Gill parasites of Schizocypris altidorsalis (Pisces: Cyprinidae), a threatened freshwater fish in Iran
Shokoofeh Shamsi A * , Javad Khedri B , Hassan Borji B , Jaydipbhai Suthar A and Nidhish Francis A
A * , Javad Khedri B , Hassan Borji B , Jaydipbhai Suthar A and Nidhish Francis A
A
B
Abstract
Schizocypris altidorsalis is a fish of very restricted distribution, with its populations being known only from few water reservoirs on the border of Iran and Afghanistan.
The aim was to investigate the occurrence and diversity of host-specific Monogenea on this fish.
In total, 400 fish were purchased from the market and examined for the presence of Monogenea. Specimens were characterised morphologically and genetically.
In total, 26.5% of fish were found to be infected with Monogenea. Further morphological and molecular studies based on the sequences of the 18S, 28S and ITS2 regions suggested that they all belong to a new species, herein named as Paradiplozoon jalalii. Our results suggest that this monogenean is a highly specific parasite of Schizocypris altidorsalis.
Monogeneans are highly specialised host-specific parasites infecting fish gill. They can adversely affect respiration and excretory functions in fish, making them more vulnerable to illness, leading to population declines.
Determining effective strategies for safeguarding endangered fish can be challenging in conflict zones, such as the area studied here. Additionally, the parasite identified in our study, with its blood-feeding habits, could contribute to the extinction of its fish host, subsequently leading to its own extinction. An unanswered question lingers regarding the potential extinction of other symbionts of Schizocypris altidorsalis if the fish faces extinction.
Keywords: conservation, Diplozoidae, fish, freshwater fish, Iran, Monogenea, new species, threatened species.
Introduction
Reports of fish mortality attributed to gill parasites are abundant (Johnsen and Jensen 1986; Obiakezie and Taege 1991; Leis et al. 2023). Gill parasites hold particular importance because they can lead to significant health issues in the infected fish. The gills play several vital roles in fish, including respiratory and excretory functions. Infections by parasites can disrupt these crucial systems, resulting in malfunctions and, consequently, significant health problems for the fish.
One of such parasite groups that poses threats to aquatic life includes Monogenea, a group comprising 6000–7000 species (Khotenovsky 1985), predominantly acting as ectoparasites on the gills and skin of fish (Sayyaf Dezfuli et al. 2021). These parasites are known to contribute to fish mortality or a decline in health, particularly when fish is under stress such as in farms with high-density stock (Sayyaf Dezfuli et al. 2021). The attachment and feeding activities of a substantial number of monogeneans on fish gills can result in injuries to epithelial cells, hyperplasia, and oedema, leading to the atrophy of capillaries and lamellae. This, in turn, adversely affects the respiratory function of the host, ultimately leading to death (Whittington 2012).
Currently, the cyprinid Schizocypris altidorsalis Bianco & Bağnağrescu, 1982 is known from a very limited distribution within the Chah Nimeh water reservoirs of Zabol, situated in the Sistan and Baluchistan Province, Iran (see Brian W. Coad’s Freshwater fishes of Iran at www.briancoad.com). This species belongs to the family Cyprinidae, which includes a diverse array of carp and minnow species. The Chah Nimeh reservoirs consist of three interconnected lakes nestled along the border between Iran and Afghanistan. These lakes are characterised by their natural and artificial origins, i.e. they are large natural holes in Sistan and Baluchistan Province into which Hirmand River water is directed, turning it to an artificial lake. The Chah Nimeh reservoirs provide the only habitat for S. altidorsalis. Known locally as ‘anjak’ (see www.briancoad.com), S. altidorsalis has become an integral part of the cultural and culinary heritage of the surrounding communities. Its delicate flavour and succulent flesh, along with limited options for other food resources, have made it a sought-after edible fish among the locals, a highly disadvantaged population (Moudi et al. 2022).
Schizocypris altidorsalis faces a multitude of threats that further compound its conservation status. Pollution, a persistent issue in many aquatic ecosystems, has been identified as one of the significant challenges for this fish. Mirnia et al. (2019) showed that industrial waste and agricultural runoff can disrupt the delicate balance of the reservoirs, affecting water quality and diminishing the fish’s ability to thrive. In addition to pollution, climate change poses a growing threat to S. altidorsalis and its habitat. Bazzi et al. (2021) shed light on the potential impacts of changing environmental conditions on the fish’s distribution, reproductive patterns and overall survival. Rising temperatures, altered rainfall patterns and shifts in water availability could disrupt the delicate ecological equilibrium that sustains this unique species.
Regrettably, the outlook for the survival of S. altidorsalis, a freshwater fish with a restricted distribution in an extremely unsafe area to conduct research, remains grim. This species has received scant international attention and is not listed as vulnerable or endangered. Inevitably, highly host-specific symbionts, including parasites of this fish, will also be subject to extinction, if their host becomes extinct. For long, ecologists have advocated for investigations aimed at comprehending the significant ecological role played by parasites, and protecting them from extinction as much as possible. Carlson et al. (2020) urged documentation of parasite declines and extinctions, as part of a more wholistic conservation practice.
In the case of our study, this circumstance extends beyond the fish itself as well. The limited distribution of S. altidorsalis poses a significant threat to the existence of unique symbionts that exhibit a high level of host specificity, residing on or within the fish. If the fish species were to disappear, these symbionts, too, face the dire prospect of extinction. Species dependent on threatened hosts may become extinct through either direct or indirect human action (Moir et al. 2012). One group of these symbionts is Monogenea, a class of ecologically and economically significant platyhelminth parasites of freshwater and marine animals (Gilbert and Avenant-Oldewage 2021), which can cause alterations at the population and organismal levels. Species of Monogenea are generally highly host-specific parasites that may face extinction when their host goes extinct, causing an ‘unintentional extinction’ (Jørgensen 2016).
Hence, the primary objective of this study was to examine the presence of monogenean parasites on the gills of S. altidorsalis and characterise them.
Materials and methods
Fish collection
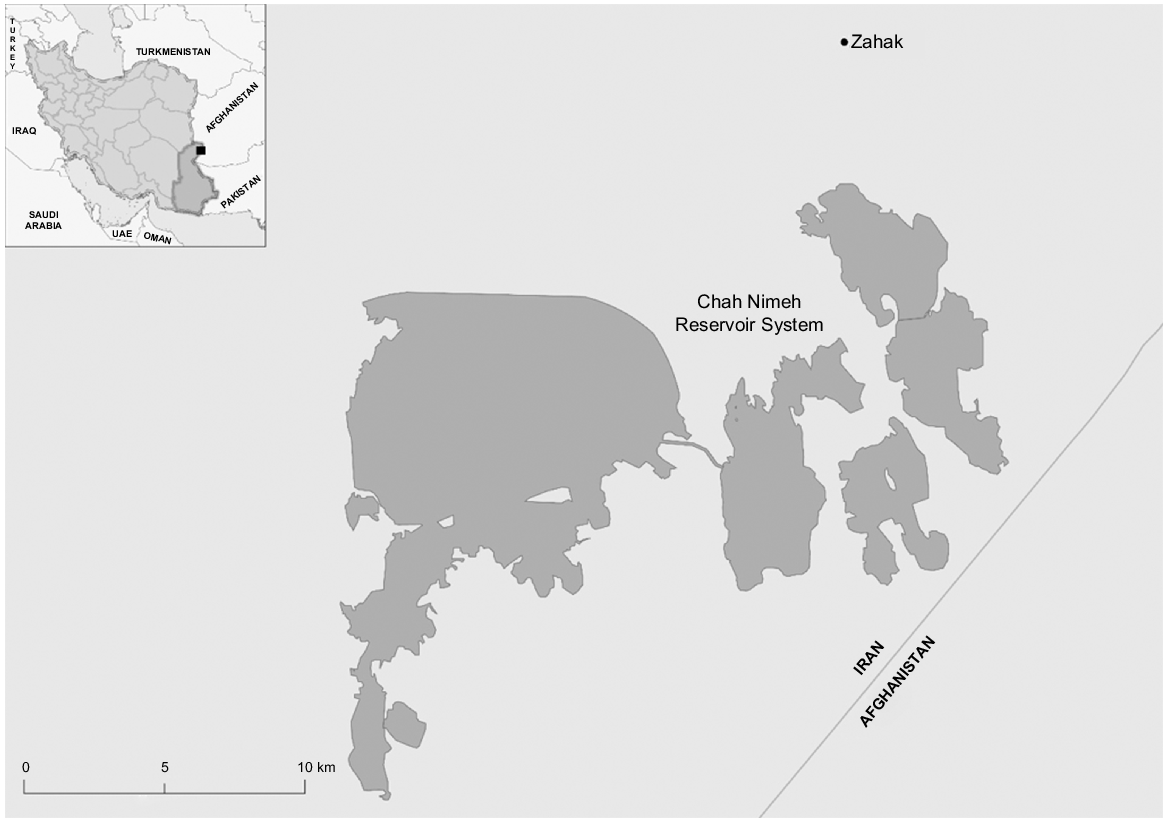
The origin of the studied fish were Chah Nimeh water reservoirs, Zabol, Iran, as depicted in Fig. 1. The Chah Nimeh reservoirs consist of three interconnected lakes nestled along the border between Iran and Afghanistan. These lakes provide a vital habitat for Schizocypris altidorsalis. In total, 400 fish (Fig. 2a) were procured from a local fish market. The sampling was conducted during two seasons, namely, autumn 2020 (October and November) and winter 2021 (December and January). Two hundred fish were examined during each season to assess any potential variations.
Map of the study area. The black box on the inset shows the study region. Map has been produced using ArcGIS Pro (ver. 3.1, ESRI, Redlands, CA, USA).
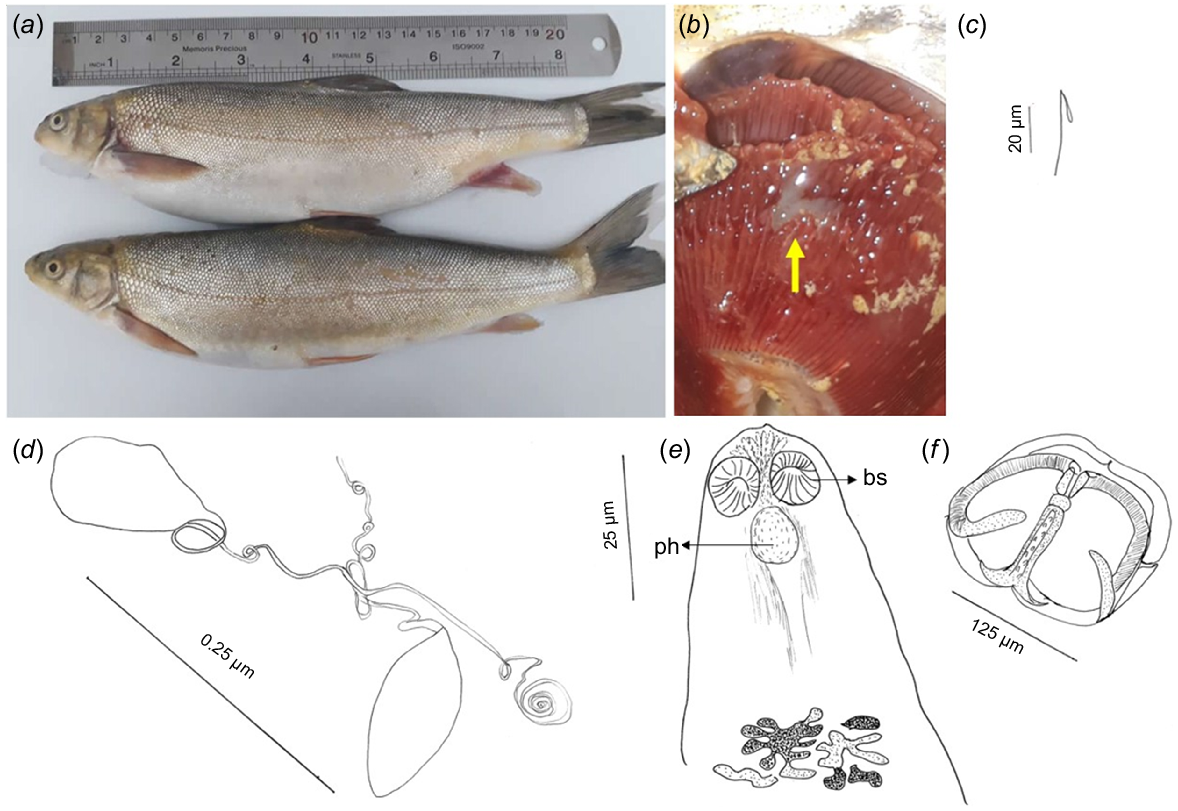
Fish and parasites that were collected in the present study, including (a) Schizothorax altidorsalis, (b) gills infected with monogenean parasite, (c) illustration of hook, (d) illustration of the eggs, (e) illustration of the taxonomically important features of the parasite in the anterior end, and (f) clamp. Abbreviations denote pharynx (ph) and buccal sucker (bs).
On acquisition, the fish were promptly transported to the laboratory on ice. Once in the laboratory, the fish were measured for their length and weight as part of the morphological analysis. Additionally, the specimens were thoroughly examined for any signs of infection or infestation with monogenean parasites (Fernando et al. 1972) on the same day as the fish’s arrival in the laboratory.
Parasite collection and identification
Within 6–12 h after fish were caught, they were examined for parasites. The excised gills were placed in separate Petri dishes filled with water. With a stereomicroscope, the surfaces of all the gills were inspected. On detection (Fig. 2b), the Monogenea parasites were extracted from the gills with fine dissection needles. Once extracted, the parasites were carefully washed, followed by counting the total number of individuals found on each gill. To enable further analysis, the collected Monogenea parasites were preserved in 70% ethanol for subsequent morphological and molecular investigations. The preserved parasites were then sent to Shamsi’s Parasitology Laboratory at Charles Sturt University, Australia, where the specimens underwent morphological examination and molecular analyses of their taxonomic classification and genetic characteristics. Parasites were morphologically examined as previously described by Gussev (1983) and Jalali et al. (2005), and identified in accordance with previous publications (Galli et al. 2010; Huang et al. 2023; Nejat et al. 2023). The terminology to describe various body-part structures is mainly in accordance with Galli et al. (2010). All measurements are given in millimetres unless otherwise stated.
A small piece of seven parasites was transferred into separate 1.5-mL autoclaved Eppendorf tubes for molecular study. Voucher material (specimens) has been deposited in the Queensland Museum under the accession numbers G241122 (holotype) and G241123–241127 (paratypes).
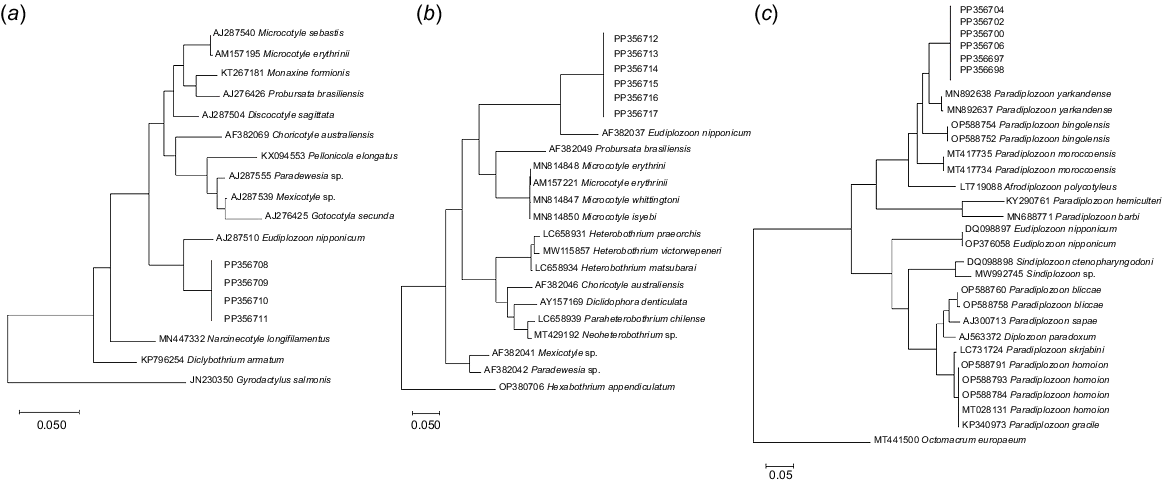
DNA was extracted using DNeasy Blood and Tissue kits (Qiagen, Hilden, Germany), as per the manufacturer’s instructions, and modified (Shamsi et al. 2018) to be eluted in 40 μL of elution buffer. Polymerase chain-reaction (PCR) amplification of the fragments of the ITS2, 18S and 28S of the rDNA region was performed as previously described (Roohi et al. 2019; Shamsi et al. 2021; Nejat et al. 2023). Representative samples were sent to the Australian Genome Research Facility (AGRF), Brisbane, Qld, Australia, and were subjected to Sanger sequencing by using the same primer sets as for PCR. Sequence data including chromatograms were observed initially through Sequence Scanner Software (Applied Biosystems Genetic Analysers). The evolutionary (pairwise) genetic distance was calculated using MEGA 10 (ver. 10.1, see https://www.megasoftware.net; Kumar et al. 2016). The phylogenetic relationships among the species were inferred using the maximum-likelihood analysis, Tamura-Nei model in MEGA 10. Closely related species of Monogenea were used as outgroup. For ITS-2 tree, analysing of phylogenetic trees built in previous works (Huang et al. 2023; Nejat et al. 2023) for Paradiplozoon spp. was useful as well. Gyrdocatus salmonis, Hexabothrium appendiculatum, and Octomacrum europaeum were used as the outgroups for 18S, 28S and ITS2 phylogenetic trees respectively. The reliability of the phylogenetic tree was assessed by the bootstrap method with 1000 replications. The pairwise comparison using the p-distance model in MEGA 10 was performed to evaluate the genetic distance between the sequences. After analysing phylogenetic trees built in previous works (Huang et al. 2023; Nejat et al. 2023) for Paradiplozoon spp., a closely related species of Monogenea, Octomacrum europaeum, was selected as an outgroup. Details of the sequences used to build phylogenetic trees in the present study can be found in Table 1.
| Taxon | Host scientific name (common name) | Locality | GenBank accession number | DNA region | References | |
|---|---|---|---|---|---|---|
| Paradiplozoon jalalii | Schizocypris altidorsalis (Anjak) | Iran | PZ203003-6 | 18S | Present study | |
| Eudiplozoon nipponicum | Cyprinus carpio (common carp) | Czechia | AJ287510 | 18S | Olson and Littlewood (2002) | |
| Discocotyle sagittata | Salmo trutta (sea trout) | Isle of Man, UK | AJ287504 | 18S | Olson and Littlewood (2002) | |
| Microcotyle sebastis | Sebastes sp. | North Sea, UK | AJ287540 | 18S | Olson and Littlewood (2002) | |
| Neomicrocotyle pacifica | Caranx hippos (black jack) | Chamela Bay, Mexico | AJ228787 | 18S | Olson and Littlewood (2002) | |
| Monaxine formionis | – | Mumbai, India | KT267181 | 18S | Verma and Verma (2022) | |
| Mexicotyle sp. | Scomberomorus sp. (mackerel) | Paraná, Brazil | AJ287539 | 18S | Olson and Littlewood (2002) | |
| Gotocotyla secunda | Scomberomorus commerson (Spanish mackerel) | Heron Island, Australia | AJ276425 | 18S | Olson and Littlewood (2002) | |
| Microcotyle erythrinii | Pagellus erythrinus | France | AM157195 | 18S | Badets et al. (2011) | |
| Paradawesia sp. | Scomberomorus sp. (mackerel) | Paraná, Brazil | AJ287555 | 18S | Olson and Littlewood (2002) | |
| Choricotyle australiensis | Rhabdosargus sarba (goldlined seabream) | Coffs Harbour, NSW, Australia | AF382069 | 18S | Olson and Littlewood (2002) | |
| Probursata brasiliensis | Oligoplites sp. (leatherjack) | Paraná, Brazil | AJ276426 | 18S | Olson and Littlewood (2002) | |
| Narcinecotyle longifilamentus | Narcine entemedor | Mexico | MN447332 | 18S | Torres-Carrera et al. (2020) | |
| Pellonicola elongatus | – | Lucknow, India | KX094553 | 18S | Unpublished | |
| Diclybothrium armatum | Acipenser schrenckii (Amur sturgeon) | Amur River, Russia | KP96254 | 18S | Rozhkovan and Shedko (2015) | |
| Gyrodactylus salmonis | Oncorhynchus mykiss (rainbow trout) | Veracruz, Mexico | JN230350 | 18S | Rubio-Godoy et al. (2012) | |
| Paradiplozoon jalalii | Schizocypris altidorsalis (Anjak) | Iran | PZ203006-11 | 28S | Present study | |
| Eudiplozoon nipponicum | Cyprinus carpio (common carp) | Czechia | AF382037 | 28S | Olson and Littlewood (2002) | |
| Microcotyle erythrini | Pagrus pagrus (red porgy) | Guardamar del Segura, Spain | MN814848 | 28S | Víllora-Montero et al. (2020) | |
| Microcotyle erythrini | Pagellus erythrinus (common pandora) | France | AM157221 | 28S | Badets et al. (2011) | |
| Microcotyle whittingtoni | Dentex dentex (common dentex) | Guardamar del Segura, Spain | MN814847 | 28S | Víllora-Montero et al. (2020) | |
| Mexicotyle sp. | Scomberomorus sp. (mackerel) | Brazil | AF382041 | 28S | Olson and Littlewood (2002) | |
| Paradawesia sp. | Scomberomorus sp. (mackerel) | Brazil | AF382042 | 28S | Olson and Littlewood (2002) | |
| Microcotyle isyebi | Boops boops (bogue) | Guardamar del Segura, Spain | MN814850 | 28S | Víllora-Montero et al. (2020) | |
| Diclidophora denticulata | Pollachius virens (saithe) | North Sea, northern Europe | AY157169 | 28S | Lockyer et al. (2003) | |
| Paraheterobothrium chilense | Hippoglossina macrops | Japan | LC658939 | 28S | Ogawa and Itoh (2022) | |
| Probursata brasiliensis | Oligoplites sp. (leatherjack) | Brazil | AF382049 | 28S | Olson and Littlewood (2002) | |
| Heterobothrium praeorchis | Takifugu flavipterus Matsuura (Japanese name: komon-fugu) | Japan | LC658931 | 28S | Ogawa and Itoh (2022) | |
| Heterobothrium matsubarai | Takifugu stictonotus (Japanese name: goma-fugu) | Japan | LC658934 | 28S | Ogawa and Itoh (2022) | |
| Neoheterobothrium sp. | Syacium papillosum (flounder) | Yucatan Shelf (Gulf of Mexico) | MT429192 | 28S | Soler-Jiménez et al. (2021) | |
| Heterobothrium victorwepeneri | Amblyrhynchotes honckenii (evileye blaasop) | South Africa | MW115857 | 28S | Acosta and Smit (2021) | |
| Choricotyle australiensis | Rhabdosargus sarba (goldlined seabream) | Australia | AF382046 | 28S | Olson and Littlewood (2002) | |
| Paradiplozoon jalalii | Schizocypris altidorsalis (Anjak) | Iran | PZ2030012-18 | ITS2 | Present study | |
| Paradiplozoon yarkandense | Schizothorax fish (Cyprinidae: Schizothoracinae) | China | MN892638 | ITS2 | Arken et al. (2022) | |
| Paradiplozoon yarkandense | Schizothorax fish (Cyprinidae: Schizothoracinae) | China | MN892637 | ITS2 | Arken et al. (2022) | |
| Paradiplozoon bingolensis | Cyprinion macrostomum | Iraq | OP588754 | ITS2 | Nejat et al. (2023) | |
| Paradiplozoon bingolensis | Cyprinion kais | Turkey | OP588752 | ITS2 | Nejat et al. (2023) | |
| Paradiplozoon krugerense | Labeo rosae | South Africa | LT574865 | ITS2 | Dos Santos and Avenant-Oldewage (2016) | |
| Paradiplozoon moroccoensis | Luciobarbus lepineyi | Morocco | MT417735 | ITS2 | Benovics et al. (2021) | |
| Paradiplozoon moroccoensis | Luciobarbus lepineyi | Morocco | MT417734 | ITS2 | Benovics et al. (2021) | |
| Afrodiplozoon polycotyleus | Labeobarbus marquensis | South Africa | LT719088 | ITS2 | Přikrylová et al. (2018) | |
| Paradiplozoon bliccae | Ladigesocypris ghigii | Turkey | OP588760 | ITS2 | Nejat et al. (2023) | |
| Paradiplozoon bliccae | Petroleuciscus ninae | Turkey | OP588758 | ITS2 | Nejat et al. (2023) | |
| Paradiplozoon homoion | Squalius cii | Turkey | OP588791 | ITS2 | Nejat et al. (2023) | |
| Paradiplozoon homoion | Garra rufa | Iraq | OP588784 | ITS2 | Nejat et al. (2023) | |
| Paradiplozoon homoion | Rhodeus amarus | Turkey | MT028131 | ITS2 | Aydoğdu et al. (2020) | |
| Paradiplozoon homoion | Squalius cii | Turkey | OP588793 | ITS2 | Nejat et al. (2023) | |
| Paradiplozoon skrjabini | Gnathopogon elongatus elongatus | Japan | LC731724 | ITS2 | Nitta and Nagasawa (2023) | |
| Diplozoon paradoxum | Abramis brama | Czechia | AJ563372 | ITS2 | Matejusová et al. (2004) | |
| Paradiplozoon hemiculteri | Hemiculter leucisculus | China | KY290761 | ITS2 | Jirsová et al. (2018) | |
| Sindiplozoon ctenopharyngodoni | Ctenopharyngodon idella | China | DQ098898 | ITS2 | Gao et al. (2006) | |
| Paradiplozoon gracile | – | – | KP340973 | ITS2 | Unpublished | |
| Paradiplozoon sapae | Abramis sapa | Czechia | AJ300713 | ITS2 | Matejusová et al. (2001) | |
| Paradiplozoon barbi | – | – | MN688771 | ITS2 | Unpublished | |
| Sindiplozoon sp. | Coreius guichenoti | China | MW992745 | ITS2 | Cao et al. (2022) | |
| Eudiplozoon nipponicum | Cyprinus carpio | China | DQ098897 | ITS2 | Gao et al. (2006) | |
| Eudiplozoon nipponicum | – | – | OP376058 | ITS2 | Unpublished | |
| Octomacrum europaeum | Alburnoides bipunctatuts | Poland | MT441500 | ITS2 | Benovics et al. (2021) |
Parasites prevalence, abundance and intensity
The prevalence (p), and mean intensity (MI) of monogeneans were calculated as follows (Bush et al. 1997):
The data were entered into an Excel spreadsheet and transferred into Stata (ver. 11, StataCorp., College Station, TX, USA). Fisher’s exact test was used to compare parasite prevalence, and mean intensity in different seasons and years. P-values of <0.05 were considered as significant.
Results
The parasite specimens found in this study were subjected to morphological examination, which placed them in the family Diplozoidae and the genus Paradiplozoon. This classification was based on several distinguishing features, including the absence of dilatations of the middle part of the posterior end of the body, absence of musculo-glandular organs anterior to the buccal suckers, and absence of folds on the posterior part of the body, and the attachment of an egg filament on the opposite end of the uterus opening. However, there were morphological, morphometric (Table 2) and molecular differences between specimens in the present study and the previously described Paradiplozoon spp., as presented below. A new species was recognised, and is described below.
| Item | Paradiplozoon jalalii sp. nov. (present study) | Paradiplozoon schizothorazi (Galli et al. 2010) | P. yarkandense (Arken et al. 2022) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of specimens measured | Minimum | Maximum | Mean | Number of specimens measured | Minimum | Maximum | Mean | Number of specimens measured | Minimum | Maximum | Mean | ||
| Body length | 7 | 2.275 | 5.200 | 4.189 | Not stated | 4.1 | 6.1 | Not stated | 58 | 1.10 | 3.05 | 2.13 | |
| Length of the anterior part | 11 | 1.575 | 4.500 | 3.030 | Not stated | 1.0 | 1.8 | Not stated | 52 | 0.57 | 2.07 | 1.39 | |
| Length of the posterior part | 11 | 0.500 | 1.200 | 0.900 | Not stated | 2.1 | 3.8 | Not stated | 52 | 0.35 | 0.93 | 0.55 | |
| Total anterior and posterior parts | 11 | 2.075 | 5.675 | 3.930 | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | |
| Length of suckers | 12 | 0.080 | 0.120 | 0.097 | Not stated | 0.11 | 0.16 | Not stated | 52 | 0.05 | 0.08 | 0.06 | |
| Width of suckers | 12 | 0.080 | 0.115 | 0.095 | Not stated | Not stated | Not stated | Not stated | 52 | 0.04 | 0.07 | 0.05 | |
| Pharynx (length) | 9 | 0.060 | 0.100 | 0.080 | Not stated | 0.06 | 0.09 | Not stated | 41 | 0.046 | 0.049 | 0.047 | |
| Pharynx (width) | 9 | 0.050 | 0.075 | 0.066 | Not stated | Not stated | Not stated | Not stated | 41 | 0.041 | 0.043 | 0.042 | |
| Clamps 1 (length) | 6 | 0.083 | 0.103 | 0.093 | Not stated | 0.12 | 0.17 | Not stated | 54 | 0.05 | 0.08 | 0.07 | |
| Clamps 1 (width) | 6 | 0.100 | 0.145 | 0.122 | Not stated | 0.17 | 0.20 | Not stated | 54 | 0.09 | 0.12 | 0.11 | |
| Clamps 2 (length) | 6 | 0.080 | 0.123 | 0.094 | Not stated | 0.12 | 0.16 | Not stated | 53 | 0.08 | 0.10 | 0.09 | |
| Clamps 2 (width) | 6 | 0.120 | 0.158 | 0.139 | Not stated | 0.21 | 0.24 | Not stated | 53 | 0.14 | 0.18 | 0.15 | |
| Clamps 3 (length) | 6 | 0.085 | 0.115 | 0.096 | Not stated | 0.13 | 0.16 | Not stated | 52 | 0.09 | 0.11 | 0.10 | |
| Clamps 3 (width) | 6 | 0.120 | 0.155 | 0.135 | Not stated | 0.21 | 0.26 | Not stated | 52 | 0.14 | 0.19 | 0.16 | |
| Clamps 4 (length) | 6 | 0.080 | 0.120 | 0.096 | Not stated | 0.13 | 0.18 | Not stated | 53 | 0.07, | 0.09 | 0.08 | |
| Clamps 4 (width) | 6 | 0.120 | 0.138 | 0.126 | Not stated | 0.22 | 0.26 | Not stated | 53 | 0.12 | 0.17 | 0.14 | |
| Central hooks length | 3 | 0.013 | 0.015 | 0.014 | Not stated | 0.019 | 0.022 | Not stated | 33 | 0.039 | 0.045 | 0.042 | |
| Shaft length | 2 | 0.038 | 0.038 | 0.038 | Not stated | 0.042 | 0.049 | Not stated | 28 | 0.019 | 0.026 | 0.023 | |
| Eggs dimensions | 2 | 0.233 × 0.153 | 0.267 × 0.113 | – | Not stated | 0.13–0.33 × 0.15–0.24 | Not stated | Not stated | 29 | 0.18 × 0.06 | 0.22 × 0.08 | 0.20 × 0.07 | |
Paradiplozoon jalalii, sp. nov.
Type host: Schizocypris altidorsalis Bianco & Bănărescu, 1982.
Chah Nimeh water reservoirs (Fig. 1), Zabol, Province of Sistan and Baluchistan, Iran.
Holotype (G241122), 5 paratypes (G241123 to G241127), deposited in Queensland Museum, Australia.
The new species is named after the late Professor Behiar Jalali in recognition of his dedicated research on monogenean parasites in Iran.
Adults forming typical couples, two adults with X-shape body (Fig. 2b, 3a, b), split into anterior and posterior sections, and smooth tegument throughout body; total body length 4.189 (2.275–5.200, n = 7); anterior part 3.030 (1.575–4.500, n = 11) long and posterior part 0.900 (0.500–1.200, n = 11) long from the fusion area to haptor end; buccal suckers (Fig. 2c, 3g, h) one pair, larger than pharynx, circular, opening subterminal, 0.097 (0.080–0.120, n = 12) × 0.095 (0.080–0.115, n = 12), glandular structures absent; pharynx (Fig. 2c, 3g, h) circular, below buccal suckers, 0.080 (0.060–0.100, n = 9) × 0.066 (0.050–0.075, n = 9), opening into branched intestine. Reproductive organs located anteriorly in the posterior part of the body; testis (Fig. 3i) circular; ovary (Fig. 3i) larger and anterior to testis; eggs (Fig. 2e, 4d) 0.325 (0.316–0.333) × 0.233 (0.233–0.233, n = 2) in size, with filament in the pointed end and rounded in the other end; up to three eggs were found in one individual; haptor with four pairs of clamps (Fig. 3b, d, i) and one pair of central hooks (Fig. 4a, c) in each haptor; posterior arch of the clamps with cross-striation; first clamp (the most posterior) 0.093 (0.083–0.103, n = 6) × 0.122 (0.100–0.145, n = 6), second clamp 0.094 (0.080–0.123, n = 6) × 0.139 (0.120–0.158, n = 6), third clamp 0.096 (0.085–0.115, n = 6) × 0.135 (0.120–0.155, n = 6), the fourth clamp 0.096 (0.080–0.120, n = 6) × 0.126 (0.120–0.137, n = 6); central hook (Fig. 4a, c) sickles 0.014 (0.013–0.015, n = 3) long, hook handles 0.038 (0.038–0.038, n = 2) long; each clamp (Fig. 4a–c) consists of sclerotised structures, including median sclerite, trapezoid outgrowth on median sclerites and jaws. The anterior end of the median sclerite has numerous perforations that extend almost the entire length of the median region.
Light microscopy images of the P. jalalii found in the present study, showing variations in the morphological characteristics such as (a, b) overall anatomy of the body, (c, d) clamps, (e) mature and (f) immature eggs, and (g, h) anterior ends among different individuals with identical ITS2, 18S and 28S sequences. Image (i) shows ovary (o) and testis (t). Other abbreviations include bs, buccal sucker; h, haptor; oe, oseophagus; ph, pharynx.
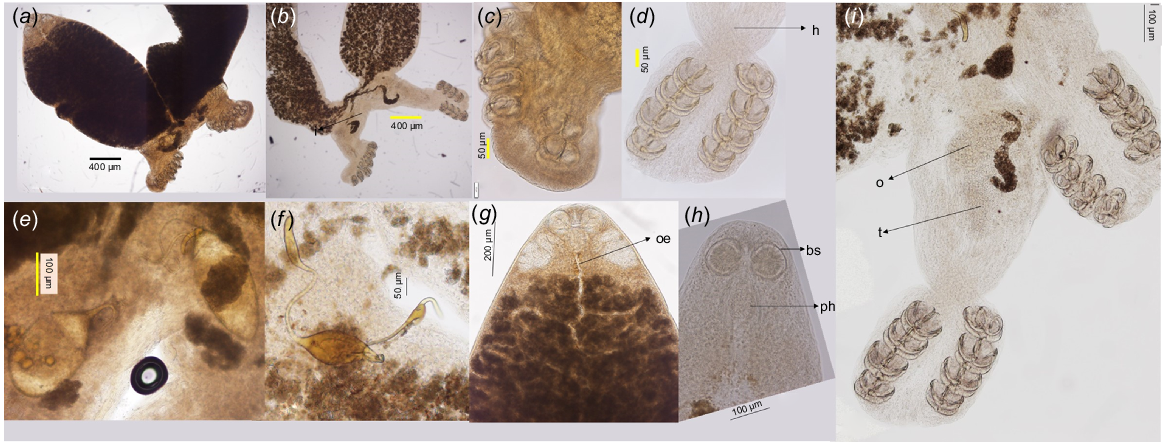
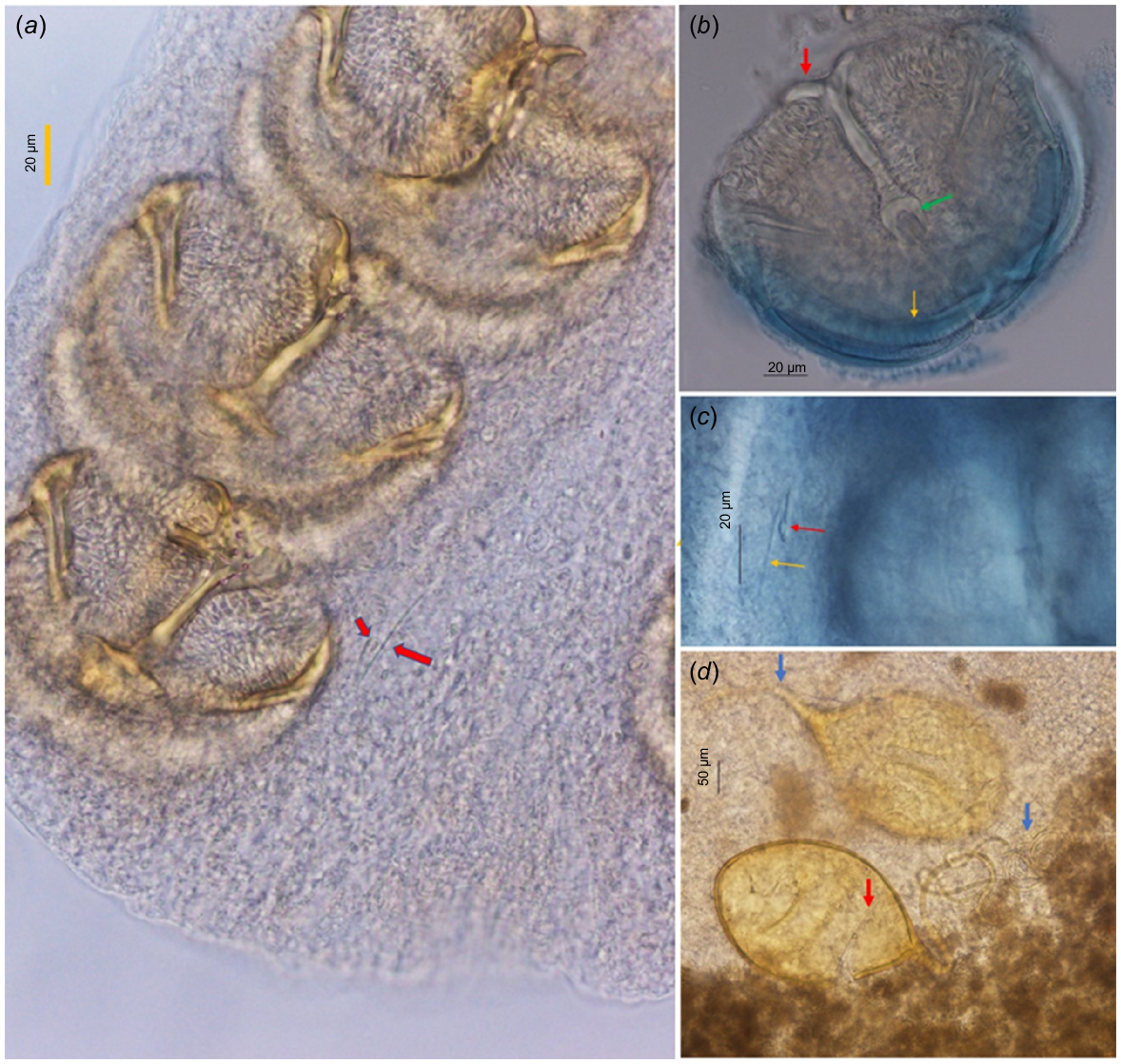
Light microscopy images of the clamps in P. jalalii found in the present study, showing details of the clamps and eggs: (a) showing the position and size of hook (red arrows) in the opisthohaptor, (b) clamp in posterior view, showing the median plate (red and green arrows) and medial sclerite of posterior jaw (yellow arrow), (c) handle (yellow arrow) and body of the central hook, and (d) egg filament (blue arrow) and operculum (red arrow).
Paradiplozoon stands out as the most diverse genus within the Diplozoinae subfamily, and its distinguishing feature for species indentification is primarily associated with its clamp structure. Specifically, the key morphological components include the median sclerite and its associated structures, such as the trapeze spur, as well as the anterior and posterior joining sclerites (Matejusová et al. 2004; Dos Santos et al. 2015; Huang et al. 2023). There are two distinct species of Paradiplozoon that parasitise Schizothorax spp., namely, Paradiplozoon schizothorazi (Iksanov, 1965) and P. yarkandense (Galli et al. 2010; Arken et al. 2022). As a result, a comparative analysis of the morphological traits of P. jalalii sp. nov. was conducted, showing notable distinctions when compared with both P. schizothorazi (Iksanov, 1965) and P. yarkandenseArken et al. (2022).
There were differences in the size of the various body parts between P. jalalii and P. yarkandense (Table 2). For example, body length of the new species was larger than that of P. yarkandense, and hooks in the new species were smaller than hooks in both P. schizothorazi and P. yarkandense. They were also different in the anatomy, with the oral sucker being oval, and testis being of irregular shape in the latter, whereas sucker and testis were circular inthe new species. P. jalalii and P. schizothorazi were very similar in the size of their body; however, the hook length (both the shaft and the hook itself) and the clamps were much smaller in the new species. The anatomy and measurements of the central hook and clamps are normally considered to be taxonomically significant, which were also different between P. jalalii and P. schizothorazi. In addition, the anterior end of the median sclerite in the new species has distinct Y-shaped projection absent in P. schizophrenic and P. yarkandense.
We generated the 18S, 28S and ITS2 sequences of the specimens investigated in our study (GenBank Accession numbers PZ203003–PZ203018). Among the six specimens, we obtained identical 18S sequences, with a length of 1033 bp. Notably, there were no 18S sequences belonging to Paradiplozoon spp. in GenBank at the time of our analysis. The BLAST analysis showed less than 95% similarity with Eudiplozoon nipponicum (AJ287510) or pairwise genetic distances ranging from 5.23 to 5.66%.
For the 28S region, sequences obtained from four specimens were also identical and spanned 1328 bp. In the BLAST results, the two most closely related diplozoids were E. nipponicum (AF382037) and Paradiplozoon sp. (KU519493), with estimated pairwise genetic distances of 13.70 and 15.87% respectively.
Additionally, we obtained ITS-2 sequences from six specimens, all of which were identical and 774 bp in length. For ITS2 comparisons, we selected 31 diplozoid sequences from GenBank. After aligning the data, the final dataset contained 882 positions, with 314-bp conserved sites, 547-bp variable sites, 424 parsimony-informative sites and 122-bp singleton sites. Similarly, for the 18S dataset, of the total 2040-bp sites, 1515 bp were conserved and 450 bp were variable. In the ITS2tree, the sequences of the newly described species formed a well-supported monophyletic group with P. yarkandense, another Monogenea species also found on Schizothorax fish, and then clustered with other Paradiplozoon spp. These findings strongly suggest that all the specimens in our study belong to a single and distinct species.
Moreover, our phylogenetic analyses (Fig. 5) provided further support for the genetic distinctiveness of the examined specimens compared with previously characterised species. This reaffirmed their classification as a distinct species within the genus Paradiplozoon, especially evident in the topography of the tree based on ITS2 sequences. Notably, only one to two diplozoid sequences from published works were available in GenBank for 18S and 28S comparisons.
Table 3 shows the details of the infection rate of fish with Paradiploozon jalalii. The maximum number of Paradiplozoon jalalii parasites found per fish in this study was two, with the majority of fish being infected by only one parasite (Table 3). Mean intensity was 1.0–1.3. This study showed no significant difference in the prevalence, mean intensity and mean abundance of Paradiplozoon jalalii between different years (Table 3).
| Season and year | Number of fish examined | Number of fish infected | Prevalence (%) | Total number of parasites | Number of fish with one parasite | Number of fish with two parasites | |
|---|---|---|---|---|---|---|---|
| Autumn 2020 (November) | 200 | 52 | 26 | 66 | 38 | 14 | |
| Winter 2021 (February) | 200 | 66 | 33 | 66 | 42 | 12 | |
| Total | 400 | 106 | 26.5 | 132 | 80 | 26 |
Discussion
This is the first study reporting monogenean parasites of Schizocypris altidorsalis. There was no opportunity in the study region where fish was collected to conduct a histopathology study. Therefore, the details of the damage caused by this parasite on fish gill remains unknown. Because, generally, the number of parasites is a crucial contributing factor to the occurrence of disease by Monogenea (Thoney and Hargis 1991), we speculate that it is unlikely that P. jalalii causes serious harm to the fish, owing to low number of the parasites found in the examined fish individuals. Although it is possible that the actual number of parasites was higher as we only collected and examined already dead fish.
In regard to the number of the parasites found in the fish host in the present study, the absence of the difference in prevalence and abundance of parasites in different seasons and years could be attributed to the narrow range of temperature fluctuations observed in the study area. Over the course of a 30-year period, the average temperatures in the area have been reported as being 7–15°C during autumn, and 1–12°C during winter (Zare Abianeh et al. 2015). Population of diplozoid Monogenea, including Paradiplozoon spp. on their fish hosts, exhibits seasonal variations, which are more pronounced during the warmer months (Gilbert and Avenant-Oldewage 2016).
There is limited knowledge about monogenean fauna of eastern regions of Iran that are influenced by Indian faunal region (Jalali et al. 2000). The present study also shows identification of a new species of diplozoid Monogenea in this region. Accurate identification and delimitation of diplozoid monogeneans have been always challenging. Morphologically they lack sclerotised genitalia and possess only small sclerites in their haptors, which are often difficult to accurately visualise owing to their orientation and position within tissue (Gläser and Gläser 1964; Khotenovsky 1985). Dos Santos and Avenant-Oldewage (2020) reviewed currently available genetic data, to uncover insights from the current sequence data, suggested improvements for future studies, and highlighted potential pitfalls to be avoided. Like several other studies about other parasitic taxa (Barton et al. 2022; Shamsi et al. 2024), Dos Santos and Avenant-Oldewage (2020) pleaded for a more integrated taxonomic approach and the inclusion of voucher material alongside the sequence data obtained from several additional markers, and concluded that analyses of a substantial amount of further morphological and genetic data are needed before an accurate study of the taxonomy and evolutionary history of diplozoid species can be achieved. These challenges over specific identification of the existing taxa makes introducing a new species challenging. With these in mind, we believe our study provides evidence for a new species of Monogenea, herein named Paradiplozoon jalalii, on a fish species, Schizocypris altidorsalis. Both fish and the parasite have been found only in a small area in the Province of Sistan and Baluchistan in Iran (R. Froese and D. Pauly, FishBase, ver. 10/2023, see www.fishbase.org). The reference materials are available in Queensland Museum for morphological examination by interested parties. The gDNA of this valuable species is also available in Shamsi’s Parasitology Laboratory at Charles Sturt University and can be handed over to interested researchers in the future.
The new species was different from previously known species on the basis of its size and also morphology of the clamps and hooks. Clamps and hooks serve as apparatus for maintaining the attachment of adult diplozoids to their hosts. The morphological characteristics and size of the clamps and hook have always been the primary basis for distinguishing among diplozoid Monogenea. However, it is important to note that factors such as host size, developmental stage, and environmental pollution can influence individual size and the shape and size of the clamp, resulting in intraspecific morphological variations within the same species of diplozoid (Pecínková et al. 2005; Arken et al. 2022). These characteristics can also be subject to different fixation and examination methods and varying observation angles by different researchers, affecting the morphological results to some extent.
Noting that not all diplozoid monogeneans have been genetically characterised yet, phylogenetic analyses using sequences of 18S, 28S and ITS2 regions also supported the distinction of our specimens from previously described species. The phylogenetic position of Paradiplozoon jalalii in the ITS2 tree (Fig. 5c) suggests that this species belongs to the genus Paradiplozoon, a paraphyletic genus (Huang et al. 2023). The present species is a sister of Eudiplozoon in the 28S and 18S trees (Fig. 5a, b); however, these trees do not include any or many other Paradiplozoon species in the analysis because of the lack of comparable data available in the GenBank. Future studies providing comparable sequences will further elucidate the taxonomic status of the specimens in the present study.
Although the phylogenetic trees constructed in the current study provide support for distinguishing the new species, they also prompt questions regarding the validity of certain previously described species. Notably, the unexpected position of Afrodiplozoon polycotyleus in Fig. 5c warrants attention. However, because of the lack of comparable sequence data for multiple regions across all diplozoids, drawing definitive conclusions about the phylogenetic relationships of these taxa has been shown to be challenging and falls outside the scope of the present study.
Finding of a highly host-specific parasite in the present study is also of significance because this parasite itself may be at risk of extinction, potentially owing to environmental factors or changes in host populations.
Although no pathological impact by the new species on its fish host can be concluded at this stage, this very parasite itself may have the potential to drive the extinction of its cyprinid fish hosts, if it was found to be pathogenic for its host. It is known that some Paradiplozoon spp. have blood-feeding habits (Smyth and Halton 1983; Rohlenová et al. 2011). The call for the conservation of parasite species began nearly three decades ago (Windsor 1995; Durden and Keirans 1996) and has persisted, although not consistently, over time. Preserving parasite species is crucial (Lymbery and Smit 2023) because they are integral components of natural ecosystems, just as important as the charismatic vertebrates that typically receive the majority of conservation attention and funding. Parasites play vital roles in maintaining the structure and functioning of ecosystems and also serve as valuable indicators of ecosystem health. Lists of threatened species, such as the International Union for Conservation of Nature’s (IUCN) Red List, continue to be widely used tools for biodiversity conservation (Moir and Brennan 2020). However, many unique species in conflict zones, such as the area studied here, often do not appear on these lists and it becomes challenging to determine effective strategies for safeguarding endangered populations. Parasite species dependent on threatened hosts may become extinct through either direct or indirect human action (Moir et al. 2012).
Author contributions
S. Shamsi was the team leader and prepared the manuscript, data analyses, drawings, light microscopy images, staining and morphometry. J. Khedri and H. Borji conducted fish dissections and collected parasites, and their relevant data. N. Francis contributed DNA extraction, PCR, sequencing and phylogenetic analyses. J. Suthar was responsible for morphology and morphometry examination of parasites.
Acknowledgements
Authors are grateful to Mr Craig Poynter at CSU for preparing the map for the study area.
References
Acosta AA, Smit NJ (2021) A first for Southern Africa: description of a new Heterobothrium (Monogenea: Diclidophoridae) parasitizing the evileye pufferfish Amblyrhynchotes honckenii (Tetraodontiformes: Tetraodontidae). Parasitology Research 120(3), 819-830.
| Crossref | Google Scholar | PubMed |
Arken K, Hao C-L, Guo A-M, Zhang W-R, Rong M-J, Kamal W, Tian S-L, Kadir M, Yue C (2022) A new species of Paradiplozoon (Monogenea: Diplozoidae), a gill parasite of the Schizothorax fish (Cyprinidae: Schizothoracinae) from the Yarkand River, Xinjiang, China. Acta Parasitologica 67, 330-339.
| Crossref | Google Scholar |
Aydoğdu N, Alemdar A, Koç HT, Erdoğan Z (2020) Susurluk Çayı (Balıkesir)’ndaki Acı Balık, Rhodeus amarus (Pallas, 1782) (Teleostei: Cyprinidae)’un Helmint Parazitleri üzerine bir araştırma: Acı Balık’ta Paradiplozoon homoion’un Türkiye’de ilk kaydı ve Helmint Parazitlerin her biri için yeni lokalite kaydı. Türk Tarım ve Doğa Bilimleri Dergisi 7(4), 1049-1056 [In Turkish].
| Google Scholar |
Badets M, Whittington I, Lalubin F, Allienne J-F, Maspimby J-L, Bentz S, Du Preez LH, Barton D, Hasegawa H, Tandon V (2011) Correlating early evolution of parasitic platyhelminths to Gondwana breakup. Systematic Biology 60(6), 762-781.
| Crossref | Google Scholar | PubMed |
Barton DP, Gherman CM, Zhu X, Shamsi S (2022) Characterization of tongue worms, Linguatula spp. (Pentastomida) in Romania, with the first record of an unknown adult Linguatula from roe deer (Capreolus capreolus Linnaeus). Parasitology Research 121(8), 2379-2388.
| Crossref | Google Scholar | PubMed |
Bazzi H, Ebrahimi H, Aminnejad B (2021) A comprehensive statistical analysis of evaporation rates under climate change in Southern Iran using WEAP (Case study: Chahnimeh Reservoirs of Sistan Plain). Ain Shams Engineering Journal 12(2), 1339-1352.
| Crossref | Google Scholar |
Benovics M, Koubková B, Civáňová K, Rahmouni I, Čermáková K, Šimková A (2021) Diversity and phylogeny of Paradiplozoon species (Monogenea: Diplozoidae) parasitising endemic cyprinoids in the peri-Mediterranean area, with a description of three new Paradiplozoon species. Parasitology Research 120, 481-496.
| Crossref | Google Scholar | PubMed |
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. The Journal of Parasitology 83(4), 575-583.
| Crossref | Google Scholar |
Cao S, Fu P, Zou H, Li M, Wu S, Wang G, Blazhekovikj-Dimovska D, Li W (2022) Sindiplozoon coreius n. sp. (Monogenea: Diplozoidae) from the gills of Coreius guichenoti (Cyprinidae) in China. Parasitology International 87, 102494.
| Crossref | Google Scholar | PubMed |
Carlson CJ, Hopkins S, Bell KC, Doña J, Godfrey SS, Kwak ML, Lafferty KD, Moir ML, Speer KA, Strona G, Torchin M, Wood CL (2020) A global parasite conservation plan. Biological Conservation 250, 108596.
| Crossref | Google Scholar |
Dos Santos QM, Avenant-Oldewage A (2016) The description of a new diplozoid species, Paradiplozoon krugerense n. sp., from Labeo rosae Steindachner, 1894 and Labeo congoro Peters, 1852 in the Kruger National Park, South Africa with notes on the effect of water quality on its infection variables. Hydrobiologia 777, 225-241.
| Crossref | Google Scholar |
Dos Santos QM, Avenant-Oldewage A (2020) Review on the molecular study of the Diplozoidae: analyses of currently available genetic data, what it tells us, and where to go from here. Parasites & Vectors 13(1), 539.
| Crossref | Google Scholar | PubMed |
Dos Santos QM, Van Vuuren BJ, Avenant-Oldewage A (2015) Paradiplozoon vaalense n. sp. (Monogenea: Diplozoidae) from the gills of moggel, Labeo umbratus (Smith, 1841), in the Vaal River System, South Africa. Journal of Helminthology 89(1), 58-67.
| Crossref | Google Scholar | PubMed |
Durden LA, Keirans JE (1996) Host–parasite coextinction and the plight of tick conservation. American Entomologist 42(2), 87-91.
| Crossref | Google Scholar |
Gao Q, Chen MX, Yao WJ, Gao Y, Song Y, Wang GT, Wang MX, Nie P (2006) Phylogeny of diplozoids in five genera of the subfamily Diplozoinae Palombi, 1949 as inferred from ITS-2 rDNA sequences. Parasitology 134(5), 695-703.
| Crossref | Google Scholar |
Gilbert BM, Avenant-Oldewage A (2016) Seasonal occurrence and microhabitat specificity of Paradiplozoon ichthyoxanthon Avenant-Oldewage in Avenant-Oldewage et al., 2014 (Monogenea: Diplozoidae) infecting Labeobarbus aeneus (Burchell) (Teleostei: Cyprinidae) from the Vaal Dam, South Africa: water quality and host size as determining factors? Folia Parasitologica 63, 2016.004.
| Crossref | Google Scholar |
Gilbert BM, Avenant-Oldewage A (2021) Monogeneans as bioindicators: a meta-analysis of effect size of contaminant exposure toward Monogenea (Platyhelminthes). Ecological Indicators 130, 108062.
| Crossref | Google Scholar |
Gläser H-J, Gläser B (1964) Zur taxonomie der gattung Diplozoon Nordmann, 1832. Zeitschrift für Parasitenkunde 25, 164-192 [In German].
| Crossref | Google Scholar |
Huang J, Zhou X, Yuan K, Ding X (2023) Paradiplozoon cirrhini n. sp. (Monogenea, Diplozoidae), a gill parasite of Cirrhinus molitorella (Cyprinidae, Labeoninae) in South China. Parasite 30, 20.
| Crossref | Google Scholar | PubMed |
Jalali B, Shamsi S, Molnar K (2000) New Dactylogyrus species (Monogenea, Dactylogyridae) from cyprinid fishes of the Bahu-Kalat River in southeast Iran. Acta Parasitologica 45(4), 289-294.
| Google Scholar |
Jalali B, Shamsi S, Barzegar M (2005) Occurrence of Gyrodactylus spp. (Monogenea: Gyrodactylidae) from Iranian freshwater fishes. Iranian Journal of Fisheries Sciences 4(2), 19-30 [In Persian].
| Google Scholar |
Jirsová D, Ding X, Civáňová K, Jirounková E, Ilgová J, Koubková B, Kašný M, Gelnar M (2018) Redescription of Paradiplozoon hemiculteri (Monogenea, Diplozoidae) from the type host Hemiculter leucisculus, with neotype designation. Parasite 25, 4.
| Crossref | Google Scholar |
Johnsen BO, Jensen AJ (1986) Infestations of Atlantic salmon, Salmo salar, by Gyrodactylus salaris in Norwegian rivers. Journal of Fish Biology 29(2), 233-241.
| Crossref | Google Scholar |
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7), 1870-1874.
| Crossref | Google Scholar | PubMed |
Leis E, Bailey J, Katona R, Standish I, Dziki S, McCann R, Perkins J, Eckert N, Baumgartner W (2023) A mortality event involving endangered pallid sturgeon (Scaphirhynchus albus) associated with Gyrodactylus conei n. sp. (Monogenea: Gyrodactylidae) effectively treated with Parasite-S (formalin). Parasitologia 3(2), 205-214.
| Crossref | Google Scholar |
Lockyer AE, Olson PD, Littlewood DTJ (2003) Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biological Journal of the Linnean Society 78(2), 155-171.
| Crossref | Google Scholar |
Lymbery AJ, Smit NJ (2023) Conservation of parasites: a primer. International Journal for Parasitology: Parasites and Wildlife 21, 255-263.
| Crossref | Google Scholar |
Matejusová I, Koubková B, D’Amelio S, Cunningham CO (2001) Genetic characterization of six species of diplozoids (Monogenea; Diplozoidae). Parasitology 123(5), 465-474.
| Crossref | Google Scholar |
Matejusová I, Koubková B, Cunningham CO (2004) Identification of European diplozoids (Monogenea, Diplozoinae) by restriction digestion of the ribosomal RNA internal transcribed spacer. Journal of Parasitology 90(4), 817-822.
| Crossref | Google Scholar | PubMed |
Mirnia N, Mirdar Harijani J, Gharaei A, Rigi M (2019) Survey of some metal elements (Cu, Zn, Ni, Pb) accumulation in muscle, liver, kidney and gill of Schizocypris altidorsalis in Chahnimeh reservoirs of Sistan. Iranian Scientific Fisheries Journal 28(5), 157-161.
| Google Scholar |
Moir ML, Brennan KEC (2020) Incorporating coextinction in threat assessments and policy will rapidly improve the accuracy of threatened species lists. Biological Conservation 249, 108715.
| Crossref | Google Scholar |
Moir ML, Vesk PA, Brennan KEC, Poulin R, Hughes L, Keith DA, McCarthy MA, Coates DJ (2012) Considering extinction of dependent species during translocation, ex situ conservation, and assisted migration of threatened hosts. Conservation Biology 26(2), 199-207.
| Crossref | Google Scholar | PubMed |
Moudi Z, Tabatabaei SM, Share Mollashahi S, Zaboli M (2022) Study factors involved in maternal deaths attributed to Covid-19 in a disadvantaged area in southeast of Iran. Journal of Family & Reproductive Health 16(1), 67-77.
| Crossref | Google Scholar | PubMed |
Nejat F, Benovics M, Řehulková E, Vukić J, Šanda R, Kaya C, Tarkan AS, Abdoli A, Aksu S, Šimková A (2023) Diversity, phylogeny and intraspecific variability of Paradiplozoon species (Monogenea: Diplozoidae) parasitizing endemic cyprinoids in the Middle East. Parasitology 150, 705-722.
| Crossref | Google Scholar |
Nitta M, Nagasawa K (2023) Gill monogeneans (Platyhelminthes) parasitic on Gnathopogon elongatus elongatus and G. caerulescens (Cypriniformes: Gobionidae) from Japan, with descriptions of one new species of Dactylogyrus and three new species of Bivaginogyrus (Dactylogyridae). Species Diversity 28(1), 69-97.
| Crossref | Google Scholar |
Obiakezie AI, Taege M (1991) Mortality in hatchery reared fry of the African catfish Clarias gariepinus (Burchell) caused by Gyrodactylus groschafti Ergens, 1973. Bulletin of the European Association of Fish Pathologists 11, 82-85.
| Google Scholar |
Ogawa K, Itoh N (2022) Five new and two known species of Heterobothrium (Monogenea: Diclidophoridae) infecting puffers of the genus Takifugu from Japanese waters. Systematic Parasitology 99(3), 317-340.
| Crossref | Google Scholar | PubMed |
Olson P, Littlewood D (2002) Phylogenetics of the Monogenea: evidence from a medley of molecules. International Journal for Parasitology 32(3), 233-244.
| Crossref | Google Scholar |
Pecínková M, Matejusova I, Koubková B, Gelnar M (2005) Classification and occurrence of abnormally developed Paradiplozoon homoion (Monogenea, Diplozoinae) parasitising gudgeon Gobio gobio. Diseases of Aquatic Organisms 64(1), 63-68.
| Crossref | Google Scholar |
Přikrylová I, Mašová Š, Gelnar M, Matla MM, Tavakol S, Luus-Powell WJ (2018) Redescription of the genus Afrodiplozoon Khotenovski, 1981 and its only known species Afrodiplozoon polycotyleus (Paperna, 1973) (Monogenea: Diplozoidae) using a combined multidisciplinary approach. Parasitology International 67(2), 245-252.
| Crossref | Google Scholar | PubMed |
Rohlenová K, Morand S, Hyršl P, Tolarová S, Flajšhans M, Šimková A (2011) Are fish immune systems really affected by parasites? An immunoecological study of common carp (Cyprinus carpio). Parasites & Vectors 4(1), 120.
| Crossref | Google Scholar |
Roohi JD, Dalimi Asl A, Pourkazemi M, Shamsi S (2019) Occurrence of dactylogyrid and gyrodactylid Monogenea on common carp, Cyprinus carpio, in the southern Caspian Sea Basin. Parasitology International 73, 101977.
| Crossref | Google Scholar |
Rozhkovan KV, Shedko MB (2015) Phylogenetic relationships of Paradiclybothrium pacificum and Diclybothrium armatum (Monogenoidea: Diclybothriidae) inferred from 18S rDNA sequence data. Parasitology International 64(5), 448-452.
| Crossref | Google Scholar | PubMed |
Rubio-Godoy M, Paladini G, Freeman MA, Garcia-Vasquez A, Shinn AP (2012) Morphological and molecular characterisation of Gyrodactylus salmonis (Platyhelminthes, Monogenea) isolates collected in Mexico from rainbow trout (Oncorhynchus mykiss Walbaum). Veterinary Parasitology 186(3–4), 289-300.
| Crossref | Google Scholar | PubMed |
Shamsi S, Steller E, Chen Y (2018) New and known zoonotic nematode larvae within selected fish species from Queensland waters in Australia. International Journal of Food Microbiology 272, 73-82.
| Crossref | Google Scholar | PubMed |
Shamsi S, Day S, Zhu X, McLellan M, Barton DP, Dang M, Nowak BF (2021) Wild fish as reservoirs of parasites on Australian Murray cod farms. Aquaculture 539, 736584.
| Crossref | Google Scholar |
Shamsi S, Nelson L, Gordon A, Markham K, Francis N, Suthar J, Zhu X (2024) Multidisciplinary approach to the diagnosis of Contracaecum magnipapillatum infections in Australian black noddies, Anous minutus (Charadriiformes: Laridae). Parasitology Research 123(1), 90.
| Crossref | Google Scholar | PubMed |
Soler-Jiménez LC, Hernández-Mena DI, Centeno-Chalé OA, Vidal-Martínez VM (2021) A new species of Neoheterobothrium Price, 1943 (Monogenea, Diclidophoridae) from Syacium papillosum (Linnaeus) (Pleuronectiformes, Paralichthyidae) in the Yucatan Shelf, with notes on the validity of the subfamilies in the Diclidophoridae. Parasitology Research 120, 887-897.
| Crossref | Google Scholar | PubMed |
Thoney DA, Hargis W, Jr (1991) Monogenea (Platyhelminthes) as hazards for fish in confinement. Annual Review of Fish Diseases 1, 133-153.
| Crossref | Google Scholar |
Torres-Carrera G, Ruiz-Escobar F, García-Prieto L, Oceguera-Figueroa A (2020) Narcinecotyle longifilamentus n. gen., n. sp. (Monogenea: Hexabothriidae), gill parasite of the numbfish Narcine entemedor (Torpediniformes: Narcinidae) from the Mexican Pacific coast. Parasitology International 76, 102095.
| Crossref | Google Scholar | PubMed |
Verma A, Verma J (2022) Redescription and new host record of Heteraxinoides atlanticus (Monogenea: Heteraxinidae) from the gills of Nemipterus japonicus (Bloch) and its systematics. Agricultural Science Digest – A Research Journal 42(2), 203-209.
| Crossref | Google Scholar |
Víllora-Montero M, Pérez-del-Olmo A, Georgieva S, Raga JA, Montero FE (2020) Considerations on the taxonomy and morphology of Microcotyle spp.: redescription of M. erythrini van Beneden & Hesse, 1863 (sensu stricto) (Monogenea: Microcotylidae) and the description of a new species from Dentex dentex (L.) (Teleostei: Sparidae). Parasites & Vectors 13(1), 45.
| Crossref | Google Scholar |
Windsor DA (1995) Equal-rights for parasites. Conservation Biology 9(1), 1-2.
| Crossref | Google Scholar |
Zare Abianeh H, Sabziparvar A, Marofi S, Ghiyami F, Mirmasoud SS, Kazemi A (2015) Analyzing and monitoring the meteorological droughts in the region of Sistan and Balouchestan. Environmental Science and Technology Quarterly 17(1), 49-61 [In Persian].
| Google Scholar |