affect the ability of wetlands to sequester C. However, appropriate management may mitigate their negative effects (Fig. 1).
Livestock grazing
The strongest effect of livestock grazing on wetlands likely occurs through the direct consumption of plant material (Robertson 1997), which is exported from wetlands in the form of stock body mass. Trampling of vegetation can also affect wetland plants and associated C negatively. The net effects of consumption and trampling include reduced C stores, and decreased wetland C capture and storage potential (Morris and Reich 2013). Stock also affect plant community composition by introducing exotic seeds (via hooves and faeces) and consuming plants, altering plant succession and competition. For example, Lunt et al. (2012) found that grazing (by native and exotic herbivores) reduced the cover of native plants in recently flooded floodplain areas. In addition, exotic species such as para grass (Urochloa muticia (Forssk.); Queensland Government 2016) are sometimes deliberately introduced into wetlands to provide fodder for livestock.
Changes in the structure and composition of wetland plant communities affect C storage potential because resource quality (chemical composition) differs among plants, affecting decomposition rates (Robertson 1997). Therefore, the chemical composition of wetland plant material determines turnover rates (Zech et al. 1997). Easily decomposed components of plant material include polysaccharides and proteins. Lignin and polyphenols are comparatively resistant to microbial breakdown. Consequently, plants that are high in phenols, waxes and lignin, decompose slowly and contribute to the recalcitrant soil organic matter pool, whereas plants high in polysaccharides and proteins turnover much faster (Zech et al. 1997). In Australia, litter from native vegetation generally decomposes more slowly than that of exotic species because of its relatively high contribution of polyphenols and tannins (Campbell and Fuchshuber 1995).
Stock trampling also affects wetland C dynamics through compaction and pugging of soil. Pugging describes the indentations left in the soil surface when stock traverse waterlogged or soft soils (Boulding and Baldwin 2006). Pugging can dislodge plants, and damage soil structure and wetland seed banks. Soil compaction in a wetland may reduce oxygen infiltration of soils, altering biogeochemical processes. Plants do not thrive in compacted areas because their roots penetrate the soil less easily, resulting in shallow root systems (Morris and Reich 2013). However, soil compaction may affect vegetation less than consumption by grazers (Greenwood and McKenzie 2001). The effects of pugging may last up to13 years following de-stocking, and the damage to the soil may be permanent (Boulding and Baldwin 2006).
Few grazing management regimes have been tested experimentally and the outcomes of such studies are usually anecdotal (Morris and Reich 2013). Some studies have involved complete exclusion of stock, but other management options, including stocking rates, timing and duration, and stock type, have received little attention. Additionally, most of these studies were undertaken in floodplain wetlands. Rain-filled wetlands have received comparatively little attention.
‘Stocking rate’ refers to the number of stock per unit area. Dry sheep equivalents per hectare (ha) or cattle class (e.g. breeder) per unit area (km2 or ha) is the typical measure for carrying capacity of a unit of land (NSW Department of Primary Industries 2016). However, in the context of wetlands, paddock-level stocking rates have little meaning when conditions are dry and stock congregate around wetlands (Robertson 1997). Reducing stocking rates eases the pressure exerted by stock on wetlands. Optimal stocking rates are difficult to define because utilisation rates depend on a range of factors, including water quality, pasture quality, age and reproductive status of stock, and ambient temperature. Also, the amount of feed available to stock elsewhere in the paddock can influence their effect on a wetland, with higher intensity of use when paddock feed is low. Holmes et al. (2009) recommended that stocking rates should be based on the most grazing-sensitive part of the wetland.
Livestock grazing in wetlands can be managed by alternating different durations of grazing with rest periods. Grazing duration can vary from short periods (crash grazing), such as 2 weeks, or longer periods of grazing (e.g. 3 months). Rest periods leading up to strategic grazing may be several months in duration (Jansen and Robertson 2001). This resting period enables vegetation to recover and seed in the soil seed bank to germinate. However, long-term resilience of vegetation to grazing will depend on grazing intensity, condition of the vegetation, and longevity of seed stores (Morris and Reich 2013).
Although stock would ideally be excluded altogether during hot dry periods, establishing alternative watering points and shelter (cattle camps) away from wetlands may reduce the amount of time stock spend at natural water bodies (Jansen and Robertson 2001). Cattle may spend less time at natural water bodies when an alternative water supply is provided (Agouridis et al. 2005), although season may influence this behaviour (Waudby et al. 2013). For example, cattle are less likely to drink from alternative watering spots in hot weather if water temperature is high, and may enter wetlands to cool off or use shade provided by wetland vegetation. However, Morris and Reich (2013) noted that placing watering points away from wetlands may be insufficient to avoid damage to wetlands by stock.
Timing of grazing is important because the influence of stock can vary seasonally. For example, pugging and soil damage will be greater when wetlands are inundated (Greenwood and McKenzie 2001) and damage to native vegetation greatest when plants are seeding. Conversely, grazing during seed set in weed species may reduce weed burden. Lunt et al. (2012) found that stock preferred wetland vegetation in recently flooded areas over dryland vegetation in unflooded areas, whereas Boulding and Baldwin (2006) suggested that wetlands should be completely de-stocked when soils are saturated. Morris and Reich (2013) referred to seasonal management of grazing as ‘seasonal tracking’, where livestock are restricted or excluded at certain times of the year (e.g. when soils are saturated, when plants are establishing, or at critical plant germination times, such as after floods).
Trampling by cattle produces greater soil compaction and pugging than that by sheep because cattle hooves maintain a higher static pressure (Greenwood and McKenzie 2001). Boulding and Baldwin (2006) suggested that sheep were preferable to cattle in riparian areas for this reason. However, sheep affect wetland vegetation differently from cattle as they graze closer to the ground and target particular species (Department of Environment and Primary Industries 2013). Pasture quality, water availability, and the presence of predators will determine the distance travelled by stock and, therefore, the extent of soil compaction and pugging (Greenwood and McKenzie 2001). Consequently, managers should consider stocking rate, animal movement and access to pasture and water when developing wetland management regimes.
Grazing management outcomes can be highly variable in terms of response of wetlands, so context is important when considering appropriate measures. Morris and Reich (2013) outline several response modifiers, or wetland attributes, which can influence a wetland’s response to grazing. Those attributes relevant to wetland C storage and sequestration are:
Sensitivity of vegetation
Currently, no metrics are available to describe the sensitivity of wetland vegetation to grazing. However, Morris and Reich (2013) infer that plant species sensitive to grazing are those that are palatable, have little structural support and foliage easily accessed by grazers, do not produce vegetatively, grow slowly, have short-lived seed banks, produce few seeds, and have specific germination requirements. Species that are less susceptible to grazing likely include canegrasses (Eragrostis spp.), rushes (Juncus spp.), and sedges (Eleocharis spp.) (I. Davidson, pers. comm., 13 April 2016).
Wetland size
Small wetlands are more susceptible to grazing effects than larger wetlands because of the larger perimeter-to-wetland-area ratio (Morris and Reich 2013) or ‘edge effect’. The edge, or littoral zone, of a wetland is the area most susceptible to damage from livestock, particularly if alternative watering points are not available.
History of grazing
Floodplains in the southern Murray–Darling Basin have been grazed for over 150 years (Robertson 1997). Historical stocking and grazing should be considered when assessing the likely response of a wetland to stock exclusion (Jansen and Robertson 2001). Lunt et al. (2007) suggested separating current grazing effects from historical effects as historical grazing may have had substantial effects on ecosystem conditions, which could be irreversible. However, the effects of historical grazing can be difficult to separate from the influence of contemporary grazing (Tiver and Andrew 1997). Although historical grazing has had a major effect on riparian habitats in south-eastern Australia, the outcome of stock removal is likely to be site-specific. In some cases, stock removal prevents further damage (Belsky et al. 1999), whereas in others, removal of stock may have undesirable effects on current ecosystem conditions (e.g. Riverina native grasslands) (Lunt et al. 2007), including the proliferation of weeds.
Natural levels of disturbance
Natural disturbances, such as fire and grazing by native animals, can increase biodiversity by providing opportunities for colonising plants. Productive wetlands that do not experience natural levels of disturbance may benefit from low intensity grazing (Morris and Reich 2013).
Timeframe of response
Timeframes for wetland responses to removal of stock from an area can vary greatly, from 12 months to 50 years (Boulding and Baldwin 2006), and it may take years before soil C improves following management. Miller et al. (2010) found that soil C did not change after 4–6 years of grazing exclusion of a stream bank. However, standing litter did increase by as much as 8-fold when stock were excluded, indicating that improved soil C may become detectable after some time following litter breakdown.
Total grazing pressure
Morris and Reich (2013) argued that total grazing pressure (i.e. native and exotic grazers), should be considered when determining stocking densities. However, total grazing pressure can be difficult to estimate. Fisher et al. (2004) also discussed total grazing pressure and suggested some management strategies for dealing with wild grazers.
Weeds
Weeds are ubiquitous in Australia and can cause a shift in vegetation community composition and in wetland C dynamics. The replacement of natives with exotics in wetlands leads to changes in the quality of organic matter entering the wetland, and often to changes in the timing of organic matter processing (Schulze and Walker 1997). Aquatic weeds are relatively uncommon in rain-filled wetlands because of their isolation from waterways, which can carry propagules downstream (Howell and Benson 2000). Terrestrial weeds, however, are a common feature of rain-filled wetlands during the dry phase.
Characteristics of organic matter in the form of plant litter differ in their lignin, tannin, polyphenol and nitrogen (N) contents, which can alter the rate of decomposition (Leff and McArthur 1990). The C : N ratio of leaves has long been suggested as being inversely correlated with decomposition rate, and high leaf N is generally associated with fast decomposition rates (Webster and Benfield 1986). Weeds in Australia generally have higher N than Australian natives, so the displacement of native vegetation in and around wetlands will shift the quality of the organic matter entering the wetland to one with a lower C : N ratio and therefore faster processing rate. The overall effect will be high turnover rates and less organic matter being stored long-term.
Typically, weed control in wetlands focuses on targeted spraying of weed species with herbicide. However, stock management, fire, biological control, and bulldozing may also be used and often in conjunction with one another. For example, a combination of biological control, herbicide, fire and bulldozing have been used for the control of Mimosa spp. (Paynter and Flanagan 2004). Similarly, the treatment of reed canary grass (Phalaris arundinacea L.) with herbicide and soil scarification led to an increase in the establishment of natives (Thomsen et al. 2012).
Livestock can be used to strategically graze areas where weeds have become a problem; subsequent stock exclusion would allow native vegetation to recover. The disadvantages of this approach include the negative effects that stock have on wetland C storage (see above). Nevertheless, areas that have been historically grazed may be most suited to this approach, as complete stock exclusion often favours weeds over native vegetation (Price et al. 2011). Jansen and Robertson (2001) found exclusion of stock for more than 50 years did not prevent the occurrence of blackberry in the Murrumbidgee catchment. Grazing may therefore play an important role in supressing weeds in these systems though careful management is required to ensure stock do not have a detrimental effect. The disturbance caused by cattle in and around temporary wetlands may favour native vegetation over exotics, as suggested by Marty (2005).
The use of fire as a management tool in wetlands is less common than livestock grazing, but surface or cool fires can influence wetland C sequestration and storage. For example, in the short term, surface fires increase CH4 emissions, soil erosion and nutrient loss, and reduce aboveground biomass. Long-term effects include reduced weed cover, increased diversity of vegetation, and increased soil and nutrient retention (Kotze 2013). Surface fires, where aboveground biomass is consumed by the fire, or ground fires, which destroy belowground plant parts and soil organic matter, are both used as management tools. Surface fires are appropriate as a management tool for C storage in wetlands. Ground fires compromise the C reservoir of wetlands and impede post-fire primary production as a result of soil nutrient depletion and reduced mycorrhizal development, negatively affecting plant growth (Kotze 2013). Vegetation responses to fire include greater light penetration through the canopy, increased nutrients, and reduced litter. Plants respond by either reseeding (germination of seeds) or resprouting via perennating buds of surviving plants. The latter method is common among Cyperaceae, Poaceae, Typhaceae and Juncaceae. Surface fires are believed to have little effect on the soil seed bank (Kotze 2013).
Some native plants are adapted to fire and recover quickly. Invasive species tend not to recover as well, which is why fire can be an effective weed control method. Species that become dormant in response to fire tend to do well after fire. Fire can also be used to increase the biodiversity of vegetation where particular species dominate (e.g. Typha and Phragmites spp.) by targeting burns at particular times of the year (Kotze 2013).
Fire may be most effective at controlling weeds when used in combination with other management tools, such as herbicide and/or mechanical interventions (Paynter and Flanagan 2004). For example, spring burns, together with C supplements and seed application, increased native perennial grasses and reduced exotic annuals in a woodland plant community (Prober et al. 2005).
Although fires are an important source of GHG emissions, the effects of wetland fires on GHG emissions is unclear. Inferences in this paper are drawn from grasslands instead. Livesley et al. (2011) found no change in CH4 or CO2 fluxes in savanna grasslands following fire, apart from a short pulse of CH4 entering the atmosphere 24 h after the fire. They suggested that fire is likely to reduce GHG emissions in the long-term, by altering soil C, nutrients, microbial activity and soil moisture (Livesley et al. 2011).
Cropping and soil disturbance
Many rain-filled wetlands in south-eastern Australia are located in agricultural landscapes and are sometimes modified for cropping. In 1997, 2.5% of rain-filled lakes in western NSW were cropped (Seddon and Briggs 1998), although this number may be higher in areas where higher rainfall facilitates more successful cropping. In recent years, cropping has increased in Australia, leading to widespread effects on aquatic ecosystems (Davis et al. 2015).
Cropping in rain-filled wetland areas is usually opportunistic, occurring following the recession of floodwaters in semi-arid areas. Soil disturbance occurs through ploughing in preparation for crop planting, reducing soil C and the water-holding capacity of the wetland, particularly when crops are planted frequently (Seddon and Briggs 1998). Additional disturbances involve the application of herbicides and fertilisers, and damage from machinery during crop cultivation and harvesting (Casanova 2012). Wetland hydrology may also be compromised by earthworks or regulators installed to control irrigation (Seddon and Briggs 1998). Cropping reduces the biodiversity and abundance of wetland plants, leading to increased open water or bare ground (Casanova 2012).
The frequency of cropping in wetlands may ultimately depend on water availability. A cessation in cropping may allow the recovery of wetland plant communities. However, recovery will depend on historical frequency of cropping. Infrequently cropped wetlands will have lost little soil C and wetland plant communities are likely to make an almost full recovery, whereas frequently cropped wetlands are more affected (Seddon and Briggs 1998; Casanova 2012). Casanova (2012) suggested that wetland plant recovery is more likely to be successful where an area of a cropped wetland remains uncultivated. Ephemeral wetland plants possess several mechanisms that make them somewhat resilient to disturbance, including the long-term viability of seeds stored in the soil, and morphological and life-history traits that allow plants to persist and germinate when optimum conditions return (Brock 2011).
Pests and non-domesticated grazers
Native and feral non-domesticated grazers such as kangaroos (Macropus spp.), rabbits (Oryctolagus cuniculus L.), hares (Lepus europaeus Pallas), goats (Capra hircus L.), pigs (Sus scrofa L.) and deer (Cervus and Dama spp.) contribute to total grazing pressure and soil compaction, although effects may differ among taxa. Despite the loss of C through the physical removal of plant biomass during grazing, grazers may play a role as C cyclers within an ecosystem if they deposit faecal material where they feed. Little is known about the role of pests and wildlife as vectors of nutrients and C across the landscape, particularly of migratory species (Post et al. 1998).
Macropods feed extensively on macrophytes following floodplain inundation. Despite macropod movement being less restricted than that of domesticated stock, stable isotope analysis of macropod scats found on floodplains suggests that faecal material is deposited locally rather than being exported elsewhere in the landscape (Iles et al. 2010). Kangaroo faecal material can reach quantities up to 193 g m−2 following flooding, and may be an important C source for wetland consumers (Kobayashi et al. 2009; Baldwin et al. 2013). However, the quality of the C in kangaroo faecal material and its contribution to wetland C stores is unclear. Feral pigs damage wetlands through their foraging behaviour, including burrowing for roots, bulbs and other detrital material. Water turbidity is greater when feral pigs forage in wetlands because macrophytes are damaged resulting in areas of bare ground and open water (Doupé et al. 2010).
Shooting, baiting and trapping may reduce total grazing pressure around wetlands. Fencing to exclude exotic animals is uncommon, and an expensive option that is not always available to wetland managers. Doupé et al. (2010) found that excluding feral pigs with fencing can improve wetland health. However, seasonal changes in wetlands were greater than any effects of fencing, raising questions about the cost-effectiveness of fencing as a management tool.
Loss of vegetation biomass
The potential for wetlands to sequester C is largely driven by primary productivity. Loss of vegetation through harvesting and timber extraction (such as firewood collection) will have a strong effect on future C storage potential.
In NSW, the Native Vegetation Act 2003 (soon to be replaced by new biodiversity legislation) currently regulates clearing of remnant vegetation. Consequently, clearing is less common than historically, when European settlement led to widespread vegetation loss (Walker et al. 1993). Firewood collection on private properties, however, is more difficult to regulate, and the extent to which this activity occurs, difficult to quantify. Firewood collection in public river red gum (Eucalyptus camaldulensis Dehn.) floodplain forests, however, is widely documented, and has resulted in the almost complete removal of fallen timber (Mac Nally et al. 2011). Non-leaf components of riparian vegetation, such as branches, decompose more slowly than leaves, and have a greater potential to contribute to the long-term storage of C in wetlands (O’Connell et al. 2000). Harvesting and firewood collection will reduce wetland C stores and timber removal with heavy machinery will likely damage remaining wetland vegetation. Higher water temperatures as a result of reduced riparian shading may alter decomposition processes (Batzer et al. 2000). Conversely, reduced shading may also lead to greater primary production of wetland macrophytes (Sweeney 1993).
Although wetland revegetation can be induced by altering the hydrological regime, revegetation through tube-stock planting or direct seeding is a commonly-used tool among managers to improve wetland health. The focus of revegetation in rain-filled wetlands is usually on fringing terrestrial vegetation, whereas aquatic vegetation usually regenerates from the seed bank or propagules carried by wetland birds. However, revegetation does not necessarily include all components of the original vegetation community even if the ‘original’ components are known, and may also be outcompeted by exotic plant species if weed management is not also undertaken.
Although less is known about regeneration from the riparian seed bank, riparian zones are usually the focus of wetland revegetation. The seed bank, which may be diverse and abundant in germinable seeds, may only restore a portion of the riparian community (Williams et al. 2008). Revegetation ensures representation of all components of the riparian zone vegetation community, and prevents exotic species from germinating.
Competing demands of management goals
Wetland managers are frequently required to make decisions with little scientific evidence for the mechanisms by which management actions will improve wetland health or how these management actions interact. A management action may have multiple outcomes, and both positive and negative effects on wetland C sequestration and storage (Fig. 1). For example, stock exclusion may affect C storage potential positively through increased vegetation biomass and improved soil condition, but the resulting weed proliferation may affect C quality and overall wetland condition negatively.
Integrated management actions are likely needed to improve wetland C storage potential. For example, Jansen and Robertson (2005) suggested that stock exclusion alone will not return riparian function and biodiversity to pre-grazing conditions, but must be done in conjunction with revegetation activities and weed control. Similarly, stock exclusion alone may have little effect in areas where weeds and non-domesticated grazers are abundant. Consequently, wetland managers must assess wetlands individually and tailor management actions accordingly to incorporate the threats and opportunities for increasing C storage for individual wetlands.
Australian arid and semi-arid rain-filled wetlands could partially mitigate the effects of increased atmospheric C if managed appropriately. The potential for active management of these wetlands for C capture and storage has not been thoroughly explored, despite the likely far-reaching role of wetlands in mitigating the effects of climate change. Additionally, several knowledge gaps clearly still exist. Given the intensive nature of agricultural activities in areas where rain-filled wetlands are common (e.g. inland NSW), further work is needed to increase our understanding of the effects of these activities on wetland C capture and storage. Key knowledge gaps relating to the effect of management actions on wetland C sequestration include: (a) the benefits of integrated wetland management; (b) the appropriateness of different grazing regimes and the effect of total grazing pressure; (c) the effects of fire; and (d) the extent to which wetland function (C storage) can be restored following agricultural activities, such as cropping.
Acknowledgements
This paper was prepared as part of the Murray Wetland Carbon Storage Project. The project was funded by the Australian Government. We thank Dr Patricia Bowen (Murray Local Land Services) and Ms Rhonda Sinclair (Murray Darling Wetlands Working Group Ltd) for useful discussions on this paper. We also thank Dr Bowen for constructive comments on an earlier version of this manuscript.
References
Adhikari, S., Bajracharaya, R. M., and Sitaula, B. K. (2009). A review of carbon dynamics and sequestration in wetlands. Journal of Wetlands Ecology 2, 42–46.| A review of carbon dynamics and sequestration in wetlands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFeru7rJ&md5=0e6592f48514c0597d493c36926b190dCAS |
Agouridis, C. T., Workman, S. R., Warner, R. C., and Jennings, G. D. (2005). Livestock grazing management impacts on stream water quality: a review. Journal of the American Water Resources Association 41, 591–606.
| Livestock grazing management impacts on stream water quality: a review.Crossref | GoogleScholarGoogle Scholar |
Baldwin, D. S., Rees, G. N., Wilson, J. S., Colloff, M. J., Whitworth, K. L., Pitman, T. L., and Wallace, T. A. (2013). Provisioning of bioavailable carbon between the wet and dry phases in a semi-arid floodplain. Oecologia 172, 539–550.
| Provisioning of bioavailable carbon between the wet and dry phases in a semi-arid floodplain.Crossref | GoogleScholarGoogle Scholar |
Baldwin, D. S., Colloff, M. J., Mitrovic, S. M., Bond, N. R., and Wolfenden, B. (2016). Restoring dissolved organic carbon subsidies from floodplains to lowland river food webs: a role for environmental flows? Marine & Freshwater Research 67, 1387–1399.
| Restoring dissolved organic carbon subsidies from floodplains to lowland river food webs: a role for environmental flows?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xhtl2ksb7K&md5=bfe07ceb0e6e4d237f0f39de5c2afd30CAS |
Batzer, D. P., Jackson, C. R., and Mosner, M. (2000). Influences of riparian logging on plants and invertebrates in small, depressional wetlands of Georgia, USA. Hydrobiologia 441, 123–132.
| Influences of riparian logging on plants and invertebrates in small, depressional wetlands of Georgia, USA.Crossref | GoogleScholarGoogle Scholar |
Belsky, A., Matzke, A., and Uselman, S. (1999). Survey of livestock influences on stream and riparian ecosystems in the western United States. Journal of Soil and Water Conservation 54, 419–431.
Bernal, B., and Mitsch, W. J. (2012). Comparing carbon sequestration in temperate freshwater wetland communities. Global Change Biology 18, 1636–1647.
| Comparing carbon sequestration in temperate freshwater wetland communities.Crossref | GoogleScholarGoogle Scholar |
Boulding, A., and Baldwin, D. (2006). Impacts of domestic grazing animals on the water quality of Lake Hume – a desktop analysis. Report prepared for Goulburn-Murray Water. Murray–Darling Freshwater Research Centre, Wodonga.
Bowen, P. M. (2010). Wetlands of the Murray Catchment: an inventory of wetlands of the NSW Murray Catchment. Murray Catchment Management Authority, NSW.
Brock, M. A. (2011). Persistence of seed banks in Australian temporary wetlands. Freshwater Biology 56, 1312–1327.
| Persistence of seed banks in Australian temporary wetlands.Crossref | GoogleScholarGoogle Scholar |
Bunn, S. E., and Arthington, A. H. (2002). Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30, 492–507.
| Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity.Crossref | GoogleScholarGoogle Scholar |
Campbell, I. C., and Fuchshuber, L. (1995). Polyphenols, condensed tannins, and processing rates of tropical and temperate leaves in an Australian stream. Journal of the North American Benthological Society 14, 174–182.
| Polyphenols, condensed tannins, and processing rates of tropical and temperate leaves in an Australian stream.Crossref | GoogleScholarGoogle Scholar |
Casanova, M. T. (2012). Does cereal crop agriculture in dry swamps damage aquatic plant communities? Aquatic Botany 103, 54–59.
| Does cereal crop agriculture in dry swamps damage aquatic plant communities?Crossref | GoogleScholarGoogle Scholar |
Casanova, M., and Brock, M. A. (2000). How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecology 147, 237–250.
| How do depth, duration and frequency of flooding influence the establishment of wetland plant communities?Crossref | GoogleScholarGoogle Scholar |
Danone Fund for Nature (2010). Achieving carbon offsets through mangroves and other wetlands. Expert Workshop Meeting Report. Danone Group/ IUCN/ Ramsar Convention Secretariat, Gland, Switzerland.
Davis, J., O’Grady, A. P., Dale, A., Arthington, A. H., Gell, P. A., Driver, P. D., Bond, N., Casanova, M., Finlayson, M., and Watts, R. J. (2015). When trends intersect: the challenge of protecting freshwater ecosystems under multiple land use and hydrological intensification scenarios. The Science of the Total Environment 534, 65–78.
| When trends intersect: the challenge of protecting freshwater ecosystems under multiple land use and hydrological intensification scenarios.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXlvVSitLk%3D&md5=f23700d9c5aa4be2d2e20f4d3542ece3CAS |
Department of Environment and Primary Industries (2013). ‘Managing Grazing on Riparian Land. Decision Support Tool and Guidelines.’ (The State of Victoria Department of Environment and Primary Industries: East Melbourne.)
Department of Sustainability, Environment, Water, Population and Communities (2012). ‘The Role of Wetlands in the Carbon Cycle.’ (Commonwealth of Australia: Canberra.)
Doupé, R. G., Mitchell, J., Knott, M. J., Davis, A. M., and Lymbery, A. J. (2010). Efficacy of exclusion fencing to protect ephemeral floodplain lagoon habitats from feral pigs (Sus scrofa). Wetlands Ecology and Management 18, 69–78.
| Efficacy of exclusion fencing to protect ephemeral floodplain lagoon habitats from feral pigs (Sus scrofa).Crossref | GoogleScholarGoogle Scholar |
Fisher, A., Hunt, L., James, C., Landsberg, J., Phelps, D., Smyth, A., and Watson, I. (2004). Management of total grazing pressure: managing for biodiversity in the rangelands. Report prepared for Australian Department of the Environment and Heritage. Desert Knowledge CRC and Tropical Savannas CRC, Alice Springs.
Greenwood, K., and McKenzie, B. (2001). Grazing effects on soil physical properties and the consequences for pastures: a review. Animal Production Science 41, 1231–1250.
| Grazing effects on soil physical properties and the consequences for pastures: a review.Crossref | GoogleScholarGoogle Scholar |
Holmes, S., Speirs, S., Berney, P., and Rose, H. (2009). Guidelines for grazing in the Gwydir Wetlands and Maquarie Marshes. Report prepared for the NSW Wetland Recovery Program. NSW Department of Primary Industries, Albury.
Howell, J., and Benson, D. (2000). Predicting potential impacts of environmental flows on weedy riparian vegetation of the Hawkesbury–Nepean River, south-eastern Australia. Austral Ecology 25, 463–475.
| Predicting potential impacts of environmental flows on weedy riparian vegetation of the Hawkesbury–Nepean River, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Iles, J., Kelleway, J., Kobayashi, T., Mazumder, D., Knowles, L., Priddel, D., and Saintilan, N. (2010). Grazing kangaroos act as local recyclers of energy on semiarid floodplains. Australian Journal of Zoology 58, 145–149.
| Grazing kangaroos act as local recyclers of energy on semiarid floodplains.Crossref | GoogleScholarGoogle Scholar |
Jansen, A., and Robertson, A. I. (2001). Relationships between livestock management and the ecological condition of riparian habitats along an Australian floodplain river. Journal of Applied Ecology 38, 63–75.
| Relationships between livestock management and the ecological condition of riparian habitats along an Australian floodplain river.Crossref | GoogleScholarGoogle Scholar |
Jansen, A., and Robertson, A. I. (2005). Grazing, ecological condition and biodiversity in riparian river red gum forests in south-eastern Australia. Proceedings of the Royal Society of Victoria 117, 85–95.
Keeley, J. E., and Zedler, P. H. (1998). Characterization and global distribution of vernal pools. In: ‘Proceedings from the 1996 Conference, Ecology, Conservation, and Management of Vernal Pool Ecosystems’. (Eds C. W. Witham, E. T. Bauder, D. Belk, W. R. Ferren Jr. and R. Ornduff.) pp. 1–14. (California Native Plant Society: Sacramento, CA.)
Kingsford, R. T., Brandis, K., Thomas, R. F., Crighton, P., Knowles, E., and Gale, E. (2003). ‘The Distribution of Wetlands in New South Wales.’ (Natural Heritage Trust, National Parks & Wildlife Service, Murray-Darling Basin Commission: Hurstville.)
Kobayashi, T., Ryder, D., Gordon, G., Shannon, I., Ingleton, T., Carpenter, M., and Jacobs, S. (2009). Short-term response of nutrients, carbon and planktonic microbial communities to floodplain wetland inundation. Aquatic Ecology 43, 843–858.
| Short-term response of nutrients, carbon and planktonic microbial communities to floodplain wetland inundation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsF2qu7fF&md5=2bf7b96c3480338d2198d79ed35d6baeCAS |
Kotze, D. (2013). The effects of fire on wetland structure and functioning. African Journal of Aquatic Science 38, 237–247.
| The effects of fire on wetland structure and functioning.Crossref | GoogleScholarGoogle Scholar |
Lawrence, A., Baker, E., and Lovelock, C. (2012). Optimising and managing coastal carbon: comparative sequestration and mitigation opportunities across Australia’s landscapes and land uses. Report prepared for Fisheries Research and Development Corporation. FRDC Report 2011/084.
Leff, L. G., and McArthur, V. J. (1990). Effect of nutrient content on leaf decomposition in a coastal plain stream: a comparison of green and senescent leaves. Journal of Freshwater Ecology 5, 269–277.
| Effect of nutrient content on leaf decomposition in a coastal plain stream: a comparison of green and senescent leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXitFersr0%3D&md5=35ea90a343daa09b685ed9191bf5d9dbCAS |
Livesley, S. J., Grover, S., Hutley, L. B., Jamali, H., Butterbach-Bahl, K., Fest, B., Beringer, J., and Arndt, S. K. (2011). Seasonal variation and fire effects on CH4, N2O and CO2 exchange in savanna soils of northern Australia. Agricultural and Forest Meteorology 151, 1440–1452.
| Seasonal variation and fire effects on CH4, N2O and CO2 exchange in savanna soils of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Lunt, I. D., Eldridge, D. J., Morgan, J. W., and Witt, G. B. (2007). Turner Review No. 13. A framework to predict the effects of livestock grazing and grazing exclusion on conservation values in natural ecosystems in Australia. Australian Journal of Botany 55, 401–415.
| Turner Review No. 13. A framework to predict the effects of livestock grazing and grazing exclusion on conservation values in natural ecosystems in Australia.Crossref | GoogleScholarGoogle Scholar |
Lunt, I. D., Jansen, A., and Binns, D. L. (2012). Effects of flood timing and livestock grazing on exotic annual plants in riverine floodplains. Journal of Applied Ecology 49, 1131–1139.
| Effects of flood timing and livestock grazing on exotic annual plants in riverine floodplains.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R., Cunningham, S. C., Baker, P. J., Horner, G. J., and Thomson, J. R. (2011). Dynamics of Murray‐Darling floodplain forests under multiple stressors: the past, present, and future of an Australian icon. Water Resources Research 47, W00G05.
| Dynamics of Murray‐Darling floodplain forests under multiple stressors: the past, present, and future of an Australian icon.Crossref | GoogleScholarGoogle Scholar |
Marty, J. T. (2005). Effects of cattle grazing on diversity in ephemeral wetlands. Conservation Biology 19, 1626–1632.
| Effects of cattle grazing on diversity in ephemeral wetlands.Crossref | GoogleScholarGoogle Scholar |
Mcleod, E., Chmura, G. L., Bouillon, S., Salm, R., Björk, M., Duarte, C. M., Lovelock, C. E., Schlesinger, W. H., and Silliman, B. R. (2011). A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment 9, 552–560.
| A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2.Crossref | GoogleScholarGoogle Scholar |
Miller, J., Chanasyk, D., Curtis, T., and Willms, W. (2010). Influence of streambank fencing on the environmental quality of cattle-excluded pastures. Journal of Environmental Quality 39, 991–1000.
| Influence of streambank fencing on the environmental quality of cattle-excluded pastures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXls1ams7k%3D&md5=bf1ec8fd2a2471b038989f2b61058acbCAS |
Morris, K., and Reich, P. (2013). Understanding the relationship between livestock grazing and wetland condition. Arthur Rylah Institute for Environmental Research Technical Report Series No. 252. Department of Environment and Primary Industry, Heidelberg, Victoria.
NSW Department of Primary Industries (2016). Using DSEs and carrying capacities to compare sheep enterprises. Available at: www.dpi.nsw.gov.au/content/agriculture/farm-business/budgets/livestock/sheep/background/dse (accessed 19 December 2016).
O’Connell, M., Baldwin, D. S., Robertson, A., and Rees, G. (2000). Release and bioavailability of dissolved organic matter from floodplain litter: influence of origin and oxygen levels. Freshwater Biology 45, 333–342.
| Release and bioavailability of dissolved organic matter from floodplain litter: influence of origin and oxygen levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXos1Omu7g%3D&md5=699c5b21d88e279528f913f21b1e6f19CAS |
Paynter, Q., and Flanagan, G. J. (2004). Integrating herbicide and mechanical control treatments with fire and biological control to manage an invasive wetland shrub, Mimosa pigra. Journal of Applied Ecology 41, 615–629.
| Integrating herbicide and mechanical control treatments with fire and biological control to manage an invasive wetland shrub, Mimosa pigra.Crossref | GoogleScholarGoogle Scholar |
Post, D. M., Taylor, J. P., Kitchell, J. F., Olson, M. H., Schindler, D. E., and Herwig, B. R. (1998). The role of migratory waterfowl as nutrient vectors in a managed wetland. Conservation Biology 12, 910–920.
| The role of migratory waterfowl as nutrient vectors in a managed wetland.Crossref | GoogleScholarGoogle Scholar |
Price, J. N., Whalley, R. D. B., van Klinken, R. D., Duggin, J. A., and Gross, C. L. (2011). Periodic rest from grazing provided no control of an invasive perennial forb. The Rangeland Journal 33, 287–298.
| Periodic rest from grazing provided no control of an invasive perennial forb.Crossref | GoogleScholarGoogle Scholar |
Prober, S. M., Thiele, K. R., Lunt, I. D., and Koen, T. (2005). Restoring ecological function in temperate grassy woodlands: manipulating soil nutrients, exotic annuals and native perennial grasses through carbon supplements and spring burns. Journal of Applied Ecology 42, 1073–1085.
| Restoring ecological function in temperate grassy woodlands: manipulating soil nutrients, exotic annuals and native perennial grasses through carbon supplements and spring burns.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xkt1aiuw%3D%3D&md5=18bf069feb439aef4f19f01339d30c75CAS |
Queensland Government (2016). Para grass. Available at: www.business.qld.gov.au/industry/agriculture/species/invasive-plants/other/para-grass (accessed 19 December 2016).
Robertson, A. (1997). Land-water linkages in floodplain river systems: the influence of domestic stock. In: ‘Frontiers in Ecology: Building the Links’. (Eds N. Klomp and I. D. Lunt.) pp. 207–218. (Elsevier Science: Oxford, UK.)
Schulze, D. J., and Walker, K. F. (1997). Riparian eucalypts and willows and their significance for aquatic invertebrates in the River Murray, South Australia. Regulated Rivers: Research and Management 13, 557–577.
| Riparian eucalypts and willows and their significance for aquatic invertebrates in the River Murray, South Australia.Crossref | GoogleScholarGoogle Scholar |
Seddon, J. A., and Briggs, S. V. (1998). Lakes and lakebed cropping in the western division of New South Wales. The Rangeland Journal 20, 237–254.
| Lakes and lakebed cropping in the western division of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Sweeney, B. W. (1993). Effects of streamside vegetation on macroinvertebrate communities of White Clay Creek in eastern North-America. Proceedings of the Academy of Natural Sciences of Philadelphia 144, 291–340.
Thomsen, M., Brownell, K., Groshek, M., and Kirsch, E. (2012). Control of reed canarygrass promotes wetland herb and tree seedling establishment in an upper Mississippi River floodplain forest. Wetlands 32, 543–555.
| Control of reed canarygrass promotes wetland herb and tree seedling establishment in an upper Mississippi River floodplain forest.Crossref | GoogleScholarGoogle Scholar |
Tiver, F., and Andrew, M. H. (1997). Relative effects of herbivory by sheep, rabbits, goats and kangaroos on recruitment and regeneration of shrubs and trees in eastern South Australia. Journal of Applied Ecology 34, 903–914.
| Relative effects of herbivory by sheep, rabbits, goats and kangaroos on recruitment and regeneration of shrubs and trees in eastern South Australia.Crossref | GoogleScholarGoogle Scholar |
UNEP and Center for International Forestry Research (2014). Guiding principles for delivering coastal wetland carbon projects. United Nations Environment Programme, Nairobi, Kenya and Center for International Forestry Research, Bogor, Indonesia.
Walker, J., Bullen, F., and Williams, B. G. (1993). Ecohydrological changes in the Murray-Darling Basin. I. The number of trees cleared over two centuries. Journal of Applied Ecology 30, 265–273.
| Ecohydrological changes in the Murray-Darling Basin. I. The number of trees cleared over two centuries.Crossref | GoogleScholarGoogle Scholar |
Waudby, H. P., Petit, S., and Brown, G. (2013). Use of creeks and gilgaied stony plains by cattle in arid rangelands during a wet summer: a case study with GPS/VHF radio collars. Range Management and Agroforestry 34, 101–107.
Webster, J., and Benfield, E. (1986). Vascular plant breakdown in freshwater ecosystems. Annual Review of Ecology and Systematics 17, 567–594.
| Vascular plant breakdown in freshwater ecosystems.Crossref | GoogleScholarGoogle Scholar |
Williams, L., Reich, P., Capon, S. J., and Raulings, E. (2008). Soil seed banks of degraded riparian zones in southeastern Australia and their potential contribution to the restoration of understorey vegetation. River Research and Applications 24, 1002–1017.
| Soil seed banks of degraded riparian zones in southeastern Australia and their potential contribution to the restoration of understorey vegetation.Crossref | GoogleScholarGoogle Scholar |
Wilson, J., Baldwin, D., Rees, G., and Wilson, B. (2011). The effects of short‐term inundation on carbon dynamics, microbial community structure and microbial activity in floodplain soil. River Research and Applications 27, 213–225.
| The effects of short‐term inundation on carbon dynamics, microbial community structure and microbial activity in floodplain soil.Crossref | GoogleScholarGoogle Scholar |
Zech, W., Senesi, N., Guggenberger, G., Kaiser, K., Lehmann, J., Miano, T. M., Miltner, A., and Schroth, G. (1997). Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 79, 117–161.
| Factors controlling humification and mineralization of soil organic matter in the tropics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXns1Cmu7Y%3D&md5=1e37c004f2e2710809dc6cc1e2fa32e6CAS |