The utility of otolith weight in growth studies of young-of-year bony bream (Nematalosa erebi), Australia’s most widespread freshwater fish
Oliver P. Pratt A * , Leah S. Beesley
A * , Leah S. Beesley  A , Bradley J. Pusey
A , Bradley J. Pusey  A , Daniel C. Gwinn
A , Daniel C. Gwinn  B , Chris S. Keogh A , Samantha A. Setterfield
B , Chris S. Keogh A , Samantha A. Setterfield  A and Michael M. Douglas
A and Michael M. Douglas  A
A
A
B
Abstract
Otoliths are calcified structures in the inner ear of fish, the analysis of which can be used to derive important life-history characteristics. Otoliths can be used to age young fish by counting daily growth increments visible in the otolith cross-section; however, this is costly and time-consuming. Otolith weight is a potential surrogate for fish age in growth analysis, providing a rapid alternative. Bony bream (Nematalosa erebi) is Australia’s most widespread freshwater fish and an important component of riverine food webs, yet its life-history characteristics are informed by few publications. We investigated the relationship between assumed fish age derived from otolith increments and otolith weight in young-of-year bony bream. We also assessed the utility of otolith weight for use in relative growth rate analysis. Linear modelling showed a significant positive relationship between increment count and otolith weight. Otolith weight when paired with body length was a reliable alternative to increment count, and thus age, for use in relative growth studies. This method can facilitate research into the factors shaping the life history of this ecologically significant species.
Keywords: biomonitoring, conservation, ecology, ecosystem processes, environmental monitoring, fish, fisheries, flooding, floodplains, flow regulation, freshwater, otoliths.
Introduction
Otoliths are calcified structures in the inner ear of fish, the analysis of which can provide species-specific life-history characteristics and inform demographic traits of fish populations (Burbank et al. 2021; Roberts et al. 2021). In particular, otolith microstructure can be used to age fish because otolith growth occurs through the daily addition of calcium carbonate increments (Campana and Thorrold 2001). Under magnification, daily increments can be counted in young fish to determine age, from which life-history characteristics such as growth rate can be estimated. The addition of daily increments is continual even during periods when somatic growth is non-existent (Williams and Bedford 1974; Maillet and Checkley 1990). Moreover, otoliths are the only calcified structures in fish that are not subject to resorption, even during periods of starvation (Campana and Thorrold 2001).
The process of aging fish by counting daily increments can be time consuming and costly. An alternative is to use otolith weight as a proxy for age. Support for the association of otolith weight and age is strong in early life-stage fish, with younger fish having lighter otoliths than older fish (Pacheco et al. 2021). Age estimation using otolith weight is low cost, quick and requires less specialised equipment and operational training than does ageing by daily increments.
The strong association between age and otolith weight lends itself to studies examining growth and growth-related life-history characteristics. In some species otolith weight can be used to assign a specific age to individuals (Cardinale et al. 2000), although this relationship can also show considerable variability, resulting in unreliable age estimates (Francis and Campana 2004; Hansen et al. 2022). However, for many applications, specific age estimates are not required, particularly when evaluating growth differences among various treatment groups, such as, habitat type or season. When controlling for bodylength, otolith weight has been shown to be a reliable method for analysing relative differences in growth rate (Templeman and Squires 1956; Strelcheck et al. 2003).
The clupeid gizzard shad bony bream (Nematalosa erebi (Günther)) is the most widespread freshwater fish species in Australia and a key component in riverine food webs (Pusey et al. 2021). It is an important prey fish for many higher-order consumers and, as a detritivore, it facilitates the movement of terrestrial carbon into the aquatic food chain (Pusey et al. 2021). The species is also an important bait fish for customary hunting (Jackson et al. 2012). Information on the life history of bony bream, particularly their growth rate, is informed by few publications, despite its ecological and cultural significance. Two studies from opposite ends of the species range have directly measured growth using otolith daily increment counts from young-of-year individuals. Stocks et al. (2019) constructed size-at-age growth models in the Macquarie River (NSW) and Pratt et al. (2023) linked growth rate to environmental factors (food availability, temperature, habitat) in the Fitzroy River (WA). Southwell et al. (2015) used otolith increments as a proxy for age to back-calculate hatch dates when evaluating the influence of environmental water release on spawning and recruitment, but used bodylength frequency analysis to assess growth. Other studies have also used length-frequency distributions or biomass to infer variability in growth and recruitment in relation to flow characteristics and food resources (Puckridge and Walker 1990; Balcombe et al. 2007, 2015; Lear et al. 2023); however, these approaches will be less reliable than using otoliths. Studies that track the size of a cohort (i.e. modal length) through time within the same site will have the precision of their growth estimate influenced by the number of fish measured (Vokoun et al. 2001; Miranda 2007). Studies that compare cohort length among treatment groups (e.g. site, year, season, habitat etc.) make the assumption that timing of spawning is uniform across treatment groups (Laslett et al. 2004), an assumption that may not hold for bony bream. Bony bream can spawn at multiple times throughout the year, including under different flow conditions (Puckridge and Walker 1990; Kerezsy et al. 2011; Stocks et al. 2021), and has been found to spawn at different times at different sites within the same catchment (Beesley 2006). Thus, these studies may mistakenly attribute cohort length differences to varied growth rate when they are simply caused by differences in age. Therefore, when assessing the influence of habitat, hydroperiod or environmental flow characteristics etc., a more robust method of inferring growth using otoliths is preferred.
Despite the wealth of literature confirming the daily addition of otolith increments in young-of-year fishes (Pannella 1971; Brown and Wooden 2007; Sponaugle 2009; Burndred et al. 2017), species-specific validation of this relationship is desirable, although not always available. Currently, this relationship has not been validated in bony bream. Here, we use the term ‘increment count’ rather than age for transparency.
Bony bream is an important component of riverine food webs across northern and central Australia. Understanding the factors that shape growth and recruitment of the species is therefore essential. This study seeks to find out whether otolith weight can be used as a surrogate for increment count (age) derived from otoliths in young-of-year bony bream. Specifically, we investigate the utility of using otolith weight paired with bodylength data in evaluating growth across different treatment groups. Given the strong association between otolith weight and increment count or age in other fish species, we hypothesise that growth patterns will be consistent whether using increment count or otolith weight as a proxy for age. Establishing this relationship will offer a fast and low-cost method of assessing relative growth among treatment groups, facilitating research into the factors affecting the life history of this ecologically and culturally important species.
Materials and methods
Animal ethics
This research was conducted under Fisheries exemption 191-2009-27 and 2974, Animal Ethics permits RA/3/100/1536 and RA/3/100/884 (Animal Ethics Committee, The University of Western Australia) and the Department of Environment and Conservation permit number SF006973. All field work was undertaken in accordance with the Animal Welfare Act (2002) (Western Australia).
Study site and field sampling
In total, 315 fish were sampled during the dry season (June–December) from the lower 350 km of the Fitzroy River (Kimberley, Western Australia) over a 3-year period (2018–2020). Fish were sampled from main channel and floodplain sites. At each site, young-of-year bony bream (<100-mm standard length) was sampled using seine nets (mesh size 7 or 9 mm) or cast nets (mesh size 15 mm). A maximum of 10 individuals spanning the size range were euthanased, frozen, and transported to the laboratory for processing. Fish remained frozen for <1 month prior to processing.
Laboratory procedures: otolith weight
Before removal of otoliths, the standard length (mm) of the body of each individual was measured. Both sagittae otoliths were removed from each fish, dried, and stored. Prior to weighing, a dissecting microscope was used to remove foreign bodies and check for chipped otoliths, which were excluded from analysis. Both otoliths from each individual were weighed using a Shimadzu AUW220D analytical balance (d = 0.00001 g). There was no difference in otolith weight between left-lateral and right-lateral sides (paired-sample Student‘s t-test: t48 = 0.224, P = 0.82).
Laboratory procedures: counting otolith increments
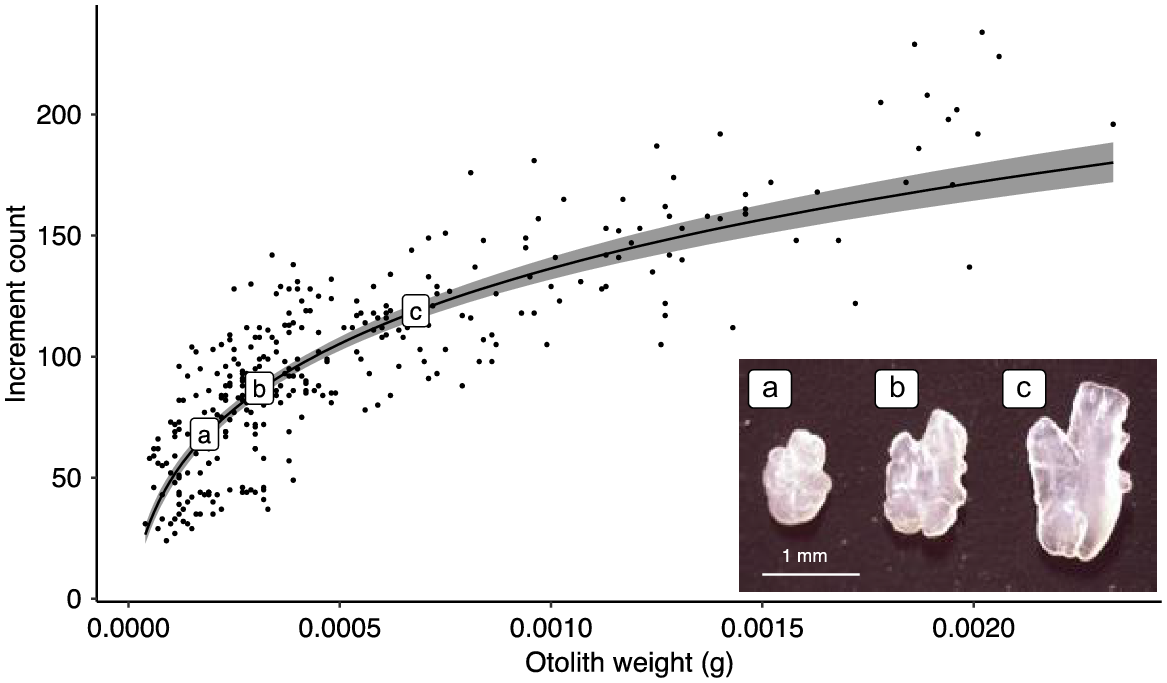
Otolith preparation followed the method described by Secor et al. (1992) for otoliths <300 μm with thermoplastic resin used as the mounting medium. The proximal and distal surfaces of each otolith were ground down by using 1200-grit lapping film to expose growth increments. Otoliths were viewed under 400× magnification by using a Leica DM-3000 microscope. Assumed age was estimated for each individual by counting increments from a hatch mark (~15 μm from the primordium) to the outer edge of the otolith section. Each otolith was ‘aged’ twice, and if increment counts were inconsistent, a third read occurred and an ‘agreed count’ determined. Increments from a subsample of 126 otoliths were counted a final time to quantify intra-reader error, expressed as the average percentage error (APE). Derived APE was 2.1%, below the 3% considered to indicate acceptable precision (Chilton and Beamish 1982). Increment count, approximating fish age (days), was recorded with corresponding otolith weight (g).
Statistical approach
Statistical analysis was divided into two parts. First, we investigated the relationship between increment count (i.e. assumed fish age) and otolith weight by using all available data (n = 315 individuals). We used least-squares linear regression to assess this relationship with otolith increment count as the dependent variable (y) and otolith weight as the independent variable (x) by using base packages within program R (ver. 4.1.2, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/). To meet assumptions of normality, both variables were power-transformed prior to analysis following the maximum-likelihood approach of Box and Cox (1964), by using the car package (ver. 3.0–12, see https://CRAN.R-project.org/package=car; Fox and Weisberg 2019) called in R. Otolith weight data were then centred on zero and scaled to one standard deviation. Model fit was diagnosed with visual inspection of residual and Q–Q plots.
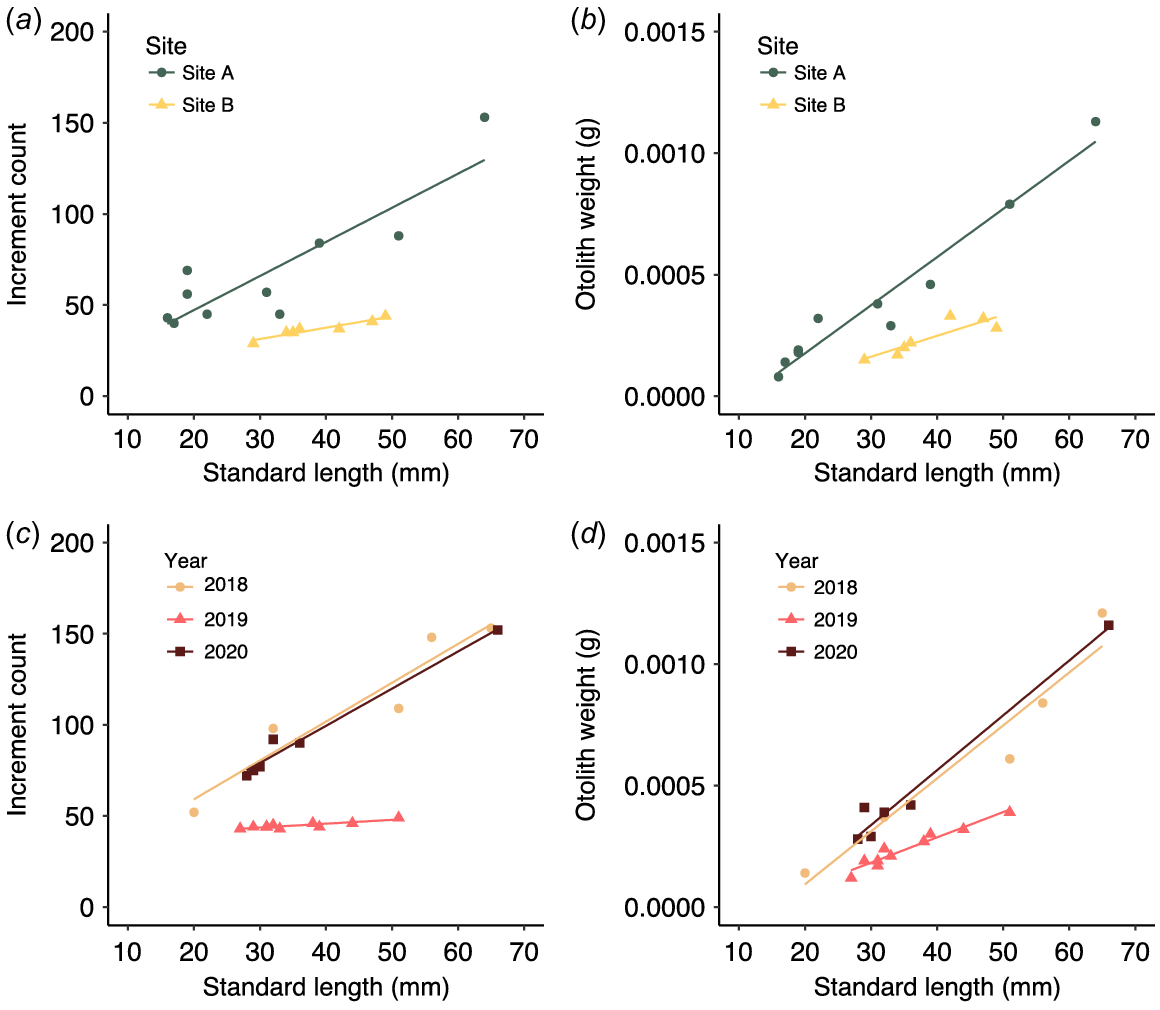
For the second part of our analysis, we aimed to demonstrate the effectiveness of using otolith weight instead of increment count as a proxy for age in relative growth analysis. For illustrative purposes and for ease of interpretation, we used a subset of data (n = 38 individuals, see Table 1) split into two treatment groups, as follows: ‘site’, a two-level factor (two floodplain pools sampled within the same week) and ‘year’, a three-level factor (the same site sampled over three consecutive years). If otolith weight is a suitable alternative to increment count, the same statistically significant or non-significant differences in growth between factor levels in our treatment groups should be detected regardless of the response variable. Patterns of relative growth in each treatment group were assessed using least-squares multiple regression. Standard length (mm) was included as a covariate to control for the effect of bodylength on the response variable (otolith weight or increment count), i.e. older fish with heavier otoliths tend to be larger. We expected that the relationship between increment count or otolith weight and standard length could change across treatment groups, so the interaction term treatment group × standard length was included in all models. Statistical analysis was conducted for each response variable in each treatment group (n = 4 models). Type III sum of squares was used because of uneven sample numbers by using the car package (ver. 3.0–12; Fox and Weisberg 2019). Prior to analysis, both otolith weight and increment count were natural-log transformed to meet the assumptions of homoscedasticity. Standard-length data were centred on zero and scaled to one standard deviation. All other parametric assumptions were evaluated using a combination of residual Q–Q plots and fitted values. In models with a three-level factor (‘year’ treatment group), comparisons among levels were assessed with Tukey’s HSD post hoc test. Comparisons were averaged over covariate effects by using the glht function in R package multcomp (ver. 1.4-25, see https://cran.r-project.org/package=multcomp; Hothorn et al. 2008).
| Item | Treatment group | |||||
|---|---|---|---|---|---|---|
| Site | Year | |||||
| Site A | Site B | 2018 | 2019 | 2020 | ||
| n individuals | 10 | 7 | 5 | 10 | 6 | |
| SL range (mm) | 16–64 | 29–49 | 20–65 | 27–51 | 28–66 | |
| mean SL ± s.d. (mm) | 31.1 ± 16.2 | 38.9 ± 7.3 | 44.8 ± 18.4 | 35.5 ± 7.5 | 36.8 ± 14.6 | |
SL, standard length; s.d., standard deviation; n, number of individuals.
Results
Increment count and otolith weight relationship
The relationship between otolith weight and otolith increment count was positive and statistically significant (R2 = 0.688, F313 = 694.4, P < 0.001), although the observed relationship was complex. For instance, an increase in otolith weight corresponded with an increase in increment count (effect size 1.646, 95% CI 1.254 and 1.769), but the association was non-linear and contemporaneous with changes in otolith morphology during development (Fig. 1). Considerable variation in otolith increment count for a given otolith weight was also observed. For example, an otolith weight of 0.0004 g had an increment count range of 95–131 (Fig. 1).
Treatment groups
Patterns of relative growth across treatment groups were consistent regardless of whether increment count or otolith weight was used as the response variable (Fig. 2). Within the treatment group ‘site’, growth was faster at Site B (Fig. 2a, b). This site effect was significant for increment count (F(1,13) = 47.765, P ≤ 0.001) and otolith weight (F(1,13) = 16.040, P = 0.001) when controlling for the significant effect of standard length (SL) (response: increment count, F(1,13) = 38.554, P ≤ 0.001; response: otolith weight, F(1,13) = 73.344, P ≤ 0.001). The relationship between response variables and standard length was consistent across sites as indicated by a non-significant interaction term in both models (P > 0.05). The year in which fish were sampled also had a significant influence on growth when controlling for standard length, regardless of response variable (response: increment count, F(2,15) = 150.405, P ≤ 0.001; response: otolith weight, F(2,15) = 24.495, P ≤ 0.001). The covariate standard length was also significant (response: increment count, F(1,15) = 86.401, P ≤ 0.001; response: otolith weight, F(1,15) = 107.487, P ≤ 0.001). The relationship between increment count and standard length was significantly different in 2019 only (P = 0.001). Tukey’s HSD post hoc tests showed significant (P < 0.001) differences in growth between 2018–2019 and 2019–2020, but non-significant (P > 0.05) differences between 2018 and 2020 for both response-variable groups.
Discussion
Bony bream is an ecologically and culturally important species that occurs across much of Australia. This study has validated a relationship between otolith weight and increment count in young-of-year individuals of the species, specifically in fish with an assumed age of less than 250 days. We demonstrated that when paired with bodylength data, otolith weight is a reliable alternative to increment count when evaluating relative growth among treatment groups. Using otolith weight is considerably less expensive and time consuming than counting otolith increments. We encourage researchers to use otolith weight paired with bodylength data to investigate the factors influencing the growth of bony bream in the early stages of its life.
The strong association between increment count and otolith weight in young-of-year bony bream demonstrated in this study is consistent with existing literature across a broad range of species (Pacheco et al. 2021). The non-linear relationship observed is likely to be a result of ontogenetic changes in otolith morphology that occur in early life stages (Radtke 1989). For example, the shape of bony bream otoliths changes during early development, which effectively alters the relationship between otolith weight and increment count while otolith morphology is developing. Despite the strong association, our results also showed that there is considerable variation in increment count for a given otolith weight, a finding observed in other species (Francis and Campana 2004; Hansen et al. 2022). This variation can be attributed to differences in growth rate among individuals caused by environmental effects. For instance, the width between successive daily (and annual) otolith increments in fish that experience rapid growth is typically greater than for those that experience slow growth (Gauldie 1991; Sponaugle 2009). Thus, individuals with higher growth rates have larger and therefore heavier otoliths than do slow-growing counterparts of the same age (Francis and Campana 2004). The growth rate of juvenile bony bream can vary substantially through space and time, depending on environmental conditions (Stocks et al. 2019). In the Fitzroy River, north-western Australia, the primary mechanism influencing the growth of juvenile bony bream was zooplankton biomass, which varied 250-fold between floodplain pools sampled within the same week (Pratt et al. 2023). Given the observed variability in the increment count–otolith weight relationship and potential differences in site-scale growth rate, we caution against the use of otolith weight to assign individual ages to young-of-year bony bream.
Understanding the mechanisms that influence growth and survival is important for the management of freshwater fisheries (Arlinghaus et al. 2015), and this is particularly true for ecologically important species such as bony bream. Our results demonstrated that when paired with bodylength data, otolith weight is a reliable metric for use in relative growth-rate analysis of young-of-year bony bream. Recent modelling of the species suggests that faster growth boosts survival, increasing the number of individuals that recruit into the adult spawning stock (Pratt et al. 2023). A greater understanding of the factors that influence growth can help shape management strategies to ensure that bony bream thrive in environments that are increasingly threatened by anthropogenic disturbance (Arthington and Pusey 2003; Brodie and Mitchell 2005; King et al. 2015) and climate change (Finlayson et al. 2013).
We acknowledge several limitations in this study. First, we recognise that increment counts are more accurate than otolith-weight measurements when approximating age. As such, we recommend that when using otolith weight, potential sources of variation are kept to a minimum to ensure appropriate statistical power and reduction of Type II errors. Second, growth studies of young-of-year fish using age derived from otolith increment counts typically require validation that increments are accrued one per day. This relationship has not been validated for bony bream. However, the vast majority of published literature demonstrate daily accrual of otolith increments across a variety of marine and freshwater fishes (Pannella 1971; Brown and Wooden 2007; Sponaugle 2009; Burndred et al. 2017), including species within the same subfamily as bony bream (Dorosoma cepedianum, Clupidae: Dorosomatinae; Davis et al. 1985). Moreover, increment count–bodylength relationships in our data set compare favourably with individuals from the opposite end of the species range (Macquarie River, Murray–Darling Basin) published by Stocks et al. (2019). Ultimately, daily age validation of otolith increments in young-of-year bony bream is desirable and should be a focus of future research. An overview of validation methods is provided in Campana (2001).
Data availability
All data associated with this study are available through the University of Western Australia’s research repository (https://research-repository.uwa.edu.au/en/datasets/).
Declaration of funding
This project was supported by funding to M. M. Douglas from the Australian Government’s National Environmental Science Program through the Northern Australia Environmental Resources Hub.
Author contributions
O. P. Pratt, L. S. Beesley, B. J. Pusey and M. M. Douglas were responsible for conceptualisation and developing methods. O. P. Pratt, L. S. Beesley and C. S. Keogh conducted the research. O. P. Pratt, L. S. Beesley and Daniel C. Gwinn contributed data analysis, data interpretation, and preparation of figures and tables. O. P. Pratt, L. S. Beesley, B. J. Pusey, D. C. Gwinn, S. A. Setterfield and M. M. Douglas were responsible for writing this manuscript.
Acknowledgements
We gratefully acknowledge the Traditional Owners of the country on which this work was undertaken, the Nyikina-Mangala, Yanunijarra and Gooniyandi people, and recognise their continuing connection to land, waters and culture. We pay our respects to their Elders, past, present and emerging. We thank the Prescribed Body Corporates of the different native title groups, and representatives from Yungngora Community, for providing us with permission to undertake research on country. Thanks go to the Indigenous Rangers and ranger co-ordinators who assisted with field work and we acknowledge the assistance of the Kimberley Land Council in project facilitation, particularly Karen Dayman. We thank the pastoral managers of Jubilee Downs, Myroodah, Mount Anderson, Millijidee, Noonkanbah and Gogo Stations for their cooperation. Thanks go to Brett Taylor for his insight and advice concerning otoliths and otolith processing.
References
Arthington AH, Pusey BJ (2003) Flow restoration and protection in Australian rivers. River Research and Applications 19, 377-395.
| Crossref | Google Scholar |
Balcombe SR, Bunn SE, Arthington AH, Fawcett JH, Mckenzie-Smith FJ, Wright A (2007) Fish larvae, growth and biomass relationships in an Australian arid zone river: links between floodplains and waterholes. Freshwater Biology 52, 2385-2398.
| Crossref | Google Scholar |
Balcombe SR, Turschwell MP, Arthington AH, Fellows CS (2015) Is fish biomass in dryland river waterholes fuelled by benthic primary production after major overland flooding? Journal of Arid Environments 116, 71-76.
| Crossref | Google Scholar |
Box GEP, Cox DR (1964) An analysis of transformations. Journal of the Royal Statistical Society – B. Methodological 26, 211-243.
| Crossref | Google Scholar |
Brodie JE, Mitchell AW (2005) Nutrients in Australian tropical rivers: changes with agricultural development and implications for receiving environments. Marine and Freshwater Research 56, 279-302.
| Crossref | Google Scholar |
Brown P, Wooden I (2007) Age at first increment formation and validation of daily growth increments in golden perch (Macquaria ambigua: Percichthyidae) otoliths. New Zealand Journal of Marine and Freshwater Research 41, 157-161.
| Crossref | Google Scholar |
Burbank J, Drake DAR, Power M (2021) Urbanization correlates with altered growth and reduced survival of a small-bodied, imperilled freshwater fish. Ecology of Freshwater Fish 30, 478-489.
| Crossref | Google Scholar |
Burndred KR, Cockayne BJ, Lou DC (2017) Early development of eel-tailed catfish, Tandanus tandanus (Mitchell) (Teleostei : Plotosidae), with validation of daily otolith increment formation. Australian Journal of Zoology 65, 12-20.
| Crossref | Google Scholar |
Campana SE (2001) Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59, 197-242.
| Crossref | Google Scholar |
Campana SE, Thorrold SR (2001) Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations. Canadian Journal of Fisheries and Aquatic Sciences 58, 30-38.
| Crossref | Google Scholar |
Cardinale M, Arrhenius F, Johnsson B (2000) Potential use of otolith weight for the determination of age-structure of Baltic cod (Gadus morhua) and plaice (Pleuronectes platessa). Fisheries Research 45, 239-252.
| Crossref | Google Scholar |
Davis RD, Storck TW, Miller SJ (1985) Daily growth increments in the otoliths of young-of-the-year gizzard shad. Transactions of the American Fisheries Society 114, 304-306.
| Crossref | Google Scholar |
Finlayson CM, Davis JA, Gell PA, Kingsford RT, Parton KA (2013) The status of wetlands and the predicted effects of global climate change: the situation in Australia. Aquatic Sciences 75, 73-93.
| Crossref | Google Scholar |
Francis RICC, Campana SE (2004) Inferring age from otolith measurements: a review and a new approach. Canadian Journal of Fisheries and Aquatic Sciences 61, 1269-1284.
| Crossref | Google Scholar |
Gauldie RW (1991) The morphology and periodic structures of the otolith of the chinook salmon (Oncorhynchus tshawytscha), and temperature-dependent variation in otolith microscopic growth increment width. Acta Zoologica 72, 159-179.
| Crossref | Google Scholar |
Hansen MJ, Nate NA, Muir AM, Chavarie L, Howland KL, Krueger CC (2022) Usefulness of otolith weight for estimating age-based life history metrics of lake trout. North American Journal of Fisheries Management 42, 1359-1371.
| Crossref | Google Scholar |
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical Journal 50, 346-363.
| Crossref | Google Scholar | PubMed |
Jackson S, Finn M, Featherston P (2012) Aquatic resource use by indigenous Australians in two tropical river catchments: the Fitzroy River and Daly River. Human Ecology 40, 893-908.
| Crossref | Google Scholar |
Kerezsy A, Balcombe SR, Arthington AH, Bunn SE (2011) Continuous recruitment underpins fish persistence in the arid rivers of far-western Queensland, Australia. Marine and Freshwater Research 62, 1178-1190.
| Crossref | Google Scholar |
King AJ, Townsend SA, Douglas MM, Kennard MJ (2015) Implications of water extraction on the low-flow hydrology and ecology of tropical savannah rivers: an appraisal for northern Australia. Freshwater Science 34, 741-758.
| Crossref | Google Scholar |
Laslett GM, Eveson JP, Polacheck T (2004) Fitting growth models to length frequency data. ICES Journal of Marine Science 61, 218-230.
| Crossref | Google Scholar |
Lear KO, Ebner BC, Fazeldean T, Whitty J, Morgan DL (2023) Inter-decadal variation in diadromous and potamodromous fish assemblages in a near pristine tropical dryland river. Ecology of Freshwater Fish 32, 444-463.
| Crossref | Google Scholar |
Maillet GL, Checkley DM, Jr (1990) Effects of starvation on the frequency of formation and width of growth increments in sagittae of laboratory-reared Atlantic menhaden Brevoortia tyrannus larvae. Fishery Bulletin 88, 155-165.
| Google Scholar |
Miranda LE (2007) Approximate sample sizes required to estimate length distributions. Transactions of the American Fisheries Society 136, 409-415.
| Crossref | Google Scholar |
Pacheco C, Bustamante C, Araya M (2021) Mass-effect: understanding the relationship between age and otolith weight in fishes. Fish and Fisheries 22, 623-633.
| Crossref | Google Scholar |
Pannella G (1971) Fish otoliths: daily growth layers and periodical patterns. Science 173, 1124-1127.
| Crossref | Google Scholar |
Pratt OP, Beesley LS, Pusey BJ, Gwinn DC, Keogh CS, Douglas MM (2023) Brief floodplain inundation provides growth and survival benefits to a young-of-year fish in an intermittent river threatened by water development. Scientific Reports 13, 17725.
| Crossref | Google Scholar | PubMed |
Puckridge JT, Walker KF (1990) Reproductive biology and larval development of a gizzard shad, Nematalosa erebi (Gunther) (Dorosomatinae: Teleostei), in the River Murray, South Australia. Marine and Freshwater Research 41, 695-712.
| Crossref | Google Scholar |
Pusey BJ, Jardine TD, Beesley LS, Kennard MJ, Ho TW, Bunn SE, Douglas MM (2021) Carbon sources supporting Australia’s most widely distributed freshwater fish, Nematalosa erebi (Günther) (Clupeidae: Dorosomatinae). Marine and Freshwater Research 72, 288-298.
| Crossref | Google Scholar |
Radtke RL (1989) Larval fish age, growth, and body shrinkage: information available from otoliths. Canadian Journal of Fisheries and Aquatic Sciences 46, 1884-1894.
| Crossref | Google Scholar |
Secor DH, Dean JM, Laban EH (1992) Otolith removal and preparation for microstructural examination. In ‘Otolith microstructure examination and analysis’. (Eds DK Stevenson, SE Campana) Canadian Special Publication of Fisheries and Aquatic Sciences 117, pp. 19–57. (Department of Fisheries & Oceans: Ottawa, ON, Canada) Available at https://publications.gc.ca/collections/collection_2016/mpo-dfo/Fs41-31-117-eng.pdf
Southwell M, Wilson G, Ryder D, Sparks P, Thoms M (2015) Monitoring the ecological response of Commonwealth Environmental Water delivered in 2013–14 in the Gwydir River system: a report to the Department of Environment. (Environmental Water Office, Commonwealth of Australia) Available at https://www.dcceew.gov.au/sites/default/files/documents/ecological-response-monitoring-gwydir-13-14.pdf
Stocks JR, Scott KF, Gilligan DM (2019) Daily age determination and growth rates of freshwater fish throughout a regulated lotic system of the Murray–Darling Basin Australia. Journal of Applied Ichthyology 35, 457-464.
| Crossref | Google Scholar |
Stocks JR, Davis S, Anderson MJ, Asmus MW, Cheshire KJM, van der Meulen DE, Walsh CT, Gilligan DM (2021) Fish and flows: abiotic drivers influence the recruitment response of a freshwater fish community throughout a regulated lotic system of the Murray–Darling Basin, Australia. Aquatic Conservation 31, 3228-3247.
| Crossref | Google Scholar |
Strelcheck AJ, Fitzhugh GR, Coleman FC, Koenig CC (2003) Otolith–fish size relationship in juvenile gag (Mycteroperca microlepis) of the eastern Gulf of Mexico: a comparison of growth rates between laboratory and field populations. Fisheries Research 60, 255-265.
| Crossref | Google Scholar |
Templeman W, Squires HJ (1956) Relationship of otolith lengths and weights in the haddock Melanogrammus aeglefinus (L.) to the rate of growth of the fish. Journal of the Fisheries Research Board of Canada 13, 467-487.
| Crossref | Google Scholar |
Vokoun JC, Rabeni CF, Stanovick JS (2001) Sample-size requirements for evaluating population size structure. North American Journal of Fisheries Management 21, 660-665.
| Crossref | Google Scholar |