Effects of prepartum oilseed supplements on subclinical endometritis, pro- and anti-inflammatory cytokine transcripts in endometrial cells and postpartum ovarian function in dairy cows
Reza Salehi A , Marcos G. Colazo B , Mohanathas Gobikrushanth A , Urmila Basu A and Divakar J. Ambrose A B CA Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta T6G 2P5, Canada.
B Livestock Research Branch, Alberta Agriculture and Forestry, Suite 307, 7000 – 113 Street NW, Edmonton, Alberta T6H 5T6, Canada.
C Corresponding author. Email: divakar.ambrose@gov.ab.ca
Reproduction, Fertility and Development 29(4) 747-758 https://doi.org/10.1071/RD15334
Submitted: 18 August 2015 Accepted: 25 November 2015 Published: 14 January 2016
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Postpartum uterine infections affect ovarian function and delay ovulation in cattle. As dietary fats can affect immune cell function, we investigated the influence of prepartum diets on postpartum uterine inflammatory status (UIS) as assessed 25 ± 1 days postpartum by endometrial cytology (normal: ≤8% polymorphonuclear cells (PMN) vs subclinical endometritis (SCE): >8% PMN) and associations between SCE, pro- and anti-inflammatory cytokine gene expression and ovarian function. During the last 5 weeks of gestation, dairy cows received a diet supplemented with 8% rolled sunflower (n = 10) or canola seed (n = 9) or no oilseed (n = 9). Ovaries were scanned until 35 days postpartum. Prepartum diets did not influence SCE, but a preovulatory-size follicle developed sooner (P ≤ 0.05), the interval to first ovulation was shorter and the proportion of cows ovulating within 35 days postpartum was greater in the sunflower seed group. Although mRNA expression of cytokines was not affected by diet, cows with SCE had higher (P ≤ 0.05) expression of interleukin-1β (IL1B), interleukin-8 (CXCL8), IL10 and tumour necrosis factor-α (TNF) than normal cows. The interval (mean ± s.e.m.) from calving to preovulatory-size follicle was shorter (P ≤ 0.05) in normal (13.2 ± 0.9 days) than SCE cows (18.7 ± 1.4 days). In summary, a prepartum diet supplemented with sunflower seed positively influenced postpartum ovarian function without affecting UIS or pro- and anti-inflammatory cytokine gene expression in endometrial cells.
Additional keywords: endometrium, fatty acid, gene expression, inflammation, ovulation.
Introduction
Delayed resumption of ovarian cyclicity in postpartum dairy cows contributes to significant economic losses (Opsomer et al. 2000). Uterine infections during the postpartum period affect ovarian follicular development and function and eventually delay ovulation in cattle (Dourey et al. 2011; Ambrose 2015). Inflammatory responses induced by bacteria during the early postpartum period cause perturbation of hypothalamic, pituitary and ovarian function, which, in turn, may contribute to impaired fertility of cows affected with uterine infection (Sheldon et al. 2009). The first response of the innate immune system is the invasion of polymorphonuclear (PMN) cells to the site of bacterial infection, i.e. the endometrium in the case of metritis and endometritis. The presence of PMN is used to diagnose subclinical endometritis (SCE; Kasimanickam et al. 2004; Gilbert et al. 2005), which is defined as an inflammation of the endometrium in clinically healthy cows, i.e. cows with no cervical or vulvar discharge. Dourey et al. (2011) found that cows with >8% PMN, when endometrial cytology was performed 25 days postpartum, had a longer interval from calving to first ovulation (45 vs 32 days) and tended to have fewer cumulative pregnancies by 270 days after calving (58 vs 80%) compared with those cows with ≤8% PMN.
Both the level of fat and the types of fatty acids present in the diet can affect immune cell functions. The fatty-acid composition of lymphocytes and other immune cells is altered according to dietary fatty-acid composition, which, in turn, alters their function. Schroit and Gallily (1979) demonstrated that macrophage phagocytic activity was enhanced when the cells were cultured with polyunsaturated fatty acids (linoleic, α-linolenic and arachidonic acids). Furthermore, significant decreases in phagocytic activity were observed in macrophages grown in the presence monounsaturated fatty acids (oleic and elaidic acids; Schroit and Gallily 1979). However, feeding a diet supplemented with rolled canola (high in oleic acid), linola (high in linoleic acid) or flax seed (high in α-linolenic acid) to dairy cows for up to 5 weeks in the immediate prepartum period did not affect postpartum uterine infection, but cows fed canola seed had delayed resumption of ovarian cyclicity (Colazo et al. 2009).
Silvestre et al. (2011) studied the effects of different types of fatty acids on postpartum immune function in dairy cows and found that feeding a diet enriched in linoleic acid during the transition period induced a pro-inflammatory state during the first week postpartum as illustrated by increased neutrophil expression of adhesion molecules, production of cytokines (tumour necrosis factor-α (TNF) and interleukin-1β (IL1B)), enhanced bactericidal activity and increased circulating acute-phase proteins. In addition, higher expression of pro-inflammatory cytokines (TNF and IL1B) in the endometrium during the first week after calving improved the activation of inflammation and clearance of bacteria, leading to reduction in the incidence of SCE, defined as cows with >10% PMN, at 5 weeks after parturition (Galvão et al. 2011).
In the present study, we hypothesised that feeding a diet supplemented with sunflower seed (high in linoleic acid) during the prepartum period would induce a pro-inflammatory state in the endometrium of cows during the early postpartum period, resulting in improved uterine health and positive effects on ovarian function. Canola (high in oleic acid), the main oilseed produced in Canada (Canadian Grain Commission Government of Canada 2014), was chosen for comparison because it is commonly included in dairy cow rations yet little is known about its effects on ovarian function in dairy cattle.
The specific objectives were to: (1) determine the effect of prepartum diets supplemented with rolled sunflower or canola seed on early postpartum uterine inflammatory status (UIS), pro- and anti-inflammatory cytokine gene expression and ovarian function and (2) further investigate the associations between UIS, pro- and anti-inflammatory cytokine gene expression and ovarian function in dairy cows.
Materials and methods
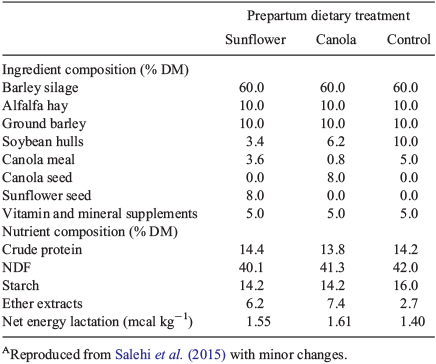
Twenty-eight non-lactating pregnant Holstein cows, parity 1 to 5 (12 primiparous, 16 multiparous), were used in the study. Approximately 35 days before the expected calving date (Week 5), cows were blocked by body condition score (BCS), parity and expected calving date and assigned to one of three dietary treatments containing oilseeds (either sunflower (high in linoleic acid, n = 10) or canola (high in oleic acid, n = 9)) or no oilseed (control, n = 9). Diets were offered ad libitum as a total mixed ration containing forage (alfalfa hay and barley silage) and concentrates (Table 1). Cows were fed a diet supplemented with 8% rolled oilseeds on dry-matter basis. Oilseeds were rolled as described previously (Ambrose et al. 2006) before incorporation in the diet. Upon calving, cows were placed on a common ration containing alfalfa hay, barley silage and concentrate balanced for a lactating dairy cow of 690 kg bodyweight (BW), producing 45 kg milk per day, according to National Research Council Committee on Animal Nutrition (2001) guidelines. The study was conducted at the Dairy Research and Technology Center of the University of Alberta, Edmonton, Canada, with all animal experimental procedures approved by the University of Alberta Animal Care and Use Committee (Protocol # AUP00000131). Cows in the study were cared for according to the Canadian Council on Animal Care Guidelines on the Care and Use of Farm Animals in Research, Teaching and Testing (2009).
|
Ultrasonography and endometrial cytology
Ovarian follicular development was monitored by transrectal ultrasonography with a real-time, B-mode scanner (MicroMAXX, colour Doppler scanner equipped with a multifrequency 5 to 10 MHz linear transducer; SonoSite Inc., Bothell, WA, USA). Ovaries were examined twice weekly from 7 ± 1 days after calving until ovulation or 35 days after calving, whichever occurred first. We recorded the intervals from calving to first appearance of a 10-mm follicle (referred to as dominant follicle in this paper) and that of a 16-mm follicle, the mean diameter of an ovulatory follicle in dairy cows (Colazo et al. 2015), which is referred to as a preovulatory-size follicle in this paper. Ovulation was confirmed by the absence of a large (≥10 mm diameter) follicle that had been detected at the previous examination and by subsequent corpus luteum formation (Pierson and Ginther 1984).
On Day 25 ± 1 postpartum two successive cytological samples were obtained from the uterine body of all cows using a cytobrush (Medscand Medical, Malmö, Sweden) modified for use in large animals as described by Kasimanickam et al. (2005). Endometrial cytological samples were collected by rotating the cytobrush approximately one-half turn to obtain cellular material while maintaining gentle contact with the wall of the uterine body. Upon completion of the sampling, the cytobrush was withdrawn into its protective sheath and the instrument removed from the uterus. One sample was used to prepare endometrial cytological smears by rolling the cytobrush on each of two clean glass slides and then fixing with a cytofixative alcohol spray (Fisher Scientific Co., Pittsburgh, PA, USA). The slides were then brought to the laboratory and stained with modified Giemsa stain (Ricca Chemical Co., Arlington, VA, USA) for 2 min, washed in distilled water, dried and stored until evaluation under 400× magnification. Cells (300–400) were counted for each cow (two slides) to calculate PMN%. Cows with >8% PMN in the cytological sample were defined as SCE and those with ≤8% PMN were defined as normal. Immediately after collection, the second endometrial cytological sample was placed in a 2-mL tube containing 1.5 mL RNA Later, kept at 4°C for 24 h and then stored at –30°C until RNA extraction.
After a voluntary waiting period of 75 ± 3 days postpartum, ovulation was synchronised with a Presynch/Ovsynch protocol for timed artificial insemination (TAI; Moreira et al. 2001) in all cows. Pregnancy diagnosis was performed by transrectal ultrasonography 32 days after TAI.
RNA extraction
Total RNA was extracted from endometrial cells with Trizol reagent (Life Technologies, Burlington, ON, Canada) according to the manufacturer’s instructions. Briefly, after adding 1 mL Trizol, samples were homogenised and kept in Trizol at –20°C overnight. Thereafter, samples were incubated at 25°C for 20 min and then centrifuged (12 000g for 10 min at 4°C). The supernatant was transferred to a new tube and 200 µL chloroform was added, vortexed and incubated at 25°C for 2 min followed by centrifugation at 12 000g for 15 min at 4°C. The clear phase was then transferred to a fresh tube and RNA was precipitated by mixing with 550 µL isopropyl alcohol, incubated at 25°C for 15 min and centrifuged (12 000g for 10 min at 4°C). After centrifugation, supernatant was discarded and the pellet washed with 75% ethanol and centrifuged at 7700g for 3 min at 4°C. The final RNA pellet was dried for 10 min at room temperature and then dissolved in 100 µL of RNase-free water by repetitive gentle pipetting. The RNA yield and purity was measured with a spectrophotometer (ND-1000; Nanodrop Technologies, Wilmington, DE, USA). The quality of RNA was determined by automated electrophoresis (2200 TapeStation; Agilent Technologies Canada Inc., Mississauga, ON, Canada).
Complementary DNA synthesis and real-time polymerase chain reaction
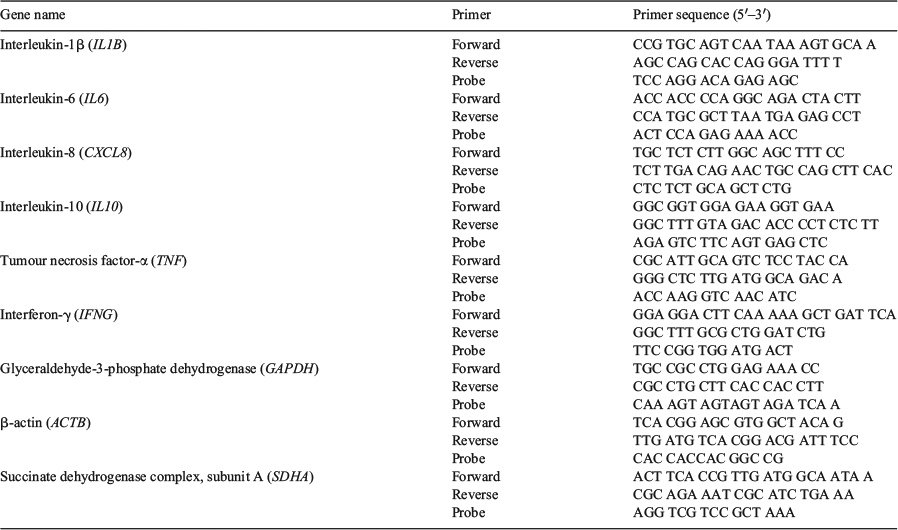
RNA was treated with DNase (QIAGEN, Toronto, ON, Canada) and then cDNA was generated by MultiScribe reverse transcription (RT; Life Technologies) and random primers (Life Technologies) adding 4 µL 10× RT buffer, 1.6 µL 25× dNTP mix, 4 µL 10× RT random primer, 2 µL MultiScribe reverse transcriptase, 2 µL RNase inhibitor and 6.4 µL nuclease-free water to 20 μL of RNA diluted with nuclease-free water, containing 1 μg total RNA. Polymerase chain reaction (PCR) incubation was performed based on the following program: 25°C for 10 min, 37°C for 120 min, 85°C for 5 min and then kept at 4°C. Real-time PCR was performed as described previously (Laarman et al. 2012; Schlau et al. 2012) with Taqman Fast Universal Master Mix (Life Technologies) in a StepOnePlus Real-Time PCR System (Life Technologies). Primers and probes were designed (Table 2) using Primer Express Version 3.0 (Life Technologies) and analysed by BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi, verified 2 December 2015) to verify primer specificity. Primer efficiency was determined using the standard-curve method (with at least five serial dilutions) in a pool of all samples.
|
The mRNA abundance of target genes was normalised using the geometric mean of three reference genes (glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin (ACTB) and succinate dehydrogenase complex, subunit A (SDHA)) as described previously (Vandesompele et al. 2002; Laarman et al. 2012; Schlau et al. 2012).
In the present study, mRNA expression of interleukin-1β (IL1B), IL6, interleukin-8 (CXCL8), tumour necrosis factor-α (TNF) and interferon-γ (IFNG) were evaluated as pro-inflammatory cytokines and IL10 as an anti-inflammatory cytokine. Additionally, the ratio of pro-inflammatory cytokines to IL10 (anti-inflammatory) was evaluated to consider inflammatory balance.
Statistical analyses
The abundance of mRNA expression, intervals (in days) from calving to the development of 10-mm (dominant) and 16-mm (preovulatory-size) follicles were analysed using the MIXED procedure of SAS (Version 9.3, 2011; SAS Institute Inc., Cary, NC, USA). The final statistical model included prepartum dietary treatment, UIS (normal (≤8% PMN) or SCE (>8% PMN)) and parity as the main effects and prepartum dietary treatment by UIS as interaction. We also defined animal as random effect. The interval to ovulation was evaluated with Kaplan–Meier survival analysis (LIFETEST procedure of SAS). Separate models were used including either dietary treatment or UIS, time (days) and ovulatory status (with the value 0 indicating censoring) to test for effects of diet and UIS. All data are reported as mean ± s.e.m.; probabilities <0.05 were considered to be significant, whereas P > 0.05 but < 0.10 were considered to be trends.
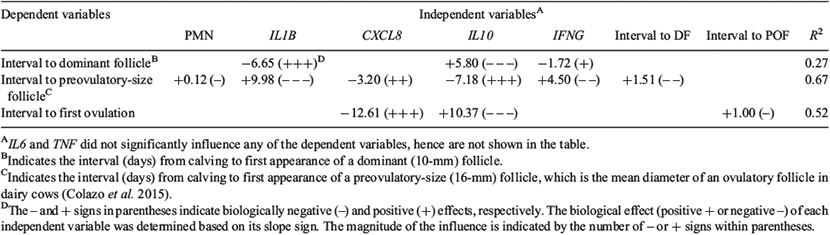
The proportion of normal cows (≤8% PMN) or those with SCE (>8% PMN) that ovulated or failed to ovulate within 35 days after calving and that conceived or failed to conceive were analysed using the GENMOD procedure of SAS. The final statistical model included prepartum dietary treatment or UIS as the main effect to evaluate prepartum dietary treatment or UIS effects, respectively. Moreover, model specifications included a binomial distribution and logit link function. Pearson’s test was used to calculate the correlation between the mRNA expression of cytokines and the percentage of PMN. Adjusted R2 analysis was used to select the effective factors among pro- and anti-inflammatory cytokines and PMN percentage (as independent variables) on interval from calving to formation of dominant follicle, preovulatory-size follicle or first ovulation (as dependent variables). Thereafter, multiple regression analysis was used to define a regression model to determine the importance and biological type effect of each selected independent variable on dependent variables.
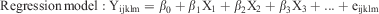
Yijklm = dependent variable (interval from calving to formation of dominant follicle, preovulatory-size follicle or first ovulation).
β0 = intercept.
X1, X2, X3, … Xn = independent variables (IL1B, IL6, CXCL8, IL10, IFNG and TNF and PMN percentage).
β1, β2, β3, … βn = slope, indicating how much change in dependent variable (Y) per unit change in independent variables (X).
The biological effect of each independent variable was defined as positive (+) or negative (–) based on whether it decreased or increased, respectively, the interval from calving to formation of a dominant or a preovulatory-size follicle or ovulation. Moreover, the intervals from calving to the formation of dominant and preovulatory-size follicles were defined as dependent variables for the interval to a preovulatory-size follicle and first ovulation, respectively.
Results
Influence of prepartum diet on uterine inflammatory status and ovarian follicle dynamics
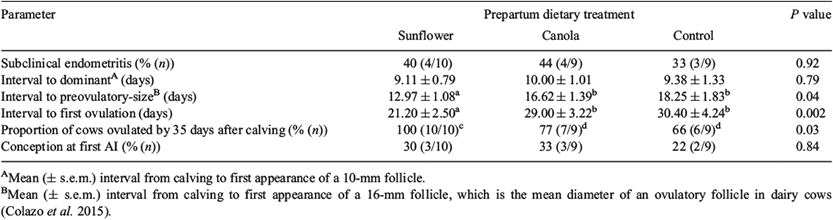
The duration of prepartum diets did not differ (P = 0.45) among the three treatment groups (sunflower, 37.3 ± 1.6; canola, 34.5 ± 1.6; control, 35.3 ± 1.6 days). Based on endometrial cytology, prepartum dietary treatments did not affect the proportion of cows with SCE (Table 3). Likewise, prepartum diets did not affect the interval from calving to the development of a dominant follicle. However, the mean intervals from calving to formation of a preovulatory-size follicle (Table 3) and ovulation (Fig. 1) were significantly shorter in cows fed a diet supplemented with sunflower seed than in those fed canola seed or control diets. Consequently, the proportion of cows ovulating by 35 days after calving was higher in cows fed a diet supplemented with sunflower seed than those fed canola or control diets (Table 3).
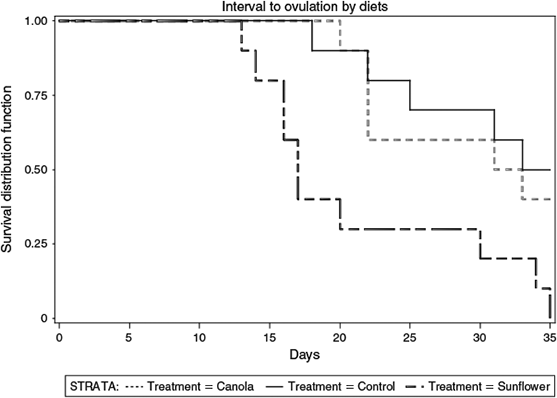
|
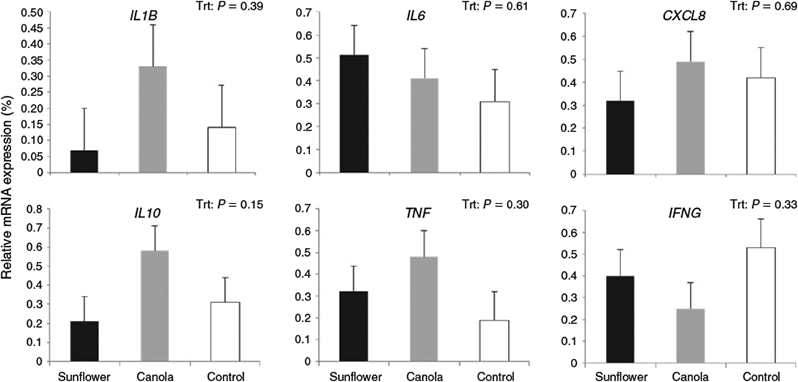
The mRNA expression of IL1B, IL6, CXCL8, IL10, TNF and IFNG was not affected either by prepartum dietary treatment (Fig. 2) or by the interaction between prepartum dietary treatment and uterine inflammatory status (data not shown). Moreover, the ratio of pro-inflammatory cytokines to IL10 (anti-inflammatory) was evaluated to consider inflammatory balance. Our results indicated that cows fed a prepartum diet supplemented with sunflower seed had a higher TNF : IL10 ratio than those fed canola seed or control. However, the mRNA expression ratios of IL1B, IL6, CXCL8 and IFNG to IL10 were not influenced by prepartum diet (Table 4).
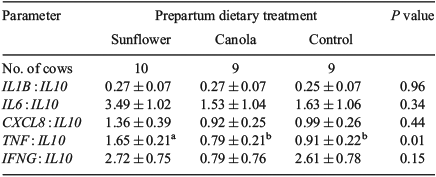
|
Influence of uterine inflammatory status based on PMN cells
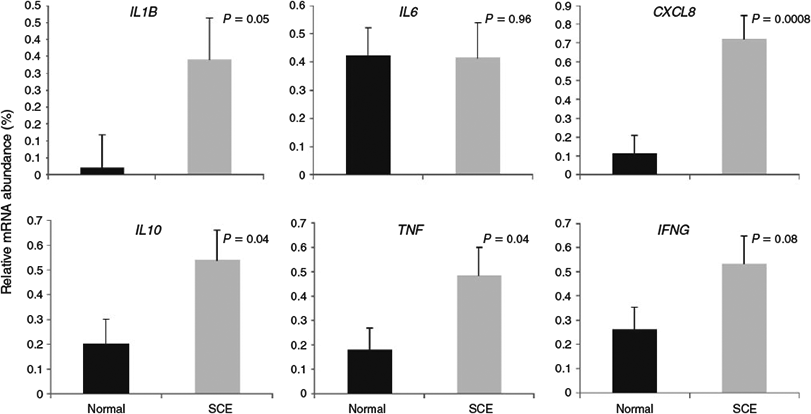
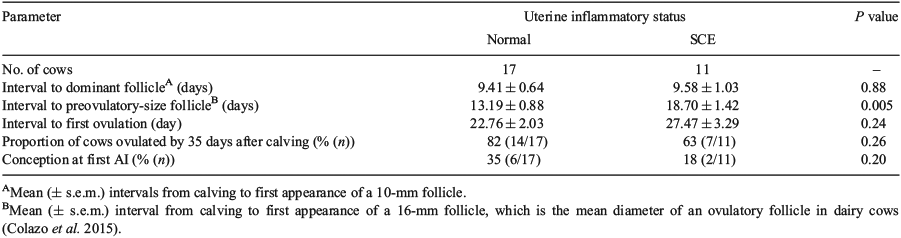
Uterine inflammatory status (based on percentage of PMN cells) was significantly associated with mRNA expression of cytokines (Fig. 3) and the interval from calving to establishment of a preovulatory-size follicle, but it did not affect the intervals from calving to formation of a dominant follicle and to first ovulation (Table 5). Cows with SCE had higher mRNA expression of IL1B, CXCL8, IL10, TNF and IFNG than normal cows; however, the mRNA expression of IL6 was not affected by uterine inflammatory status (Fig. 3). The ratio of pro-inflammatory cytokines to IL10 mRNA expression was also affected by uterine inflammatory status, with the ratio of IL1B and CXCL8 to IL10 being significantly higher in cows with SCE than in those considered to be normal (Table 6). The IL6 : IL10 ratio also tended (P = 0.09) to be higher in cows with SCE (Table 6).
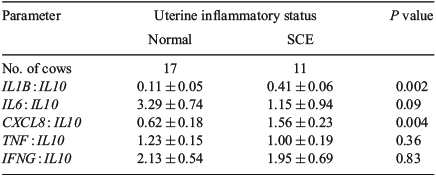
|
Correlation and multiple regression analysis
Correlations between the mRNA expression of cytokines and PMN percentage are presented in Table 7. The mRNA expression of IL1B, CXCL8, IL10 and TNF were correlated with each other. Moreover, CXCL8 and IL10 were correlated with the proportion of PMN, with the strongest correlation evident between PMN and CXCL8. TNF and IL1B mRNA expression also tended (P = 0.06) to be correlated with the proportion of PMN. In contrast, IL6 and IFNG mRNA expression had no correlation with other cytokines or PMN percentage.
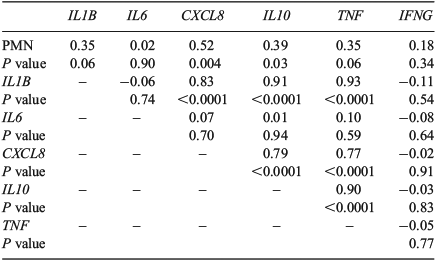
|
Multiple regression analysis indicated the importance and biological effect of each independent variable (selected pro- and anti-inflammatory cytokines and PMN percentage) on the dependent variables, i.e. intervals from calving to the formation of dominant or preovulatory-size follicles or first ovulation (Table 8). The regression model (e.g. interval to a dominant follicle = 9.53 – 6.65 IL1B + 5.80 IL10 – 1.72 IFNG) contains dependent variable (interval to a dominant follicle), intercept (9.53), independent variables (IL1B, IL10 and IFNG) and slopes (–6.65, +5.80 and –1.72 for IL1B, IL10 and IFNG, respectively). The slope indicates by how much the dependent variable (e.g. interval to a dominant follicle) changes per unit change in the independent variables (IL1B, IL10 and IFNG). We defined the biological effect (positive + or negative –) of each independent variable based on its slope sign. The independent variable has either a positive or negative biological effect based on whether it decreased or increased, respectively, the interval from calving to formation of dominant or preovulatory-size follicles or first ovulation. For example, the IL1B slope in the regression model for the interval to a dominant follicle was –6.65. The negative slope sign means that an increased expression of IL1B was associated with a reduction in the interval from calving to establishment of a dominant follicle; therefore, IL1B exerted a positive biological effect. The interval to the formation of a dominant follicle was associated with the mRNA expression of IL1B, IL10 and INFG; however, these factors collectively explained only 27% of the variation. The interval from calving to formation of a preovulatory-size follicle (interval to a preovulatory-size follicle = –0.60 + 9.98 IL1B – 3.20 CXCL8 – 7.18 IL10 + 4.50 IFNG + 0.12 PMN + 1.51 interval to a dominant follicle) was determined by IL1B, CXCL8, IL10, IFNG expression, PMN percentage and the interval to formation of dominant follicles. These factors explained 67% of variation. Furthermore, the predicted slope of IL1B and IL10 indicated that they are the most significant factors with negative and positive biological effects, respectively (Table 8). The least-associated factors were PMN and interval to a dominant follicle. The interval from calving to first ovulation (interval to ovulation = 9.16 – 12.61 CXCL8 + 10.37 IL10 + 1.00 interval to a preovulatory-size follicle) was mainly influenced by CXCL8 and IL10, with positive and negative biological effects, respectively, explaining 52% of variation. Out of these three factors, the interval to formation of a preovulatory-size follicle was the least-influential factor to determining interval to ovulation.
Discussion
Influence of prepartum diet on uterine inflammatory status and ovarian follicle dynamics
In the present study, the supplementation of sunflower seed prepartum did not affect the percentage of PMN in the uterus on Day 25 postpartum. Our results agree with those of Amaral (2008) who found that the concentrations of live white blood cells, dead white blood cells and cell viability in the uterine flushing at 37 ± 3 days postpartum did not differ among cows fed different fatty acid diets. Moreover, neutrophil concentration in uterine flushing, neutrophils as a proportion of total cells or as a proportion of white blood cells also did not differ. In that study (Amaral 2008), Holstein cows were fed a diet supplemented with calcium salts of safflower oil (high in linoleic acid), a mix of palm oil (high in saturated fatty acids) and fish oil (high in eicosapentaenoic and docosahexaenoic acids) or a control diet (no added fat) from 34 days before until 49 days after calving.
Prepartum dietary treatments per se did not have a significant effect on the mRNA expression of IL1B, IL6, CXCL8, IL10, TNF or INFG. A previous study (Renner et al. 2013) showed that adding linoleic acid to peripheral blood mononuclear cells did not alter concanavalin A-induced mRNA expression of IL4, IL10, IFNG and TNF. Amaral (2008) evaluated the production of concanavalin A-induced TNF-α and IFN-γ production by lymphocytes isolated at 10, 20 and 30 days postpartum from the blood of Holstein cows fed a control diet (no fat supplemented), diet supplemented with calcium salts of safflower oil or a mix of palm oil and fish oil from 34 days before to 49 days after calving and found that lymphocytes from cows fed supplemental fats had reduced secretion of TNF-α and IFN-γ compared with cows fed the control diet. Conversely, when the influence of fat supplementation (both oilseed diets combined) was compared against control (no oilseed diet) neither uterine inflammatory status nor the expression of IL1B, IL6, CXCL8, IL10, TNF or IFNG was affected in our study (data not shown). However, cows fed a diet supplemented with sunflower seed had a higher TNF : IL10 ratio than those fed canola or control, which is an indicator of greater inflammatory status in sunflower-fed cows postpartum. Silvestre et al. (2011) found that lipopolysaccharide (LPS)-induced TNF-α and IL-1β production increased in the culture medium of neutrophils collected at 35 days after calving from cows fed a diet supplemented with calcium salt of safflower oil compared with those fed palm oil. Discrepancies among experiments could be due to differences in the amount and source of fatty acids in the diets, physiological state of the animals and experimental model (in vitro vs in vivo).
In the present study, cows fed a prepartum diet supplemented with sunflower seed had a shorter interval to formation of a preovulatory-size follicle and first ovulation than those fed the control diet or canola seed. Also, a higher proportion of cows fed the sunflower seed-based diet ovulated within 35 days postpartum relative to cows fed control or canola diets during the prepartum period. Our results are in agreement with those of Colazo et al. (2009), who found that cows fed diets supplemented with linola (high in linoleic acid, comparable to sunflower seed) or flax seed (high in α-linolenic acid) had a shorter interval from calving to first ovulation than those fed a diet supplemented with canola seed. However, prepartum dietary treatment did not affect the diameter of the largest follicle at 7 days postpartum (Colazo et al. 2009). Additionally, our previous study (Salehi et al. 2015) with similar experimental design indicated that prepartum dietary treatment did not affect prepartum energy balance, but cows fed the control diet entered a deeper state of negative energy balance during the second week postpartum than those fed diets supplemented with canola or sunflower seed. Moreover, Bilby et al. (2006) found that different types of fatty acids had different effects on follicular development. Cows fed polyunsaturated fatty acid (18 : 2 or 18 : 3)-enriched diets had a larger preovulatory follicle at insemination compared with those fed monounsaturated fatty acid (18 : 1 cis or trans) diets. Therefore, feeding a diet supplemented with sunflower seed naturally enriched in linoleic acid, a polyunsaturated fatty acid, may improve energy balance during the early postpartum period with more favourable ovarian effects for supporting the early resumption of cyclicity.
Influence of uterine inflammatory status on endometrial cytokine gene expression and ovarian follicle dynamics
There is considerable debate regarding the percentage of PMN that should be used as a threshold (Kasimanickam et al. 2004; Barlund et al. 2008; Fischer et al. 2010) for diagnosing SCE. Different researchers have used different thresholds of 5, 8 and 18% (Kasimanickam et al. 2004; Barlund et al. 2008; Fischer et al. 2010). In the present study, the 8% threshold was selected based on a previous report (Dourey et al. 2011) from our laboratory that indicated that cows with >8% PMN had significantly longer intervals from calving to first ovulation and conception and reduced first-service conception rate. Based on the 8% PMN threshold, our results indicated that there were 11 cows with SCE and 17 normal cows. However, lowering the PMN threshold to 5% resulted in 14 cows in each category. Based on a higher threshold of 18% PMN, we had 9 SCE and 19 normal cows. However, changing PMN threshold from 8% to 5% or 18% did not alter gene expression results. Therefore, the use of an 8% PMN threshold at Day 25 postpartum was considered appropriate for SCE diagnosis.
Our findings that the mRNA expression of IL1B, CXCL8, TNF and IFNG (P = 0.08) was higher in the endometrium of cows with SCE than in normal cows is in agreement with the results from other studies that have used different (5 and 18%) PMN thresholds (Fischer et al. 2010; Ghasemi et al. 2012). Kasimanickam et al. (2013) found greater IL-6 serum concentration in cows with SCE (>18% PMN in endometrial cytology at 28–35 days postpartum), but serum concentrations of cytokines were not measured in the present study. Although mRNA expression of IL6 was detected in the bovine endometrium its expression was not associated with uterine health status, in agreement with the findings of Fischer et al. (2010) and Kasimanickam et al. (2014). Interleukin-6 is an important pro-inflammatory cytokine, involved in several aspects of inflammation such as induction of fever, increase in vascular permeability and induction of acute-phase proteins by the liver (Van Snick 1990). Interleukin-6 is also produced by fibroblasts, endothelial cells, keratinocytes, mast cells, monocytes/macrophages, tumour cell lines (Van Snick 1990) and, interestingly, by adipocytes, with adipose tissue contributing up to 35% of circulating IL-6 (Mohamed-Ali et al. 1997). Therefore, IL6 expression in endometrial cells of cows that develop endometritis might be influenced by differences in body condition and might not be an indicator of SCE.
Correlation analyses in the present study support the concept that pro-inflammatory cytokines have a role in PMN recruitment and infiltration of the endometrium after infection, as previously reported by Fischer et al. (2010). The highest association observed was between CXCL8 and PMN percentage, which is in agreement with previous reports (Fischer et al. 2010; Ghasemi et al. 2012). Zerbe et al. (2003) reported that an infusion of human recombinant IL-8 into the uterus of mares and cows resulted in PMN recruitment, whereas anti-IL-8 treatment prevented PMN-dependent tissue damage and PMN infiltration. Together, these results suggest that PMN are chemo-attracted by substances secreted by epithelial cells and the latter substances may be acting together directing PMN to the site of inflammation (Hoch et al. 1996).
The control of pro-inflammatory responses to avoid excessive immune activation by bacteria, including the effects of IL-1B, is dependent on anti-inflammatory mediators such as IL-10 (Herath et al. 2009). There was higher mRNA expression of IL10 in cows with SCE than normal cows. Moreover, the IL1B or CXCL8 to IL10 ratios was also higher in cows with SCE. Herath et al. (2009) compared the ratio of IL1A or B to IL10 in fertile versus non-fertile cows during the first, third, fifth and seventh weeks postpartum and found that the IL1A or B to IL10 ratio was higher in non-fertile than fertile cows during the first week postpartum. Therefore, they suggested that IL10 has a role in limiting the pro-inflammatory response in the endometrium and improving postpartum cattle fertility.
In the present study, we found that uterine health status did not affect the interval from calving to formation of a dominant follicle. From antral to 8-mm diameter, follicles are FSH dependent (Xu et al. 1995) and previous reports found that cows with higher uterine contamination (Sheldon et al. 2002; Williams et al. 2007) or those given intrauterine infusion of LPS (Williams et al. 2008) did not disrupt FSH secretion. In support of the previous findings, we found that the interval to formation of dominant follicles was associated with mRNA expression of IL1B, IL10 and INFG; however, these factors only explained 27% of the variation. Based on these results, we infer that follicle development until 10 mm size could occur independent of uterine inflammation.
In follicles greater than 8 mm in diameter, granulosa cells express LH receptors and they would require pulsatile LH stimulation to continue to grow (Xu et al. 1995). Cows with SCE had a longer mean interval from calving to the formation of a preovulatory-size follicle than normal cows, suggesting that SCE might affect LH pulsatility. This finding is in agreement with previous studies (Sheldon et al. 2002; Williams et al. 2007) wherein uterine bacterial infections perturbed ovarian follicular growth and function. Those authors (Sheldon et al. 2002; Williams et al. 2007) found that genital tract microbial infections contributed to a slower growth of dominant follicles after 8–10 mm diameter and lower peripheral plasma oestradiol concentrations. In a subsequent study, Williams et al. (2008) evaluated the effect of LPS challenge on bovine pituitary LH and FSH production (in vivo) or theca and granulosa cell androstenedione and oestradiol production (in vitro). They found that LPS challenge did not affect pituitary LH, FSH or theca cell androstenedione production. However, adding LPS to granulosa cells reduced oestradiol production in vitro. They suggested that rather than affecting pituitary FSH production or secretion, LPS reduces the ability of granulosa cells to respond to FSH or to aromatise androstenedione to oestradiol (Williams et al. 2008).
Based on predicted slopes in the regression model (Table 5) in the present study, the interval from calving to formation of preovulatory-size follicle was largely influenced by uterine inflammatory status. More interestingly, IL10 and IL1B had the highest slopes with positive and negative biological effects, respectively. The positive biological effect and high predicted slope in the regression model of IL10 demonstrated how reduced uterine inflammation improved preovulatory-size follicle formation postpartum. Uterine inflammatory status did not affect the interval from calving to first spontaneous ovulation and ovulation rate in the present study, although cows with SCE had numerically greater interval to first ovulation, lower ovulation rate and lower conception rate. Similarly, Williams et al. (2007) showed that the interval from calving to ovulation and the fate of that first dominant follicle did not differ significantly between cows with high or low uterine pathogen contamination at Day 7 postpartum. In contrast, Sheldon et al. (2002) found that preovulatory-size follicles in cows with higher pathogen contamination are less likely to ovulate. Studies in sheep and cattle have identified reduced GnRH and LH secretion in animals challenged with LPS (Gilbert et al. 1990; Battaglia et al. 2000; Karsch et al. 2002). It is worth noting that these effects are seen at concentrations of LPS that induce systemic illness in the animals treated. Therefore, these contradictory results call for further investigations to understand the mechanisms of how uterine contamination affects first ovulation in postpartum dairy cows with different levels of PMN, rather than using animal models subjected to LPS challenge.
It has been discussed that infiltration of neutrophils in the endometrium before and after ovulation has to be regarded as a physiological process (Butt et al. 1991; Cobb and Watson 1995). In uterine lavage collected from cows 8 h after ovulation, the concentration of PMN was determined to be ~3% of all cells collected (Butt et al. 1991). An increased percentage of PMN in the endometrium of cows was also detected between Days 2 and 8 after ovulation (Kluciński et al. 1990a, 1990b). This can be interpreted as a first cellular defence against pathogens expected to infiltrate the uterus at mating due to the open cervix during oestrus. Based on multiple regression results, expression of CXCL8 and IL10 were mainly related to interval to ovulation with positive and negative biological effects, respectively. It is likely that CXCL8 modulates the timely recruitment of neutrophils in the endometrium to increase maternal immunity, whereas, an increase in IL10 helps to avoid excessive immune activation by bacteria to support pregnancy. However, this notion needs to be validated.
In conclusion, feeding cows a diet supplemented with sunflower seed (high in linoleic acid) during the prepartum period reduced the interval from calving to preovulatory-size follicle formation and increased the proportion of cows ovulating within the first 5 weeks after calving without affecting postpartum uterine inflammatory status. Furthermore, the observed increases in the mRNA expression of inflammatory cytokines and their impacts on follicular development and reproductive performance supported using a threshold of 8% PMN in endometrial cytology (cytobrush technique) for diagnosis of SCE when dairy cows are examined at 25 days postpartum. Higher mRNA expression of TNF, IL1B and CXCL8 was associated with SCE and is likely to play a key role in the pathogenesis of the latter condition and contribute to delayed establishment of a preovulatory-size follicle. Moreover, IL10 apparently has a role to limit the pro-inflammatory response in the endometrium to improve follicular development and preovulatory-size follicle formation during the early postpartum period.
Acknowledgements
Research supported by Alberta Livestock and Meat Agency, Alberta Innovates – BioSolutions, Alberta Milk and Livestock Research Branch, Alberta Agriculture and Forestry. The authors acknowledge Prof. Masahito Oba (University of Alberta) for his expertise and assistance in diet formulation.
References
Amaral, B. C. D. (2008). Effect of supplemental fat source on production, immunity, hepatic gene expression and metabolism of periparturient dairy cows. PhD Thesis, University of Florida, Gainesville.Ambrose, D. J. (2015). Postpartum anoestrus and its management in dairy cattle. In ‘Bovine Reproduction’. (Ed. R. M. Hopper.) pp. 456–470. (John Wiley & Sons, Inc.: Ames, IA, USA.)
Ambrose, D. J., Kastelic, J. P., Corbett, R., Pitney, P. A., Petit, H. V., Small, J. A., and Zalkovic, P. (2006). Lower pregnancy losses in lactating dairy cows fed a diet enriched in alpha-linolenic acid. J. Dairy Sci. 89, 3066–3074.
| Lower pregnancy losses in lactating dairy cows fed a diet enriched in alpha-linolenic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1Cltb8%3D&md5=45b39c68c706bf2da5cae2590e9b4c09CAS | 16840624PubMed |
Barlund, C. S., Carruthers, T. D., Waldner, C. L., and Palmer, C. W. (2008). A comparison of diagnostic techniques for postpartum endometritis in dairy cattle. Theriogenology 69, 714–723.
| A comparison of diagnostic techniques for postpartum endometritis in dairy cattle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c7mvVChsA%3D%3D&md5=8c8bb6d30ae6b311be392a5ac0a1e0b9CAS | 18242670PubMed |
Battaglia, D. F., Krasa, H. B., Padmanabhan, V., Viguie, C., and Karsch, F. J. (2000). Endocrine alterations that underlie endotoxin-induced disruption of the follicular phase in ewes. Biol. Reprod. 62, 45–53.
| Endocrine alterations that underlie endotoxin-induced disruption of the follicular phase in ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhslKlsg%3D%3D&md5=63abeb87fdbf63efa4ad45190e6174a3CAS | 10611066PubMed |
Bilby, T. R., Block, J., do Amaral, B. C., Sa Filho, O., Silvestre, F. T., Hansen, P. J., Staples, C. R., and Thatcher, W. W. (2006). Effects of dietary unsaturated fatty acids on oocyte quality and follicular development in lactating dairy cows in summer. J. Dairy Sci. 89, 3891–3903.
| Effects of dietary unsaturated fatty acids on oocyte quality and follicular development in lactating dairy cows in summer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVWgsb%2FO&md5=9fef7a4bb7c2636cd0a995b15e6979d9CAS | 16960065PubMed |
Butt, B. M., Senger, P. L., and Widders, P. R. (1991). Neutrophil migration into the bovine uterine lumen following intrauterine inoculation with killed Haemophilus somnus. J. Reprod. Fertil. 93, 341–345.
| Neutrophil migration into the bovine uterine lumen following intrauterine inoculation with killed Haemophilus somnus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK387lslSlsQ%3D%3D&md5=d2037763d686cad1a2e831179ac5a9ddCAS | 1787453PubMed |
Canadian Council on Animal Care Guidelines on the Care and Use of Farm Animals in Research, Teaching and Testing (2009). http://www.ccac.ca/Documents/Standards/Guidelines/Farm_Animals.pdf [Verified 29 December 2015].
Cobb, S. P., and Watson, E. D. (1995). Immunohistochemical study of immune cells in the bovine endometrium at different stages of the oestrous cycle. Res. Vet. Sci. 59, 238–241.
| Immunohistochemical study of immune cells in the bovine endometrium at different stages of the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK287lsFSqsg%3D%3D&md5=6dd378a3cf1a618578eb8c22cbeb4058CAS | 8588099PubMed |
Colazo, M. G., Behrouzi, A., Ambrose, D. J., and Mapletoft, R. J. (2012). Diameter of the ovulatory follicle at timed artificial insemination as a predictor of pregnancy status in lactating dairy cows subjected to GnRH-based protocols. Theriogenology 84, 377–383.
Colazo, M. G., Hayirli, A., Doepel, L., and Ambrose, D. J. (2009). Reproductive performance of dairy cows is influenced by prepartum feed restriction and dietary fatty-acid source. J. Dairy Sci. 92, 2562–2571.
| Reproductive performance of dairy cows is influenced by prepartum feed restriction and dietary fatty-acid source.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmsFenu78%3D&md5=dd874c3d07a01d14881e95b8c7256469CAS | 19447988PubMed |
Dourey, A., Colazo, M. G., Barajas, P. P., and Ambrose, D. J. (2011). Relationships between endometrial cytology and interval to first ovulation and pregnancy in postpartum dairy cows in a single herd. Res. Vet. Sci. 91, e149–e153.
| Relationships between endometrial cytology and interval to first ovulation and pregnancy in postpartum dairy cows in a single herd.Crossref | GoogleScholarGoogle Scholar | 21176928PubMed |
Fischer, C., Drillich, M., Odau, S., Heuwieser, W., Einspanier, R., and Gabler, C. (2010). Selected pro-inflammatory factor transcripts in bovine endometrial epithelial cells are regulated during the oestrous cycle and elevated in case of subclinical or clinical endometritis. Reprod. Fertil. Dev. 22, 818–829.
| Selected pro-inflammatory factor transcripts in bovine endometrial epithelial cells are regulated during the oestrous cycle and elevated in case of subclinical or clinical endometritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlsVCqtL0%3D&md5=08c8a732f127d060505086d85696785cCAS | 20450834PubMed |
Galvão, K. N., Santos, N. R., Galvão, J. S., and Gilbert, R. O. (2011). Association between endometritis and endometrial cytokine expression in postpartum Holstein cows. Theriogenology 76, 290–299.
| Association between endometritis and endometrial cytokine expression in postpartum Holstein cows.Crossref | GoogleScholarGoogle Scholar | 21496904PubMed |
Ghasemi, F., Gonzalez-Cano, P., Griebel, P. J., and Palmer, C. (2012). Pro-inflammatory cytokine gene expression in endometrial cytobrush samples harvested from cows with and without subclinical endometritis. Theriogenology 78, 1538–1547.
| Pro-inflammatory cytokine gene expression in endometrial cytobrush samples harvested from cows with and without subclinical endometritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Kju73L&md5=a44447e03900bd7d17ad73542c663de2CAS | 22925636PubMed |
Gilbert, R. O., Bosu, W. T. K., and Peter, A. T. (1990). The effect of Escherichia coli endotoxin on luteal function in Holstein heifers. Theriogenology 33, 645–651.
| The effect of Escherichia coli endotoxin on luteal function in Holstein heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkt1Wmtb4%3D&md5=0cb360efa9fe150959efedfcbfd62d21CAS | 16726760PubMed |
Gilbert, R. O., Shin, S. T., Guard, C. L., Erb, H. N., and Frajblat, M. (2005). Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 64, 1879–1888.
| Prevalence of endometritis and its effects on reproductive performance of dairy cows.Crossref | GoogleScholarGoogle Scholar | 15961149PubMed |
Herath, S., Lilly, S. T., Santos, N. R., Gilbert, R. O., Goetze, L., Bryant, C. E., White, J. O., Cronin, J., and Sheldon, I. M. (2009). Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility. Reprod. Biol. Endocrinol. 7, 55.
| Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility.Crossref | GoogleScholarGoogle Scholar | 19476661PubMed |
Hoch, R. C., Schraufstätter, I. U., and Cochrane, C. G. (1996). In vivo, in vitro and molecular aspects of interleukin-8 and the interleukin-8 receptors. J. Lab. Clin. Med. 128, 134–145.
| In vivo, in vitro and molecular aspects of interleukin-8 and the interleukin-8 receptors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xls1GksL8%3D&md5=f4fe212ad519af9e94df6868de4d41daCAS | 8765209PubMed |
Karsch, F. J., Battaglia, D. F., Breen, K. M., Debus, N., and Harris, T. G. (2002). Mechanisms for ovarian cycle disruption by immune–inflammatory stress. Stress 5, 101–112.
| Mechanisms for ovarian cycle disruption by immune–inflammatory stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmvFChs7k%3D&md5=faa49445113b92c7bc2eab884fdc2738CAS | 12186688PubMed |
Kasimanickam, R., Duffield, T. F., Foster, R. A., Gartley, C. J., Leslie, K. E., Walton, J. S., and Johnson, W. H. (2004). Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 62, 9–23.
| Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c3mtFSkuw%3D%3D&md5=48a405b987d6621daab42bafa8c800d5CAS | 15159097PubMed |
Kasimanickam, R., Duffield, T. F., Foster, R. A., Gartley, C. J., Leslie, K. E., Walton, J. S., and Johnson, W. H. (2005). A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can. Vet. J. 46, 255–259.
| 15884649PubMed |
Kasimanickam, R. K., Kasimanickam, V. R., Olsen, J. R., Jeffress, E. J., Moore, D. A., and Kastelic, J. P. (2013). Associations among serum pro- and anti-inflammatory cytokines, metabolic mediators, body condition and uterine disease in postpartum dairy cows. Reprod. Biol. Endocrinol. 11, 103.
| Associations among serum pro- and anti-inflammatory cytokines, metabolic mediators, body condition and uterine disease in postpartum dairy cows.Crossref | GoogleScholarGoogle Scholar | 24209779PubMed |
Kasimanickam, R., Kasimanickam, V., and Kastelic, J. P. (2014). Mucin 1 and cytokines mRNA in endometrium of dairy cows with postpartum uterine disease or repeat breeding. Theriogenology 81, 952–958.e2.
| Mucin 1 and cytokines mRNA in endometrium of dairy cows with postpartum uterine disease or repeat breeding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjsFyrtL0%3D&md5=312a3bf04226dd3e0e838c53470042eaCAS | 24576715PubMed |
Kluciński, W., Targowski, S. P., Miernik-Degorska, E., and Winnicka, A. (1990a). The phagocytic activity of polymorphonuclear leucocytes isolated from normal uterus and that with experimentally induced inflammation in cows. Zentralbl. Veterinarmed. A 37, 506–512.
| The phagocytic activity of polymorphonuclear leucocytes isolated from normal uterus and that with experimentally induced inflammation in cows.Crossref | GoogleScholarGoogle Scholar | 2123052PubMed |
Kluciński, W., Targowski, S. P., Winnicka, A., and Miernik-Degorska, E. (1990b). Immunological induction of endometritis-model investigations in cows. Zentralbl. Veterinarmed. A 37, 148–153.
| Immunological induction of endometritis-model investigations in cows.Crossref | GoogleScholarGoogle Scholar | 2113751PubMed |
Laarman, A. H., Ruiz-Sanchez, A. L., Sugino, T., Guan, L. L., and Oba, M. (2012). Effects of feeding a calf starter on molecular adaptations in the ruminal epithelium and liver of Holstein dairy calves. J. Dairy Sci. 95, 2585–2594.
| Effects of feeding a calf starter on molecular adaptations in the ruminal epithelium and liver of Holstein dairy calves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvFyhtLY%3D&md5=3b035f191c75dd77ea077e000cd5042dCAS | 22541487PubMed |
Mohamed-Ali, V., Goodrick, S., Rawesh, A., Katz, D. R., Miles, J. M., Yudkin, J. S., Klein, S., and Coppack, S. W. (1997). Subcutaneous adipose tissue releases interleukin-6, but not tumour necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 82, 4196–4200.
| 1:CAS:528:DyaK2sXnvFyrsrw%3D&md5=63dc4f62651c7ad0704951d8b503e335CAS | 9398739PubMed |
Moreira, F., Orlandi, C., Risco, C. A., Mattos, R., Lopes, F., and Thatcher, W. W. (2001). Effects of presynchronisation and bovine somatotrophin on pregnancy rates to a timed artificial insemination protocol in lactating dairy cows. J. Dairy Sci. 84, 1646–1659.
| Effects of presynchronisation and bovine somatotrophin on pregnancy rates to a timed artificial insemination protocol in lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvFamu7w%3D&md5=3872662f6f4e0c8e4a6653011d3d046dCAS | 11467815PubMed |
National Research Council Committee on Animal Nutrition (2001). ‘Nutrient Requirements of Dairy Cows’. (National Academy Press: Washington, DC.)
Opsomer, G., Grohn, Y. T., Hertl, J., Coryn, M., Deluyker, H., and de Kruif, A. (2000). Risk factors for postpartum ovarian dysfunction in high-producing cows in Belgium: a field study. Theriogenology 53, 841–857.
| Risk factors for postpartum ovarian dysfunction in high-producing cows in Belgium: a field study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7ptlansg%3D%3D&md5=86324ae5004ed06a0207a408a043b38cCAS | 10730974PubMed |
Pierson, R. A., and Ginther, O. J. (1984). Ultrasonography of the bovine ovary. Theriogenology 21, 495–504.
| Ultrasonography of the bovine ovary.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283pvVKksw%3D%3D&md5=d1176296e84f5617f239e6ff7b5628c8CAS | 16725899PubMed |
Renner, L., Kersten, S., Duevel, A., Schuberth, H. J., and Dänicke, S. (2013). Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid, linoleic acid, phytanic acid and the combination of various fatty acids on proliferation and cytokine expression of bovine peripheral blood mononuclear cells. Nutrients 5, 2667–2683.
| Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid, linoleic acid, phytanic acid and the combination of various fatty acids on proliferation and cytokine expression of bovine peripheral blood mononuclear cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFymtL7K&md5=0d708aa792194104d6adc614bf797802CAS | 23857174PubMed |
Salehi, R., Colazo, M. G., Oba, M., and Ambrose, D. J. (2015). A prepartum diet supplemented with oilseeds high in oleic or linoleic acid reduced GnRH-induced LH release in dairy cows during second week postpartum. Reprod. Biol. Endocrinol. 13, 69–78.
| A prepartum diet supplemented with oilseeds high in oleic or linoleic acid reduced GnRH-induced LH release in dairy cows during second week postpartum.Crossref | GoogleScholarGoogle Scholar | 26138920PubMed |
Schlau, N., Guan, L. L., and Oba, M. (2012). The relationship between rumen acidosis resistance and expression of genes involved in regulation of intracellular pH and butyrate metabolism of ruminal epithelial cells in steers. J. Dairy Sci. 95, 5866–5875.
| The relationship between rumen acidosis resistance and expression of genes involved in regulation of intracellular pH and butyrate metabolism of ruminal epithelial cells in steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVCns7vK&md5=d6ae48f5bc316ed6d760a1e86a9ac823CAS | 22863095PubMed |
Schroit, A. J., and Gallily, R. (1979). Macrophage fatty-acid composition and phagocytosis: effect of unsaturation on cellular phagocytic activity. Immunology 36, 199–205.
| 1:CAS:528:DyaE1MXksFegsLw%3D&md5=dc4a9d8d7c9e02cb9469c9092402d72aCAS | 374248PubMed |
Sheldon, I. M., Noakes, D. E., Rycroft, A. N., Pfeiffer, D. U., and Dobson, H. (2002). Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 123, 837–845.
| Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvVCiu7s%3D&md5=630e6c429de0f0630f23189907e13649CAS | 12052238PubMed |
Sheldon, I. M., Cronin, J., Goetze, L., Donofrio, G., and Schuberth, H. J. (2009). Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 81, 1025–1032.
| Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2lt7vP&md5=f15fe16ed69bfa2c2a34c7c1318c90ffCAS | 19439727PubMed |
Silvestre, F. T., Carvalho, T. S., Crawford, P. C., Santos, J. E., Staples, C. R., Jenkins, T., and Thatcher, W. W. (2011). Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: II. Neutrophil fatty acids and function, and acute-phase proteins. J. Dairy Sci. 94, 2285–2301.
| Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: II. Neutrophil fatty acids and function, and acute-phase proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvFCgs70%3D&md5=a8a5a8d88bef312f5610c298ee642a31CAS | 21524518PubMed |
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., and Speleman, F. (2002). Accurate normalisation of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034–research0034.11.
| Accurate normalisation of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes.Crossref | GoogleScholarGoogle Scholar | 12184808PubMed |
Van Snick, J. (1990). Interleukin-6: an overview. Annu. Rev. Immunol. 8, 253–278.
| Interleukin-6: an overview.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkvFGksL0%3D&md5=0e30b5a01a03e1f5b72e954183df6401CAS | 2188664PubMed |
Williams, E. J., Fischer, D. P., Noakes, D. E., England, G. C., Rycroft, A., Dobson, H., and Sheldon, I. M. (2007). The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 68, 549–559.
| The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotlWqu7w%3D&md5=8fe081b089b10ad9ca9ed77f3867d910CAS | 17574659PubMed |
Williams, E. J., Sibley, K., Miller, A. N., Lane, E. A., Fishwick, J., Nash, D. M., Herath, S., England, G. C., Dobson, H., and Sheldon, I. M. (2008). The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function. Am. J. Reprod. Immunol. 60, 462–473.
| The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVGgt7fK&md5=f6e7019524df03d0ab48a8acb6efebbeCAS | 19238751PubMed |
Xu, Z., Garverick, H. A., Smith, G. W., Smith, M. F., Hamilton, S. A., and Youngquist, R. S. (1995). Expression of messenger ribonucleic acid encoding cytochrome P450 side-chain cleavage, cytochrome p450 17 alpha-hydroxylase and cytochrome P450 aromatase in bovine follicles during the first follicular wave. Endocrinology 136, 981–989.
| 1:CAS:528:DyaK2MXjvFCjsbo%3D&md5=8835b50b5a599d167a03dc9356c36b3dCAS | 7867608PubMed |
Zerbe, H., Schuberth, H. J., Engelke, F., Frank, J., Klug, E., and Leibold, W. (2003). Development and comparison of in vivo and in vitro models for endometritis in cows and mares. Theriogenology 60, 209–223.
| Development and comparison of in vivo and in vitro models for endometritis in cows and mares.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3szhtVejsw%3D%3D&md5=22637468367923a2bc96516aefb8a7beCAS | 12749935PubMed |