Humpback whale (Megaptera novaeangliae) behaviour determines habitat use in two Australian bays
S. McCulloch A B H , J.-O. Meynecke B C D I , T. Franklin E F , W. Franklin E F and A. L. M. Chauvenet A G
B C D I , T. Franklin E F , W. Franklin E F and A. L. M. Chauvenet A G
A School of Environment and Science, Griffith University, Gold Coast, Qld 4222, Australia. Email: sarah.mcculloch2@alumni.griffithuni.edu.au; a.chauvenet@griffith.edu.au
B Centre for Coastal and Marine Research, Griffith University, Gold Coast, Qld 4222, Australia.
C Humpbacks & High-rises Inc., Gold Coast, Qld 4222, Australia.
D Whales and Climate Research Program, Griffith University, Gold Coast, Qld 4222, Australia.
E The Oceania Project, PO Box 646, Byron Bay, NSW 2481, Australia. Email: trish.franklin@oceania.org.au; wally@oceania.org.au
F Marine Ecology Research Centre, Southern Cross University, Lismore, NSW 2480, Australia.
G Environmental Futures Research Institute, Griffith University, Gold Coast, Qld 4222, Australia.
H Present address: Humpbacks & High-rises Inc., Gold Coast, Qld 4222, Australia.
I Corresponding author. Email: o.meynecke@griffith.edu.au
Marine and Freshwater Research 72(9) 1251-1267 https://doi.org/10.1071/MF21065
Submitted: 23 February 2021 Accepted: 8 March 2021 Published: 22 April 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Humpback whales (Megaptera novaeangliae) require a suite of essential habitats during their long migration. Therefore, the identification of critical habitats is important for continuation of their successful recovery. In this study we investigated the behaviours and habitat usage exhibited by humpback whales in two known aggregation sites on the east coast of Australia. Using a combined 5400 humpback whale records collected from Hervey Bay between 1999 and 2009 and from the Gold Coast Bay between 2011 and 2018, we analysed different types of behavioural categories. We found that humpback whales in Hervey Bay primarily exhibited surface travel and non-aggressive social behaviour, whereas both sites appeared to be similarly important for resting. Our results suggest that the Gold Coast Bay provides habitat for a wide range of critical humpback whale activities, in particular for resting mother–calf pairs, mature males seeking copulation and socialising immature whales. Hervey Bay had a higher number of mother–calf pair sightings, confirming the area as an important resting site. This study demonstrates that the two regions are critical habitats for humpback whales during their annual migration, but for different essential activities, and should be considered as a whale protection area.
Keywords: humpback whales, Megaptera novaeangliae, migration, behaviour, conservation, critical habitat.
Introduction
Global environmental changes, including increases in water temperature and decreases in sea ice cover (Learmonth et al. 2006), and anthropogenic threats, such as water (Bengtson Nash et al. 2013) and noise (Rossi-Santos 2015) pollution, entanglements (Groom and Coughran 2012) and ship strikes (Smith et al. 2020), are affecting cetacean species worldwide (Simmonds and Eliott 2009; Derville et al. 2018; Riekkola et al. 2019; Sousa et al. 2019). Migratory species, such as humpback whales (Megaptera novaeangliae Borowski, 1781), risk being highly affected by these threats as they migrate past cities and shipping channels, and may require access to alternative habitats for breeding, feeding and during migration. It is therefore important to better understand the habitat use of humpback whales along their migration pathways.
Recently, the focus of humpback whale research has shifted from population estimates to understanding the habitat areas used by this species (Cartwright and Sullivan 2009; Félix and Botero-Acosta 2011; Franklin et al. 2018; Thums et al. 2018). There are distinct temporal and spatial patterns in humpback whale migration, depending on the sex, age and reproductive status of the whale (Craig et al. 2003). Separate cohorts (lactating females, immature whales, mature males, pregnant females) will depart their feeding grounds at various times and arrive in different habitat types at times, which increases their reproductive success (Chittleborough 1965; Dawbin 1966, 1997; Burns 2010).
Different habitat types and environmental preferences of humpback whales can be identified for breeding, calving and resting grounds, aggregation areas and migratory pathways, and these can be classified by several environmental factors, such as water depth, currents and water temperature (Reinke et al. 2016). Migratory paths are the ranges traversed by humpback whales between their feeding and breeding grounds (Félix and Guzmán 2014; Clapham and Zerbini 2015). Breeding grounds are typically identified by shallow, calm waters close to the coast (Bruce et al. 2014; Irvine et al. 2018). Calving grounds are expanses of suitable habitat for lactating mothers and their calves (Irvine et al. 2018) and overlap with breeding areas as they are in warm and sheltered waters (Bruce et al. 2014), with shallow depths (<20 m; Ersts and Rosenbaum 2003). Calving can occur in any area along the expanse of the migratory corridor, wherever environmental conditions are suitable, rather than in precise identified locations (Irvine et al. 2018). Protected coastal waters with shallow depths and calm surface conditions are preferred resting grounds, particularly for maternal females, for protection against rough ocean conditions and to reduce energetically expensive associations with competitive male groups (Ersts and Rosenbaum 2003; Franklin et al. 2018).
Humpback whales engage in small, unstable groups that typically have short periods of association and socialisation (Tyack and Whitehead 1983; Baker and Herman 1984; Mattila et al. 1994; Clapham 2000). Agonistic and non-aggressive social behaviours occur along stretches of the migratory pathways, and may be influenced by the density of whales in an area or the reproductive states of the mature whales involved (Baker and Herman 1984). Comparing observed behaviours between aggregation sites of humpback whales has not frequently been used as a technique of identifying the function of habitats. However, recent studies have shown that social organisation can dictate the habitat types used by particular groups of whales, and which behavioural patterns occur (Ersts and Rosenbaum 2003; Lunardi et al. 2010). Previous research has used humpback whale behavioural data to determine whether whale watching and vessel presence have effects on whale behaviour (Fiori et al. 2019; Schuler et al. 2019; Amrein et al. 2020) and to assess how humpback whales change their communication in response to increasing noise levels (Dunlop et al. 2010).
The population of humpback whales that migrates annually along the east coast of Australia from its high-latitude feeding grounds to low-latitude breeding areas (E1 population; Chittleborough 1965; see International Whaling Commission at https://iwc.int/humpback-whale) was believed to be increasing at an estimated rate of 10% per annum until 2015, and is predicted to be at 30 000 humpback whales (Noad et al. 2019). Most research on the humpback whale aggregation areas along the E1 population migration route has focused primarily on the pod characteristics (Corkeron and Brown 1995; Franklin et al. 2011) and migratory movements (Burns et al. 2014) of the whales. Several recent studies have demonstrated that breeding and calving behaviours have occurred outside recognised habitat areas for humpback whale populations (Bruce et al. 2014; Lucena et al. 2016; Irvine et al. 2018; Torre-Williams et al. 2019; Valani et al. 2020), underlining the need to further determine critical habitats, which we define as any habitat relevant for maintaining a stable population through feeding, breeding and resting.
Hervey Bay, on the east coast of Australia, has been recognised as a vital resting habitat for humpback whale mother–calf pairs (Chaloupka et al. 1999; Franklin et al. 2011, 2018; Martinez et al. 2015). It is estimated that ~34% of whales in the E1 population deviate from their southern migration to enter the bay (Chaloupka et al. 1999; Franklin 2014). The Gold Coast Bay (GCB), in south-east Queensland, is a known aggregation area for the E1 population (Department of Environment and Heritage 2005). Few behavioural studies have been conducted in this location to determine the use of the bay by humpback whales (Meynecke et al. 2013; Reinke et al. 2016; Valani et al. 2020) and there is still limited knowledge about its exact function for socialisation and breeding. Recent research has demonstrated that parturition occurs outside the stated breeding grounds in the Great Barrier Reef, and evidence of newborn calves in the GCB suggests it is a suitable calving ground (Torre-Williams et al. 2019; Valani et al. 2020).
The monitoring of Australian populations of humpback whales has increasingly been done through citizen science to find spatiotemporal and behavioural trends for this species (Bruce et al. 2014; Thums et al. 2018; Torre-Williams et al. 2019), including in the GCB and Hervey Bay (Franklin et al. 2011; Meynecke et al. 2013; Valani et al. 2020). Due to the limitations of systematic, long-term research (Silvertown 2009), large-scale ecological studies are difficult to facilitate, and even more so for migratory animals with a large spatial range (Dunn et al. 2019). Citizen science can overcome these research challenges and be used to enable the continuous and large-scale monitoring of humpback whale populations (Tonachella et al. 2012). Citizen science has been used to capture various types of data on humpback whales, including abundance (Bertulli et al. 2018; Pirotta et al. 2020), distribution (Bruce et al. 2014) and population dynamics (Franklin et al. 2018; Torre-Williams et al. 2019).
The aim of this study was to compare behavioural patterns between Hervey Bay and the GCB along the migratory route of the E1 population of humpback whales to identify the habitat usage of the GCB. We hypothesised that the GCB functions as a socialising area and resting ground for this population of humpback whales and is an important habitat area along their migration route, particularly for mother–calf pairs during their northern and southern migrations.
Materials and methods
Study area
Gold Coast Bay
The GCB in south-east Queensland, Australia (Fig. 1), is an open embayment reaching from Point Lookout, North Stradbroke Island in the north (27.45°S, 153.55°E) to Tweed Heads in the south (28.16°S, 153.55°E), then 15 nautical miles (~28 km) offshore to the east from the Gold Coast Seaway (153.75°E). It is a sickle-shaped, shallow bay that is open to the Coral Sea. The bay has an annual sea surface temperature ranging from 20.4 to 28.2°C (see SeaTemperature.org at https://www.seatemperature.org/australia-pacific/australia/). The water depth ranges from 20 to 80 m, and decreases slowly over the narrow continental shelf. During the winter months, from June to October, the GCB experiences moderate- to high-energy south to south-east swells, and a mean wave height of 0.8–1.4 m (Strauss et al. 2007). The dominant winds are easterly to south-easterly trade winds, and the area experiences a calm, mild dry season (May–October) and a windier, hot and humid wet season (November–April).
Hervey Bay
Hervey Bay is a bowl- or U-shaped bay, situated on the south-east Queensland coast (25°S, 152.84°E; Fig. 1), ~350 km north of the GCB. Hervey Bay lies between mainland Australia and Fraser Island. The bay opens ~80 km wide to the north and narrows southward (Ribbe 2014), with an area of ~4000 km2 (Vang 2002). Hervey Bay has a mean depth of ~20 m (Ribbe 2014) and minimum and maximum sea surface temperatures of 20 and 28.1°C respectively (see SeaTemperature.org at https://www.seatemperature.org/australia-pacific/australia/). The region has a dry winter and a humid, wet summer, and experiences east to south-easterly trade winds (Ribbe 2014). The study area was predominately the eastern side of Hervey Bay, west of Fraser Island (Franklin et al. 2018), where it the mainly has a sand and mud bottom (Vang 2002).
Data collection
Ethical considerations
Under Australian animal ethics guidelines, the collection of whale data from commercial whale watch vessels did not require animal ethics approval from the Griffith University Animal Ethics Committee because it did not involve the use of animals as defined by the Australian Code for the Care and Use of Animals for Scientific Purposes 2013 (National Health and Medical Research Council 2013) and the Animal Care and Protection Act 2001 (Qld). The Hervey Bay data are covered by permits associated with previously published data (e.g. Franklin et al. 2011).
Gold Coast Bay
Surveys were conducted aboard multiple commercial whale-watching vessels from 2011 to 2018, during the whale migration season (from the start of June to early November). The area covered in the GCB was between Jumpinpin, South Stradbroke Island (27.75°S, 153.44°E) and Tugun (28.15°S, 153.5°E), and summed ~1125 km2 (Fig. 1). Trained volunteers from Humpbacks & High-Rises (HHR), a Gold Coast-based citizen science research organisation, used platforms of opportunity to collect data on the E1 population of humpback whales. The tour vessels were motorised boats ranging from 12 to 30 m in length. The tours were operated out of the Gold Coast Southport Seaway. Tour departure times ranged between early morning (0730 hours) to late afternoon (1500 hours), with each tour lasting an average of 3 h. Three HHR volunteer researchers would conduct a survey every day of the humpback whale migration season (from the start of June to the start of November on the Gold Coast), on one of the whale watching company boats. However, occasionally surveys were unable to be conducted daily due to poor weather or booked-out tours. The research volunteers used the continuous scanning method to locate humpback whales (Mann 1999). Volunteers used this method throughout the whale watching tour until a humpback whale pod was located, then data collection commenced.
Data collection began on a pod of whales when the vessel was within 300 m. A this point, the date, global positioning system (GPS) coordinates and time of location were recorded. The vessels travelled alongside the pod for any length of time, ranging from 10 min to 2 h (on board commercial whale watching vessels this was at the discretion of the skipper). Upon departure of a pod, the time and GPS coordinates were recorded again. The pod size and group composition (i.e. the presence or absence of calves) were determined. Calves were considered any whale of a size less than 50% of the accompanying whale, with which it was maintaining a constant and close relationship (Chittleborough 1965; Tyack and Whitehead 1983). All other whales were classified as adults for the purposes of this study, yet this did not imply sexual maturity.
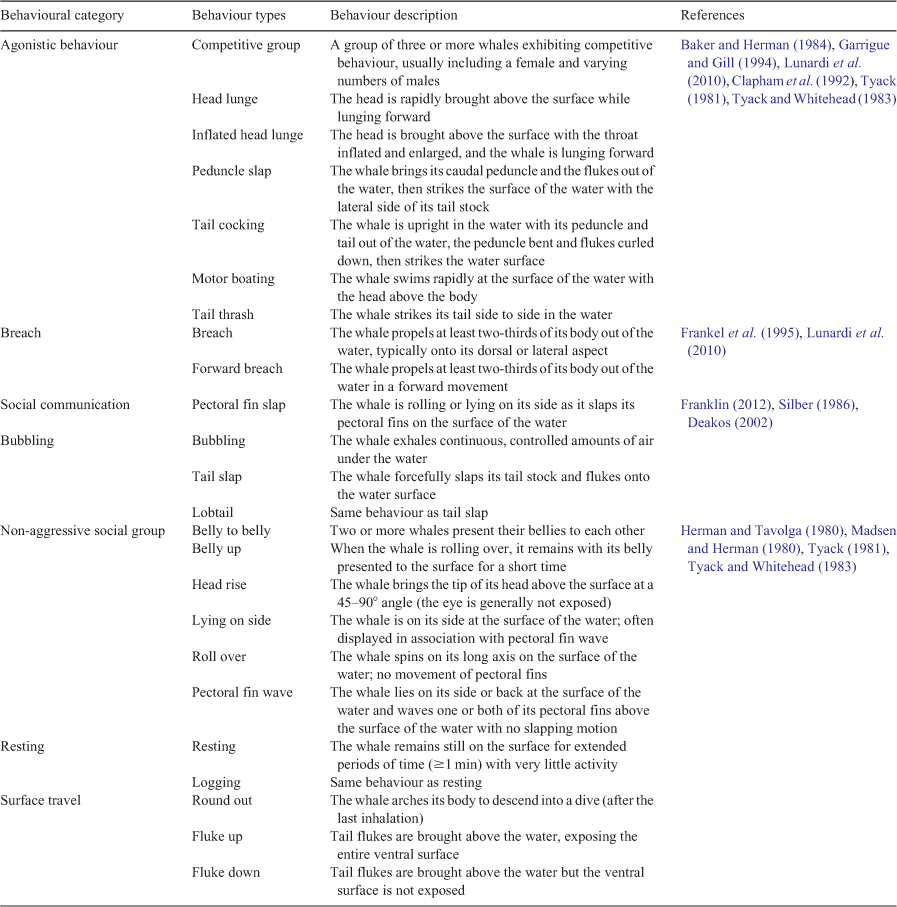
Behavioural data were also collected on each pod, using a standardised field data sheet (Mann 1999). This data sheet included 22 widely recognised behavioural categories, as well as dive and resting times (Meynecke et al. 2013). These behaviours were grouped into categories for the purposes of this study (Table 1). Behavioural data were collected for the entire duration of the time spent with a pod and every activity performed by the pod was tallied on data sheets.
In accordance with the Australian Environment Protection and Biodiversity Conservation Act 1999, a distance of 100 m had to be kept between the whales and the vessels, and 300 m between the vessel if a calf was present or if three boats were in the 100-m exclusion zone. In the GCB, the average time of each survey at sea was 2.5 h, regardless of departure time.
Hervey Bay
Data were collected by a research organisation, The Oceania Project, between 1999 and 2009 through semisystematic surveys aboard four different motorised vessels that would undertake 6 days of fieldwork per week. Surveys would begin in the mornings in a random direction transect until the first pod was spotted and observations commenced, or local whale watching operators would alert the research team to an available pod. Surveys were assisted by research students who were undergraduates or graduates from an environmental or marine sciences degree. As reported by Franklin et al. (2011), hours spent surveying in Hervey Bay varied between 6.5 and 10 h per survey, and averaged 9 h per survey. The total area surveyed in Hervey Bay was ~1050 km2 (Fig. 1).
Once a pod was located, data collection commenced, including the date, number of whales and sex identification where possible. Time and GPS locations were recorded upon locating, every 15 min during observations and when departing a pod. Behaviours were recorded using a yes or no response for the presence or absence of particular behaviours observed. The behaviour categories paralleled those collected in the GCB. For detailed methods see Franklin et al. (2011, 2018).
In both the GCB and Hervey Bay whale identification photographs of individual humpback whale flukes were taken. However, the purpose of the present study was to investigate the relative behaviour between the two study sites, and studying resighted individuals will be subject to further research. Currently, there is no published work for resident times of humpback whales on the Gold Coast; however, from anecdotal reports based on fluke matching and resightings, the authors suggest 1- to 2-day resident times for the GCB. By contrast, humpback whales in Hervey Bay have a typical residency time of 1.4–2.0 weeks (Franklin 2014).
Data preparation
Mother–calf pair sighting data
The data from the GCB and Hervey Bay were merged into one dataset by collating the following variables: number of mother–calf pairs, the date and time of data collection and the start and finish times of each survey. These variables were grouped into intervals of calendar week within a year. Unit effort was found by summing the number of surveys conducted each calendar week and then multiplying this by the average hours spent on survey (i.e. mean daily survey times of 9 and 2.5 h for Hervey Bay and the GCB respectively).
Behavioural data
Behavioural data were compiled into broader behavioural categories (Table 1). The categories were widened to reduce inflation of absence values and to allow better comparison of the datasets, which did not include identical types of records for all behaviour classes. Behaviours in the Hervey Bay dataset were recorded using yes or no responses, whereas in the GCB the data were tallied; consequently, all behavioural data had to be converted to presence or absence records.
The decision as to which behaviour types would be designated into the behavioural categories was based on semantics and current available knowledge (Table 1). It is recognised that some behaviours, such as breaching and flippering, can represent communication between individuals or pods of humpback whales (Frankel et al. 1995; Lunardi et al. 2010). Surface behaviours that were observed during diverse social interactions between humpbacks were grouped into social communication. These behaviours have been identified as less aggressive and not conducive to mating (Franklin 2012; Silber 1986) and include lob tailing, fluke slapping and pectoral fin slapping. Previous research has shown that pectoral fin slapping is performed by mature females to encourage competition in male competitive groups (Clapham 2000). However, it is also performed by subadults as a form of socialisation and to increase their development and coordination, as well as by males in attempts to maintain non-agonistic associations with other males when disaffiliating from competitive groups (Deakos 2002). Because this behaviour was contingent on the age, sex and socialisation state of the performers, it was included in the broader ‘social communication’ category. ‘Breaching’ has been identified as a signalling behaviour and used for intergroup communication (Whitehead 1985; Dunlop et al. 2008). ‘Surface travel’ included behaviours that indicated a humpback whale surfacing for only breath, generally associated with travelling. Male–male competition for reproductively mature females results in aggressive behaviours in competitive groups (Baker and Herman 1984; Clapham et al. 1992). Agonistic behaviours associated with these groups include head lunges, physical displacement, charge strikes, peduncle slaps and tail slashes (Tyack 1981; Tyack and Whitehead 1983; Baker and Herman 1984; Garrigue and Gill 1994; Lunardi et al. 2010). Any behavioural types in the data that indicated aggression, displacement or whales in competition were grouped into ‘agonistic behaviour’. This study included bubbling as a separate category from ‘agonistic behaviour’, despite it being identified as a common aggressive and competitive trait (Tyack and Whitehead 1983), because it was observed in cases that were assumed to be whales undertaking precopulatory behaviours (two adults present, rolling, pectoral waves). Like bubbling, other behaviours may be performed in different circumstances depending on the age, sex and social role of the whale (Deakos 2002). Behavioural patterns by humpback whales have also been associated with non-aggressive social behaviour and mating, and include rolling, lying on their side, showing their ventral surface, flippering, pectoral fin extensions, head rising and waving (Herman and Tavolga 1980; Madsen and Herman 1980; Tyack 1981; Tyack and Whitehead 1983). Any behaviours that inferred that precopulatory actions occurred were grouped into ‘non-aggressive social behaviour’. In the present study, ‘resting’ was defined as periods where the whales were stationary on the surface or not travelling in any direction (e.g. logging behaviour).
Data analysis
Mother–calf pair sighting data
The number of whale sightings in both Hervey Bay and the GCB was standardised to sighting per unit effort (SPUE). This was achieved by dividing the total number of whales counted in each calendar week by observation time at sea. The proportion of mother–calf pairs recorded in Hervey Bay and the GCB was calculated by dividing the number of mother–calf pair SPUE by the total number of whale SPUE at each site. Mother–calf pairs travelled either alone or within a larger pod.
Behavioural data
A cross-correlation (Pearson) was conducted on the set of behavioural data. The correlations between behaviours assisted in deciding the categories of behaviours. A correlation of r > 0.3 was considered significant for the purpose of attributing categories to the same group. Behaviours that had positive correlation coefficients between 0.3 and 0.5 (Calkins 2005) and have been previously reported as similar were assigned to the same categories. The behavioural data were grouped into categories to increase the power of analyses and to account for cross-correlation. Categories from Hervey Bay and the GCB that showed positive correlation values >0.3 and matched definitions were joined. This included behaviours commonly associated with competitive groups exhibiting agonistic behaviours, such as head lunge, inflated head lunge and high-speed chase (Tyack and Whitehead 1983; Baker and Herman 1984; Garrigue and Gill 1994; Lunardi et al. 2010). Behaviours often observed during non-aggressive social behaviour, including roll over, belly up and lying on side, were also correlated. Behaviours with similar appearances and definition, such as ‘side lobtail’ and ‘lobtail’, as well as ‘inflated head lunge’ and ‘head lunge’, were highly correlated.
The behavioural dataset was analysed in R (ver. 1.3.1093, RStudio, Inc., Boston, MA, USA). We used a mixed-effects logistic regression model with a logit-link function (for binomial distribution) to analyse the likelihood of each behavioural category being observed in Hervey Bay and the GCB. The likelihood of observing the behavioural categories (‘agonistic behaviour’, ‘breach’, ‘bubbling’, ‘non-aggressive social behaviour’, ‘resting’, ‘social communication’ and ‘surface travel’) at the two locations (Hervey Bay and GCB) was modelled with density (on a scale of 1 (lowest) to 3 (highest)), year and effort (in minutes) as fixed effects and included weeks nested within year as a random effect. Because data collection did not occur during the same time period at both study locations, the behavioural data were split between the two sites to analyse them separately. The ‘effort’ variable was created by splitting the duration of each survey into four categorical bins. Based on the data, these bins were selected as low (≤15 min), medium (16–30 min), high (31–50 min) and extra-high (>50 min). The density of whales at Hervey Bay and the GCB was calculated per calendar week. Densities were sorted into three ordinal categories (1, 2 and 3). The number of whales counted each calendar week was calculated, and the 33rd and 66th percentiles of number of whales counted each calendar week were calculated for both locations. Calendar weeks falling below the 33rd percentile of the number of whales counted were grouped into Density Category 1, those within the 33rd–66th percentiles were grouped into Density Category 2, and those above the 66th percentile were grouped in Density Category 3. To compare the observations of behaviour between the two sites, a generalised linear mixed model (GLMM) with a logit-link function was run to analyse each behavioural category. This model used the same behavioural categories as the mixed-effects logistic regression model, but this time with density and effort (in minutes) as fixed effects, and weeks as the random variable.
Results
The total estimated survey effort in the GCB was 1432 h over 672 days, with a mean of 2 h at-sea survey time. In Hervey Bay, the total estimated survey effort was 4932 h over 533 days and a mean survey time of 9 h, based on recorded start and end times. The total number of whale sightings was 3610, including 555 calves, between 2011 and 2018 in the GCB, and 8885 whales, including 1776 calves, between 1999 and 2009 in Hervey Bay (Table 2). Hervey Bay had an estimated average of 8 whales per km2 over the 11-year study period within the study range. The GCB had an estimated average of 4 whales per km2 over 8 years of study within the area of observation (Fig. 1). The GCB recorded 32% of sightings as mother–calf pairs based on SPUE, and Hervey Bay recorded 39% mother–calf pairs.
Behavioural data analysis
Results from the mixed-effects regression (see Fig. A1 of Appendix 1) showed that effort was the most common significant predictor for observing behaviour categories in both the GCB and Hervey Bay, with increased effort resulting in a higher likelihood of observation. However, effort was not a significant predictor of the likelihood of observing resting behaviour in Hervey Bay. In all but resting and surface travel behaviour categories, medium, high and extra-high effort was a significant predictor of the likelihood of observing behaviour categories in Hervey Bay. In the GCB, only high and extra-high effort increased the likelihood of observing all behaviours except for bubbling (only extra-high) and surface travel (all effort levels).
In the GCB, density was not a significant predictor for any behaviour categories. In Hervey Bay, there was a significantly greater chance of observing resting behaviour at higher density levels, and at the highest density level there was a significantly lower likelihood of observing non-aggressive social behaviour.
Year was not a significant predictor of almost all behaviour categories observed in the GCB, except in 2018, when there was a higher likelihood of observing non-aggressive social behaviour. In Hervey Bay, there were more significant yearly variations in the data, with all behaviour categories except breach having at least one year that was a predictor of the likelihood of observing behaviour categories. Surface travel was the most highly affected by year, where for 4 years (2004, 2005, 2006, 2008) there was a significantly lower chance of observing the behaviour.
Non-aggressive social behaviour had a significantly higher probability of observation with higher effort in Hervey Bay, with close to a 75% chance of observing this behaviour when the effort exceeded 50 min (Fig. 2). In the GCB, there was close to a 50% chance of observing a breach behaviour when the effort was extra-high, and there was a significantly greater chance of observing breaching behaviour in the GCB, particularly with high and extra-high effort (Fig. 2). Resting behaviour had a greater likelihood of observation in the GCB when the effort was high (Fig. 2). There was a significantly greater chance of observing agonistic behaviour in the GCB when the effort was high or extra-high (Fig. 2).
The results of the GLMM showed very similar outcomes to the mixed-effect regression. Breaching, social communication and agonistic behaviour were more likely to be observed in the GCB than Hervey Bay (Table 3). Conversely, surface travel, non-aggressive social behaviour and bubbling were more likely to be recorded at Hervey Bay (Table 3). The observed differences in resting behaviour between the two sites were not significant. Density was a significant predictor of non-aggressive social behaviour, resting and surface travel, but not behaviours (Table 3).
Discussion
Using only behavioural data, this study demonstrates the importance of the GCB as a critical habitat for the E1 humpback whale population and confirms the role of Hervey Bay as a habitat for nursing and courtship. Differences in the behaviour categories observed show that both habitats have similarities in their usage, particularly of resting behaviour, but also distinct differences: more agonistic behaviour, breaching and social communication in the GCB, and higher rates of non-aggressive social behaviour displays in Hervey Bay. The evidence of a high proportion of mother–calf pairs and observations of different behavioural displays in the GCB over the entire season suggest that the bay is used by all age classes of humpback whales. It expands upon the evidence that the GCB is frequently used for resting and agonistic behaviours, and is in line with previous findings for the GCB (Meynecke et al. 2013; Torre-Williams et al. 2019; Valani et al. 2020) that it also serves as a resting area for the E1 population.
Importance of behaviour in identifying habitat use
Trends in habitat preferences and distributions of species are typically recognised by environmental parameters (Trudelle et al. 2016; Oña et al. 2017) or through the use of technologies such as satellite tags (Guzman and Félix 2017; Bejder et al. 2019). However, as we begin to better interpret specific behaviour displays and understand how and when they are exhibited (Kavanagh et al. 2017), it is possible to interpret the use of a habitat area through these behaviours, in conjunction with environmental parameters and physiological requirements (Ersts and Rosenbaum 2003). Behavioural data have previously been used to assist with the identification of habitat use, with most marine mammal studies focusing on foraging, travelling, resting and socialising as the primary behavioural states (Karczmarski et al. 2000; Barendse and Best 2014; Noren and Hauser 2016). Less frequently have specific behaviour categories been used to describe habitat use and preferences (Geise et al. 1999).
Resting and mother–calf pairs
In this study we found that the occurrence of resting behaviour in Hervey Bay and the GCB is very similar (Table 3). Research from other breeding grounds has shown that lactating females spend, on average, 35% of their time resting (Bejder et al. 2019). Although it was not possible to measure the proportion of time spent at rest in this study, previous studies from Hervey Bay have shown its significance as a resting ground (Stack et al. 2020), and the GCB is likely of similar importance to resting whales.
Resting behaviour is specifically important for lactating females and their calves (Cartwright and Sullivan 2009). Mother–calf pairs must ensure they maintain a low energy expenditure over their migration to reduce decline in body composition for the mother and optimise growth for the calf (Bejder et al. 2019).
Hervey Bay is recognised as an important habitat ground for resting humpbacks, particularly mother–calf pairs (Stack et al. 2019). Studies have reported that more mother–calf pods are observed there than in other regions (Franklin 2012), with the proportion of all pods that included one or more calf to be 40% (Franklin et al. 2011), and mother–calf dyad pods representing 42.2% of all pods observed during September (Martinez et al. 2015). A recent study from the GCB found that up to 30% of all humpback whales sighted over the migration season were mother–calf pairs (Valani et al. 2020). The present study found a similar proportion of mother–calf pairs observed in both Hervey Bay and the GCB, despite the data collection occurring over different time periods. In the GCB, over the 8 years of data collection for this study, 39 calves were recorded in June, and evidence of newly born calves migrating north have been documented (Torre-Williams et al. 2019). The number of mother–calf pairs recorded in the GCB indicates a relatively high presence compared with known resting and breeding grounds of other populations, such as Madagascar (Ersts and Rosenbaum 2003), Ecuador (Scheidat et al. 2000), the Dominican Republic (Mattila et al. 1994) and the West Indies (Mattila et al. 1989).
Lactating humpback whales allocate significant periods of time on their migration to rest in order to reduce their energy expenditure (Bejder et al. 2019). Currently, the recognised breeding and calving grounds of the E1 population of humpback whales are situated in the southern Great Barrier Reef, south of 21°S (Simmons and Marsh 1986; Chaloupka and Osmond 1999); however, the exact parameters are ill-defined (Smith et al. 2012). Evidence of expanded calving ranges, south of the putative calving grounds, is becoming frequently documented (Guidino et al. 2014; Thums et al. 2018; Torre-Williams et al. 2019). Habitat areas along migration routes have continuously been recognised to be of more importance for humpback whales, particularly for maternal care and resting (Bruce et al. 2014; Guidino et al. 2014). It is now clear that the habitat boundaries have more plasticity than once believed, and it is very likely the areas of suitability for breeding and calving will continue to change (Derville et al. 2018). The large number of mother–calf pairs observed in the GCB in this study indicates a resting area for the E1 population. Consequently, the importance of the GCB for resting behaviours in the latter half of the migration season needs to be recognised and considered when managing conservation of the area in the future.
Non-aggressive social behaviour
The population characteristics of humpback whales in Hervey Bay are favourable to encourage mating behaviours. The occurrence of non-aggressive social behaviour was recorded in both study locations; however, there was a greater likelihood of observing these precopulatory behaviours in Hervey Bay (Table 3; Fig. 2). There is temporal segregation of separate cohorts entering the region, with mainly newly pregnant females and immature whales present earlier in the season (Franklin et al. 2018). Franklin (2012) found that most non-aggressive social behaviours occurred earlier in the migration season, coinciding with the arrival of immature males and females, and likely pertaining to the development of courtship-related behaviours. Large numbers of mature male whales are absent in Hervey Bay in August (Franklin et al. 2018), so social interactions and mating attempts can more easily occur, without large competitive groups present. The results of the mixed-effects regression showed that with higher densities of humpback whales in Hervey Bay there was a predicted decrease of the occurrence of non-aggressive social behaviours (Table 3). With the arrival of lactating females later in the season, mature males are taking advantage of more mating opportunities, with postpartum oestrus occurring in a small percentage of females (Chittleborough 1958). The GCB also recorded the presence of precopulatory behaviours, and mating is known to occur over the entire migration route (Brown et al. 1995). However, the shallow nature of Hervey Bay (on average <20 m; Ribbe 2014) is not conducive to competition in males, so large competitive pods are less likely to occur, allowing for smaller pods of mature whales to socially interact.
Agonistic behaviour and competitive groups
Previous studies largely agree on the suite of behaviours that function as agonistic communication in humpback whale populations (Baker and Herman 1984; Lunardi et al. 2010). Surface activities that are interpreted as aggressive commonly involve collisions or strikes between individuals (Silber 1986; Garrigue and Gill 1994; Félix and Botero-Acosta 2012) and include head lunges, displacement of other competing escorts, side tail slashes and peduncle slaps (Baker and Herman 1984; Lunardi et al. 2010). Some literature has discussed bubbling as an aggressive behaviour (Tyack and Whitehead 1983; Lunardi et al. 2010). It seems likely that bubbling behaviour, although occurring in agonistic displays, may also occur during non-aggressive social behaviour. Hervey Bay has been found to have a low proportion of competition pods (6.3% of pods observed; Franklin 2012), yet, in the present study, we reported a higher probability of humpback whale bubbling behaviour (Table 3); the recorded bubbling may have been in association with non-aggressive social behaviour, as observed by us.
Our results suggest that there was a greater chance of observing more agonistic behaviour in the GCB than in Hervey Bay (Fig. 2). Competitive groups typically occur in deeper waters (Herman et al. 2007) because they allow greater movement within the water column and facilitate these aggressive behaviours (Ersts and Rosenbaum 2003). Hervey Bay is very shallow in nature (mean depth 20 m), making it a favourable habitat for non-aggressive social behaviours and lower rates of agonistic behaviour. The GCB is located on the continental shelf, so has depths ranging from 20 to 80 m, encouraging competitive groups to enter the GCB and allowing aggressive behaviours in the deeper waters further away from shore. Furthermore, competitive groups have been found to associate more with females without than with a calf (Lunardi et al. 2010). The greater presence of mother–calf pairs in Hervey Bay, particularly later in the migration season (Franklin et al. 2011), may discourage male–male competitive behaviours. In addition, the closed embayment of Hervey Bay requires a diversion from the migratory path.
The mating strategy of humpback whales shows seasonal changes in levels of aggression between individuals, linked to alterations in abundance and pod size (Baker and Herman 1984; Lunardi et al. 2010). However, we did not find any significant differences in the effect of density on agonistic behaviour, similar to Kavanagh et al. (2017).
Breaching and social communication
Breaching behaviour is not thought to be associated with competitive or non-aggressive social behaviour (Franklin 2012). Previous research has shown that it is used for inter- rather than intragroup communication (Dunlop et al. 2008) and acts to accentuate communication signals (Whitehead 1985). Breaching can be influenced by the spatial organisation of whales, with the distance to the nearest pod altering the rate of this behaviour, decreasing when whales are within 4000 m compared with beyond that distance (Kavanagh et al. 2017). The present study did not find density to be a predictive variable for observing breaching behaviour at either site, yet it was observed significantly more in the GCB than Hervey Bay. In the GCB there was a higher chance of observing agonistic behaviour and social communication than in Hervey Bay, likely with more competitive groups socialising together. Fluke slapping and pectoral fin slapping are thought to be used during the splitting or joining of groups (Deakos 2002; Kavanagh et al. 2017); therefore, there is likely a much higher change of humpback whale groups in the GCB, indicating the area’s importance for intergroup communication.
Use of citizen science data
Although citizen science data is often the only available long-term data, it has its limitations. Unlike systematic surveys, the inability to synchronise the data collection time of citizen science projects means data from different time periods may be analysed. In this study, the datasets from the two locations were analysed separately and, when evaluating year as a predictive variable, there was no indication of a large shift in behavioural observations over time. In addition, we are comparing the same population of humpback whales and there were no major changes in habitat use reported from the study sites between 1999 and 2018.
External factors, such as wind speed, sea state and background noise levels, can also affect humpback whale behaviour (Whitehead 1985; Dunlop et al. 2010; Dunlop 2016). Humpback whales use both vocal communication (songs and social sounds) and surface-generated acoustic signals to communicate (Dunlop et al. 2008). With increasing wind speeds and sea state, humpback whales switch from predominately vocal to surface-generated communication (Dunlop et al. 2010). It is possible that environmental parameters can affect the rate of observation of these surface behaviours. Taking into account both vocal communication and environmental variables in future studies could provide a more comprehensive insight into humpback whale social communication and behaviour in the study areas.
Another limitation is the effect that vessels may have had on the behaviours performed by the whales. Humpback whale behaviours can change in the presence of boats, including horizontal avoidance, increased dive times, changes in path predictability, decreased respiration rates or even approaching vessels (Corkeron 1995; Schaffar et al. 2009, 2013; Stamation et al. 2010; Garcia-Cegarra et al. 2019; Sprogis et al. 2020). Yet, Amrein et al. (2020) found that some whales do not change their behaviour in the presence of vessels. The data in this study were collected aboard motorised vessels at both sites, so there is no effect on the comparison of the data. Furthermore, collecting data of this detail without the use of vessels in close proximity to the whales would be difficult to accomplish. The potential impact from diurnally varying behavioural patterns (Helweg and Herman 1994) exhibited by the humpback whales may also restrict the outcomes of this research. In the GCB, research was conducted on commercial whale watching vessels so the timing of daily surveys could not be controlled for and depended entirely on the operators (data collection could take place anytime between 0730 and 1800 hours). Humpback whale behavioural patterns have been shown to vary diurnally (Helweg and Herman 1994), which could affect the suite of behaviours recorded. This could be remedied if funding for a systematic study was granted. Still, the use of citizen science for marine mammal monitoring has been proven effective on numerous occasions (Foster-Smith and Evans 2003; Davies et al. 2012; Tonachella et al. 2012; Ward et al. 2015; Bertulli et al. 2018) and is potentially the only practical method to collect long-term data needed to document ecological patterns and broad-scale population trends (Dickinson et al. 2010). Not only can citizen science produce viable and beneficial information, but it can also provide environmental education and encourage the conservation of nature in participants (Kobori et al. 2016).
Conclusion
The increase in potential for human–animal conflict is particularly concerning with the presence of many mothers and calves in the GCB over the migration season. The E1 population faces many anthropogenic pressures when migrating through the GCB (e.g. whale watching tours, recreational and commercial fisheries, expansion of tourism activities, shark nets from the Queensland Shark Control Program). Humpback whales have been shown to change their behaviour in the presence of vessels (Corkeron 1995; Garcia-Cegarra et al. 2019), so increased disturbance from commercial and recreational vessels poses a risk to their fitness and survivorship (Stack et al. 2019). Disturbances to this population’s typical behaviours can have significant implications for socialisation and survivorship. Lactating whales and their calves have to carefully manage their energy expenditure and balance migration and movement with resting periods to reduce the decline in body composition for the mothers and to optimise the muscle development and motor skills of the calf (Cartwright and Sullivan 2009; Sullivan and Cartwright 2009; Bejder et al. 2019). Continued monitoring of the interaction between this population and human activities is important to maintain a healthy population. Identifying the function of these locations, particularly the GCB, is relevant when considering management strategies and conservation initiatives. This study has confirmed that the GCB is important for the E1 population of humpback whales and is used for many behavioural states: resting, calving, mating and socialising.
Further research into habitat use is particularly important in light of climate change impacts (Derville et al. 2018), which include variations in spatial distribution, timing of migration, behaviour (Simmonds and Eliott 2009; Cartwright et al. 2019; Sousa et al. 2019), reduced calving rates (Kershaw et al. 2021) and likely increased mortality. The use of behavioural data provides a unique opportunity to compare habitat use between different regions along a migratory pathway, as well as between separate populations. Fluctuations in habitat use demonstrate how important it is to assess both the species’ and anthropogenic use of these areas more regularly. Habitats are likely to change as the dynamics of the population also alter in an evolving ocean environment. Management policies and conservation strategies must also evolve as rapidly to allow the continued protection of humpback whales.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding. J. O. Meynecke received funding from a charitable trust under the Whales & Climate Research program.
Acknowledgements
The authors thank Humpbacks & High-Rises Inc. and The Oceania Project for valuable contribution of data to this study and for their ongoing support of whale research and conservation. The authors thank all the volunteers who were involved in the whale surveys with both organisations and the contribution from whale watch operators on the Gold Coast: Sea World Cruises, Spirit of Gold Coast, Tall Ships, Sea the Gold Coast.
References
Amrein, A. M., Guzman, H. M., Surrey, K. C., Polidoro, B., and Gerber, L. R. (2020). Impacts of whale watching on the behavior of humpback whales (Megaptera novaeangliae) in the coast of Panama. Frontiers in Marine Science 7, 601277.| Impacts of whale watching on the behavior of humpback whales (Megaptera novaeangliae) in the coast of Panama.Crossref | GoogleScholarGoogle Scholar |
Baker, C. S., and Herman, L. M. (1984). Aggressive behavior between humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters. Canadian Journal of Zoology 62, 1922–1937.
| Aggressive behavior between humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters.Crossref | GoogleScholarGoogle Scholar |
Barendse, J., and Best, P. B. (2014). Shore-based observations of seasonality, movements, and group behavior of southern right whales in a nonnursery area on the South African west coast. Marine Mammal Science 30, 1358–1382.
| Shore-based observations of seasonality, movements, and group behavior of southern right whales in a nonnursery area on the South African west coast.Crossref | GoogleScholarGoogle Scholar |
Bejder, L., Videsen, S., Hermannsen, L., Simon, M., Hanf, D., and Madsen, P. T. (2019). Low energy expenditure and resting behaviour of humpback whale mother–calf pairs highlights conservation importance of sheltered breeding areas. Scientific Reports 9, 771.
| Low energy expenditure and resting behaviour of humpback whale mother–calf pairs highlights conservation importance of sheltered breeding areas.Crossref | GoogleScholarGoogle Scholar | 30683890PubMed |
Bengtson Nash, S. M., Waugh, C. A., and Schlabach, M. (2013). Metabolic concentration of lipid soluble organochlorine burdens in the blubber of southern hemisphere humpback whales through migration and fasting. Environmental Science & Technology 47, 9404–9413.
| Metabolic concentration of lipid soluble organochlorine burdens in the blubber of southern hemisphere humpback whales through migration and fasting.Crossref | GoogleScholarGoogle Scholar |
Bertulli, C. G., Guéry, L., McGinty, N., Suzuki, A., Brannan, N., Marques, T., Rasmussen, M. H., and Gimenez, O. (2018). Capture-recapture abundance and survival estimates of three cetacean species in Icelandic coastal waters using trained scientist-volunteers. Journal of Sea Research 131, 22–31.
| Capture-recapture abundance and survival estimates of three cetacean species in Icelandic coastal waters using trained scientist-volunteers.Crossref | GoogleScholarGoogle Scholar |
Brown, M. R., Corkeron, P. J., Hale, P. T., Schultz, K. W., and Bryden, M. M. (1995). Evidence for a sex-segregated migration in the humpback whale (Megaptera novaeangliae). Proceedings of the Royal Society of London – B. Biological Sciences 259, 229–234.
| Evidence for a sex-segregated migration in the humpback whale (Megaptera novaeangliae).Crossref | GoogleScholarGoogle Scholar |
Bruce, E., Albright, L., Sheehan, S., and Blewitt, M. (2014). Distribution patterns of migrating humpback whales (Megaptera novaeangliae) in Jervis Bay, Australia: a spatial analysis using geographical citizen science data. Applied Geography 54, 83–95.
| Distribution patterns of migrating humpback whales (Megaptera novaeangliae) in Jervis Bay, Australia: a spatial analysis using geographical citizen science data.Crossref | GoogleScholarGoogle Scholar |
Burns, D. (2010) Population characteristics and migratory movements of humpback whales (Megaptera novaeangliae) identified on their southern migration past Ballina, eastern Australia. Ph.D. Thesis, Southern Cross University, Lismore, NSW.
Burns, D., Brooks, L., Harrison, P., Franklin, T., Franklin, W., Paton, D., and Clapham, P. (2014). Migratory movements of individual humpback whales photographed off the eastern coast of Australia. Marine Mammal Science 30, 562–578.
| Migratory movements of individual humpback whales photographed off the eastern coast of Australia.Crossref | GoogleScholarGoogle Scholar |
Calkins, K. G. (2005) Correlation coefficients. In ‘Applied Statistics - Lesson 5’. (Andrews University: Berrien Springs, MI, USA.) Available at https://www.andrews.edu/~calkins/math/edrm611/edrm05.htm [Verified 15 May 2020].
Cartwright, R., and Sullivan, M. (2009). Behavioral ontogeny in humpback whale (Megaptera novaeangliae) calves during their residence in Hawaiian waters. Marine Mammal Science 25, 659–680.
| Behavioral ontogeny in humpback whale (Megaptera novaeangliae) calves during their residence in Hawaiian waters.Crossref | GoogleScholarGoogle Scholar |
Cartwright, R., Venema, A., Hernandez, V., Wyels, C., Cesere, J., and Cesere, D. (2019). Fluctuating reproductive rates in Hawaii’s humpback whales, Megaptera novaeangliae, reflect recent climate anomalies in the North Pacific. Royal Society Open Science 6, 181463.
| Fluctuating reproductive rates in Hawaii’s humpback whales, Megaptera novaeangliae, reflect recent climate anomalies in the North Pacific.Crossref | GoogleScholarGoogle Scholar | 31032006PubMed |
Chaloupka, M., and Osmond, M. (1999). Spatial and seasonal distribution of humpback whales in the Great Barrier Reef region. American Fisheries Society Symposium 1999, 89–106.
Chaloupka, M., Osmond, M., and Kaufman, G. (1999). Estimating seasonal abundance trends and survival probabilities of humpback whales in Hervey Bay (east coast Australia). Marine Ecology Progress Series 184, 291–301.
| Estimating seasonal abundance trends and survival probabilities of humpback whales in Hervey Bay (east coast Australia).Crossref | GoogleScholarGoogle Scholar |
Chittleborough, R. (1958). The breeding cycle of the female humpback whale, Megaptera nodosa (Bonnaterre). Marine and Freshwater Research 9, 1–18.
| The breeding cycle of the female humpback whale, Megaptera nodosa (Bonnaterre).Crossref | GoogleScholarGoogle Scholar |
Chittleborough, R. (1965). Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski). Marine and Freshwater Research 16, 33–128.
| Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski).Crossref | GoogleScholarGoogle Scholar |
Clapham, P. J. (2000) The humpback whale. In ‘Cetacean Societies, Field Studies of Dolphins And Whales’. (Eds J. Mann, R. C. Connor, P. L. Tyack, and H. Whitehead.) pp. 173–196. (University of Chicago Press: Chicago, IL, USA.)
Clapham, P. J., and Zerbini, A. N. (2015). Are social aggregation and temporary immigration driving high rates of increase in some Southern Hemisphere humpback whale populations? Marine Biology 162, 625–634.
| Are social aggregation and temporary immigration driving high rates of increase in some Southern Hemisphere humpback whale populations?Crossref | GoogleScholarGoogle Scholar |
Clapham, P. J., Palsboll, P. J., Mattila, D. K., and Vasquez, O. (1992). Composition and dynamics of humpback whale competitive groups in the West Indies. Behaviour 122, 182.
Corkeron, P. J. (1995). Humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland: behaviour and responses to whale-watching vessels. Canadian Journal of Zoology 73, 1290–1299.
| Humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland: behaviour and responses to whale-watching vessels.Crossref | GoogleScholarGoogle Scholar |
Corkeron, P., and Brown, M. (1995). Pod characteristics of migrating humpback whales (Megaptera novaeangliae) off the east Australian Coast. Behaviour 132, 163–179.
| Pod characteristics of migrating humpback whales (Megaptera novaeangliae) off the east Australian Coast.Crossref | GoogleScholarGoogle Scholar |
Craig, A., Gabriele, C., Herman, L., and Pack, A. (2003). Migratory timing of humpback whales (Megaptera novaeangliae) in the Central North Pacific varies with age, sex and reproductive status. Behaviour 140, 981–1001.
| Migratory timing of humpback whales (Megaptera novaeangliae) in the Central North Pacific varies with age, sex and reproductive status.Crossref | GoogleScholarGoogle Scholar |
Davies, T. K., Stevens, G., Meekan, M. G., Struve, J., and Rowcliffe, J. M. (2012). Can citizen science monitor whale-shark aggregations? Investigating bias in mark–recapture modelling using identification photographs sourced from the public. Wildlife Research 39, 696–704.
| Can citizen science monitor whale-shark aggregations? Investigating bias in mark–recapture modelling using identification photographs sourced from the public.Crossref | GoogleScholarGoogle Scholar |
Dawbin, W. H. (1966) The seasonal migratory cycle of humpback whales. In ‘Whales, Dolphins, and Porpoises’. (Ed. K. S. Norris.) pp. 145–170. (University of California Press: Berkeley, CA, USA.)
Dawbin, W. H. (1997). Temporal segregation of humpback whales during migration in southern hemisphere waters. Memoirs of the Queensland Museum 42, 105–138.
Deakos, M. (2002) Humpback whale (Megaptera novaeangliae) communication: the context and potential functions of pec-slapping behavior on the Hawaiian wintering grounds. M.A. Thesis, University of Hawaii at Manoa, HI, USA.
Department of Environment and Heritage (2005) Humpback Whale Recovery Plan 2005–2010. (Department of Environment and Heritage, Wildlife Conservation Branch: Canberra, ACT, Australia.)
Derville, S., Torres, L. G., and Garrigue, C. (2018). Social segregation of humpback whales in contrasted coastal and oceanic breeding habitats. Journal of Mammalogy 99, 41–54.
| Social segregation of humpback whales in contrasted coastal and oceanic breeding habitats.Crossref | GoogleScholarGoogle Scholar |
Dickinson, J. L., Zuckerberg, B., and Bonter, D. N. (2010). Citizen science as an ecological research tool: challenges and benefits. Annual Review of Ecology, Evolution, and Systematics 41, 149–172.
| Citizen science as an ecological research tool: challenges and benefits.Crossref | GoogleScholarGoogle Scholar |
Dunlop, R. A. (2016). The effect of vessel noise on humpback whale, Megaptera novaeangliae, communication behaviour. Animal Behaviour 111, 13–21.
| The effect of vessel noise on humpback whale, Megaptera novaeangliae, communication behaviour.Crossref | GoogleScholarGoogle Scholar |
Dunlop, R. A., Cato, D. H., and Noad, M. J. (2008). Non-song acoustic communication in migrating humpback whales (Megaptera novaeangliae). Marine Mammal Science 24, 613–629.
| Non-song acoustic communication in migrating humpback whales (Megaptera novaeangliae).Crossref | GoogleScholarGoogle Scholar |
Dunlop, R. A., Cato, D. H., and Noad, M. J. (2010). Your attention please: increasing ambient noise levels elicits a change in communication behaviour in humpback whales (Megaptera novaeangliae). Proceedings of the Royal Society of London – B. Biological Sciences 277, 2521–2529.
| Your attention please: increasing ambient noise levels elicits a change in communication behaviour in humpback whales (Megaptera novaeangliae).Crossref | GoogleScholarGoogle Scholar |
Dunn, D. C., Harrison, A.-L., Curtice, C., DeLand, S., Donnelly, B., Fujioka, E., Heywood, E., Kot, C. Y., Poulin, S., Whitten, M., Åkesson, S., Alberini, A., Appeltans, W., Arcos, J. M., Bailey, H., Ballance, L. T., Block, B., Blondin, H., Boustany, A. M., Brenner, J., Catry, P., Cejudo, D., Cleary, J., Corkeron, P., Costa, D. P., Coyne, M., Crespo, G. O., Davies, T. E., Dias, M. P., Douvere, F., Ferretti, F., Formia, A., Freestone, D., Friedlaender, A. S., Frisch-Nwakanma, H., Froján, C. B., Gjerde, K. M., Glowka, L., Godley, B. J., Gonzalez-Solis, J., Granadeiro, J. P., Gunn, V., Hashimoto, Y., Hawkes, L. M., Hays, G. C., Hazin, C., Jimenez, J., Johnson, D. E., Luschi, P., Maxwell, S. M., McClellan, C., Modest, M., Sciara, G. N. d., Palacio, A. H., Palacios, D. M., Pauly, A., Rayner, M., Rees, A. F., Salazar, E. R., Secor, D., Sequeira, A. M. M., Spalding, M., Spina, F., Parijs, S. V., Wallace, B., Varo-Cruz, N., Virtue, M., Weimerskirch, H., Wilson, L., Woodward, B., and Halpin, P. N. (2019). The importance of migratory connectivity for global ocean policy. Proceedings of the Royal Society of London – B. Biological Sciences 286, 20191472.
| The importance of migratory connectivity for global ocean policy.Crossref | GoogleScholarGoogle Scholar |
Ersts, P. J., and Rosenbaum, H. C. (2003). Habitat preference reflects social organization of humpback whales (Megaptera novaeangliae) on a wintering ground. Journal of Zoology 260, 337–345.
| Habitat preference reflects social organization of humpback whales (Megaptera novaeangliae) on a wintering ground.Crossref | GoogleScholarGoogle Scholar |
Félix, F., and Botero-Acosta, N. (2011). Distribution and behaviour of humpback whale mother–calf pairs during the breeding season off Ecuador. Marine Ecology Progress Series 426, 277–287.
| Distribution and behaviour of humpback whale mother–calf pairs during the breeding season off Ecuador.Crossref | GoogleScholarGoogle Scholar |
Félix, F., and Botero-Acosta, N. (2012). Evaluating humpback whale (Megaptera novaeangliae) social behaviour through sexing active individuals. Aquatic Mammals 38, 311.
| Evaluating humpback whale (Megaptera novaeangliae) social behaviour through sexing active individuals.Crossref | GoogleScholarGoogle Scholar |
Félix, F., and Guzmán, H. M. (2014). Satellite tracking and sighting data analyses of Southeast Pacific humpback whales (Megaptera novaeangliae): is the migratory route coastal or oceanic? Aquatic Mammals 40, 329–340.
| Satellite tracking and sighting data analyses of Southeast Pacific humpback whales (Megaptera novaeangliae): is the migratory route coastal or oceanic?Crossref | GoogleScholarGoogle Scholar |
Fiori, L., Martinez, E., Orams, M. B., and Bollard, B. (2019). Effects of whale-based tourism in Vava’u, Kingdom of Tonga: behavioural responses of humpback whales to vessel and swimming tourism activities. PLoS One 14, e0219364.
| Effects of whale-based tourism in Vava’u, Kingdom of Tonga: behavioural responses of humpback whales to vessel and swimming tourism activities.Crossref | GoogleScholarGoogle Scholar | 31276544PubMed |
Foster-Smith, J., and Evans, S. M. (2003). The value of marine ecological data collected by volunteers. Biological Conservation 113, 199–213.
| The value of marine ecological data collected by volunteers.Crossref | GoogleScholarGoogle Scholar |
Frankel, A. S., Clark, C. W., Herman, L. M., and Gabriele, C. M. (1995). Spatial distribution, habitat utilization, and social interactions of humpback whales, Megaptera novaeangliae, off Hawai’i, determined using acoustic and visual techniques. Canadian Journal of Zoology 73, 1134–1146.
| Spatial distribution, habitat utilization, and social interactions of humpback whales, Megaptera novaeangliae, off Hawai’i, determined using acoustic and visual techniques.Crossref | GoogleScholarGoogle Scholar |
Franklin, T. (2012) The social and ecological significance of Hervey Bay Queensland for eastern Australian humpback whales (Megaptera novaeangliae). Ph.D. Thesis, Southern Cross University, Lismore, NSW, Australia.
Franklin, W. (2014) Abundance, population dynamics, reproduction, rates of population increase and migration linkages of eastern Australian humpback whales (Megaptera novaeangliae) utilising Hervey Bay, Queensland. Ph.D. Thesis, Southern Cross University, Lismore, NSW, Australia.
Franklin, T., Franklin, W., Brooks, L., Harrison, P., Baverstock, P., and Clapham, P. (2011). Seasonal changes in pod characteristics of eastern Australian humpback whales (Megaptera novaeangliae), Hervey Bay 1992–2005. Marine Mammal Science 27, E134–E152.
| Seasonal changes in pod characteristics of eastern Australian humpback whales (Megaptera novaeangliae), Hervey Bay 1992–2005.Crossref | GoogleScholarGoogle Scholar |
Franklin, T., Franklin, W., Brooks, L., and Harrison, P. (2018). Site-specific female-biased sex ratio of humpback whales (Megaptera novaeangliae) during a stopover early in the southern migration. Canadian Journal of Zoology 96, 533–544.
| Site-specific female-biased sex ratio of humpback whales (Megaptera novaeangliae) during a stopover early in the southern migration.Crossref | GoogleScholarGoogle Scholar |
Garcia-Cegarra, A. M., Villagra, D., Gallardo, D. I., and Pacheco, A. S. (2019). Statistical dependence for detecting whale-watching effects on humpback whales. The Journal of Wildlife Management 83, 467–477.
| Statistical dependence for detecting whale-watching effects on humpback whales.Crossref | GoogleScholarGoogle Scholar |
Garrigue, C., and Gill, P. C. (1994). Observations of humpback whales Megaptera novaeangliae in new Caledonian waters during 1991–1993. Biological Conservation 70, 211–218.
| Observations of humpback whales Megaptera novaeangliae in new Caledonian waters during 1991–1993.Crossref | GoogleScholarGoogle Scholar |
Geise, L., Gomes, N., and Cerqueira, R. (1999). Behaviour, habitat use and population size of Sotalia fluviatilis (Gervais, 1853) (Cetacea, Delphinidae) in the Cananéia estuary region, São Paulo, Brazil. Revista Brasileira de Biologia 59, 183–194.
| Behaviour, habitat use and population size of Sotalia fluviatilis (Gervais, 1853) (Cetacea, Delphinidae) in the Cananéia estuary region, São Paulo, Brazil.Crossref | GoogleScholarGoogle Scholar |
Groom, C. J., and Coughran, D. K. (2012). Entanglements of baleen whales off the coast of Western Australia between 1982 and 2010: patterns of occurrence, outcomes and management responses. Pacific Conservation Biology 18, 203–214.
| Entanglements of baleen whales off the coast of Western Australia between 1982 and 2010: patterns of occurrence, outcomes and management responses.Crossref | GoogleScholarGoogle Scholar |
Guidino, C., Llapapasca, M. A., Silva, S., Alcorta, B., and Pacheco, A. S. (2014). Patterns of spatial and temporal distribution of humpback whales at the southern limit of the Southeast Pacific breeding area. PLoS One 9, e112627.
| Patterns of spatial and temporal distribution of humpback whales at the southern limit of the Southeast Pacific breeding area.Crossref | GoogleScholarGoogle Scholar | 25391137PubMed |
Guzman, H. M., and Félix, F. (2017). Movements and habitat use by Southeast Pacific humpback whales (Megaptera novaeangliae) satellite tracked at two breeding sites. Aquatic Mammals 43, 139–155.
| Movements and habitat use by Southeast Pacific humpback whales (Megaptera novaeangliae) satellite tracked at two breeding sites.Crossref | GoogleScholarGoogle Scholar |
Helweg, D. A., and Herman, L. M. (1994). Diurnal patterns of behaviour and group membership of humpback whales (Megaptera novaeangliae) wintering in hawaiian waters. Ethology 98, 298–311.
| Diurnal patterns of behaviour and group membership of humpback whales (Megaptera novaeangliae) wintering in hawaiian waters.Crossref | GoogleScholarGoogle Scholar |
Herman, L. M., and Tavolga, W. N. (1980) The communication systems of cetaceans. In ‘Cetacean Behavior’. (Ed. L. M. Herman.) pp. 149–209. (Wiley: New York, NY, USA.)
Herman, E. Y., Herman, L. M., Pack, A. A., Marshall, G., Shepard, M. C., and Bakhtiari, M. (2007). When whales collide: crittercam offers insight into the competitive behavior of humpback whales on their Hawaiian wintering grounds. Marine Technology Society Journal 41, 35–43.
| When whales collide: crittercam offers insight into the competitive behavior of humpback whales on their Hawaiian wintering grounds.Crossref | GoogleScholarGoogle Scholar |
Irvine, L. G., Thums, M., Hanson, C. E., McMahon, C. R., and Hindell, M. A. (2018). Evidence for a widely expanded humpback whale calving range along the Western Australian coast. Marine Mammal Science 34, 294–310.
| Evidence for a widely expanded humpback whale calving range along the Western Australian coast.Crossref | GoogleScholarGoogle Scholar |
Karczmarski, L., Cockcroft, V. G., and Mclachlan, A. (2000). Habitat use and preferences of indo-pacific humpback dolphins Sousa chinensis in Algoa Bay, South Africa. Marine Mammal Science 16, 65–79.
| Habitat use and preferences of indo-pacific humpback dolphins Sousa chinensis in Algoa Bay, South Africa.Crossref | GoogleScholarGoogle Scholar |
Kavanagh, A. S., Owen, K., Williamson, M. J., Blomberg, S. P., Noad, M. J., Goldizen, A. W., Kniest, E., Cato, D. H., and Dunlop, R. A. (2017). Evidence for the functions of surface-active behaviors in humpback whales (Megaptera novaeangliae). Marine Mammal Science 33, 313–334.
| Evidence for the functions of surface-active behaviors in humpback whales (Megaptera novaeangliae).Crossref | GoogleScholarGoogle Scholar |
Kershaw, J. L., Ramp, C. A., Sears, R., Plourde, S., Brosset, P., Miller, P. J. O., and Hall, A. J. (2021). Declining reproductive success in the Gulf of St Lawrence’s humpback whales (Megaptera novaeangliae) reflects ecosystem shifts on their feeding grounds. Global Change Biology 27, 1027–1041.
| Declining reproductive success in the Gulf of St Lawrence’s humpback whales (Megaptera novaeangliae) reflects ecosystem shifts on their feeding grounds.Crossref | GoogleScholarGoogle Scholar |
Kobori, H., Dickinson, J. L., Washitani, I., Sakurai, R., Amano, T., Komatsu, N., Kitamura, W., Takagawa, S., Koyama, K., and Ogawara, T. (2016). Citizen science: a new approach to advance ecology, education, and conservation. Ecological Research 31, 1–19.
| Citizen science: a new approach to advance ecology, education, and conservation.Crossref | GoogleScholarGoogle Scholar |
Learmonth, J. A., MacLeod, C. D., Santos, M. B., Pierce, G. J., Crick, H., and Robinson, R. (2006) Potential effects of climate change on marine mammals. In ‘Oceanography and Marine Biology: An Annual Review’. (Eds R. N. Gibson, R. J. A. Atkinson, and J. D. M. Gordon.) Vol. 44, pp. 431–464. (Taylor & Francis: Boca Raton, FL, USA.)
Lucena, M. B., Barbosa, M. C., Sissini, M. N., and Sazima, I. (2016). Out of the mainstream: humpback whale calving site and associated fishes at an oceanic island off Brazil. Marine Biodiversity 46, 27–28.
| Out of the mainstream: humpback whale calving site and associated fishes at an oceanic island off Brazil.Crossref | GoogleScholarGoogle Scholar |
Lunardi, D. G., Engel, M. H., Marciano, J. L., and Macedo, R. H. (2010). Behavioural strategies in humpback whales, Megaptera novaeangliae, in a coastal region of Brazil. Journal of the Marine Biological Association of the United Kingdom 90, 1693–1699.
| Behavioural strategies in humpback whales, Megaptera novaeangliae, in a coastal region of Brazil.Crossref | GoogleScholarGoogle Scholar |
Madsen, C. J., and Herman, L. (1980) Social and ecological correlates of cetacean vision and visual appearance. In ‘Cetacean Behavior: Mechanisms and Functions’. pp. 101–146. (Wiley: New York, NY, USA.)
Mann, J. (1999). Behavioral sampling methods for cetaceans: a review and critique. Marine Mammal Science 15, 102–122.
| Behavioral sampling methods for cetaceans: a review and critique.Crossref | GoogleScholarGoogle Scholar |
Martinez, E., Currie, J., Stack, S., Easterly, S., and Kaufman, G. (2015) Note on patterns of area use by humpback whales (Megaptera novaeangliae) in 2013 in Hervey Bay, Australia, with an emphasis on mother–calf dyads. Working paper SC/66a/SH2 submitted to the Scientific Committee of the International Whaling Commission., San Diego, CA, USA.
Mattila, D. K., Clapham, P. J., Katona, S. K., and Stone, G. S. (1989). Population composition of humpback whales, Megaptera novaeangliae, on Silver Bank, 1984. Canadian Journal of Zoology 67, 281–285.
| Population composition of humpback whales, Megaptera novaeangliae, on Silver Bank, 1984.Crossref | GoogleScholarGoogle Scholar |
Mattila, D. K., Clapham, P. J., Vásquez, O., and Bowman, R. S. (1994). Occurrence, population composition, and habitat use of humpback whales in Samana Bay, Dominican Republic. Canadian Journal of Zoology 72, 1898–1907.
| Occurrence, population composition, and habitat use of humpback whales in Samana Bay, Dominican Republic.Crossref | GoogleScholarGoogle Scholar |
Meynecke, J. O., Vindenes, S., and Teixeira, D. (2013) Monitoring humpback whale (Megaptera novaeangliae) behaviour in a highly urbanised coastline: Gold Coast, Australia. In ‘Global Challenges in Integrated Coastal Zone Management’. (Eds E. Moksness, E. Dahl, and J. Støttrup.) pp. 101–113. (Wiley: Chichester, UK.)
National Health and Medical Research Council (2013). ‘Australian Code for the Care and Use of Animals for Scientific Purposes’, 8th edn. (NHMRC: Canberra, ACT, Australia.)
Noad, M. J., Kniest, E., and Dunlop, R. A. (2019). Boom to bust? Implications for the continued rapid growth of the eastern Australian humpback whale population despite recovery. Population Ecology 61, 198–209.
| Boom to bust? Implications for the continued rapid growth of the eastern Australian humpback whale population despite recovery.Crossref | GoogleScholarGoogle Scholar |
Noren, D. P., and Hauser, D. D. (2016). Surface-based observations can be used to assess behavior and fine-scale habitat use by an endangered killer whale (Orcinus orca) population. Aquatic Mammals 42, 168–183.
| Surface-based observations can be used to assess behavior and fine-scale habitat use by an endangered killer whale (Orcinus orca) population.Crossref | GoogleScholarGoogle Scholar |
Oña, J., Garland, E. C., and Denkinger, J. (2017). Southeastern Pacific humpback whales (Megaptera novaeangliae) and their breeding grounds: distribution and habitat preference of singers and social groups off the coast of Ecuador. Marine Mammal Science 33, 219–235.
| Southeastern Pacific humpback whales (Megaptera novaeangliae) and their breeding grounds: distribution and habitat preference of singers and social groups off the coast of Ecuador.Crossref | GoogleScholarGoogle Scholar |
Pirotta, V., Reynolds, W., Ross, G., Jonsen, I., Grech, A., Slip, D., and Harcourt, R. (2020). A citizen science approach to long-term monitoring of humpback whales (Megaptera novaeangliae) off Sydney, Australia. Marine Mammal Science 36, 472–485.
| A citizen science approach to long-term monitoring of humpback whales (Megaptera novaeangliae) off Sydney, Australia.Crossref | GoogleScholarGoogle Scholar |
Reinke, J., Lemckert, C., and Meynecke, J.-O. (2016). Coastal fronts utilized by migrating humpback whales, Megaptera novaeangliae, on the Gold Coast, Australia. Journal of Coastal Research 75, 552–556.
| Coastal fronts utilized by migrating humpback whales, Megaptera novaeangliae, on the Gold Coast, Australia.Crossref | GoogleScholarGoogle Scholar |
Ribbe, J. (2014) Hervey Bay and its estuaries. In ‘Estuaries of Australia in 2050 and Beyond’. (Ed. E. Wolanski.) Vol. 5, pp. 185–201. (Springer: Dordrecht, Netherlands.)
Riekkola, L., Andrews-Goff, V., Friedlaender, A., Constantine, R., and Zerbini, A. N. (2019). Environmental drivers of humpback whale foraging behavior in the remote Southern Ocean. Journal of Experimental Marine Biology and Ecology 517, 1–12.
| Environmental drivers of humpback whale foraging behavior in the remote Southern Ocean.Crossref | GoogleScholarGoogle Scholar |
Rossi-Santos, M. R. (2015). Oil industry and noise pollution in the humpback whale (Megaptera novaeangliae) soundscape ecology of the Southwestern Atlantic breeding ground. Journal of Coastal Research 31, 184–195.
| Oil industry and noise pollution in the humpback whale (Megaptera novaeangliae) soundscape ecology of the Southwestern Atlantic breeding ground.Crossref | GoogleScholarGoogle Scholar |
Schaffar, A., Madon, B., Garrigue, C., and Constantine, R. (2009). Avoidance of whale watching boats by humpback whales in their main breeding ground in New Caledonia. Report to the Scientific Committee of the International Whaling Commission SC61/WW6. Available at http://www.operationcetaces.nc/uploads/IWC%20papers/SC-61-WW6%20Schaffar%20et%20al.pdf [Verified 19 March 2021].
Schaffar, A., Madon, B., Garrigue, C., and Constantine, R. (2013). Behavioural effects of whale-watching activities on an Endangered population of humpback whales wintering in New Caledonia. Endangered Species Research 19, 245–254.
| Behavioural effects of whale-watching activities on an Endangered population of humpback whales wintering in New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Scheidat, M., Castro, C., Denkinger, J., González, J., and Adelung, D. (2000). A breeding area for humpback whales (Megaptera novaeangliae) off Ecuador. The Journal of Cetacean Research and Management 2, 165–172.
Schuler, A. R., Piwetz, S., Di Clemente, J., Steckler, D., Mueter, F., and Pearson, H. C. (2019). Humpback whale movements and behavior in response to whale-watching vessels in Juneau, AK. Frontiers in Marine Science 6, 710.
| Humpback whale movements and behavior in response to whale-watching vessels in Juneau, AK.Crossref | GoogleScholarGoogle Scholar |
Silber, G. K. (1986). The relationship of social vocalizations to surface behavior and aggression in the Hawaiian humpback whale (Megaptera novaeangliae). Canadian Journal of Zoology 64, 2075–2080.
| The relationship of social vocalizations to surface behavior and aggression in the Hawaiian humpback whale (Megaptera novaeangliae).Crossref | GoogleScholarGoogle Scholar |
Silvertown, J. (2009). A new dawn for citizen science. Trends in Ecology & Evolution 24, 467–471.
| A new dawn for citizen science.Crossref | GoogleScholarGoogle Scholar |
Simmonds, M. P., and Eliott, W. J. (2009). Climate change and cetaceans: concerns and recent developments. Journal of the Marine Biological Association of the United Kingdom 89, 203–210.
| Climate change and cetaceans: concerns and recent developments.Crossref | GoogleScholarGoogle Scholar |
Simmons, M. L., and Marsh, H. (1986). Sightings of humpback whales in Great Barrier Reef waters. Scientific Reports of the Whales Research Institute 37, 31–46.
Smith, J. N., Grantham, H. S., Gales, N., Double, M. C., Noad, M. J., and Paton, D. (2012). Identification of humpback whale breeding and calving habitat in the Great Barrier Reef. Marine Ecology Progress Series 447, 259–272.
| Identification of humpback whale breeding and calving habitat in the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Smith, J. N., Kelly, N., Childerhouse, S., Redfern, J. V., Moore, T. J., and Peel, D. (2020). Quantifying ship strike risk to breeding whales in a multiple-use marine park: the Great Barrier Reef. Frontiers in Marine Science 7, 67.
| Quantifying ship strike risk to breeding whales in a multiple-use marine park: the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Sousa, A., Alves, F., Dinis, A., Bentz, J., Cruz, M., and Nunes, J. (2019). How vulnerable are cetaceans to climate change? Developing and testing a new index. Ecological Indicators 98, 9–18.
| How vulnerable are cetaceans to climate change? Developing and testing a new index.Crossref | GoogleScholarGoogle Scholar |
Sprogis, K. R., Videsen, S., and Madsen, P. T. (2020). Vessel noise levels drive behavioural responses of humpback whales with implications for whale-watching. eLife 9, e56760.
| Vessel noise levels drive behavioural responses of humpback whales with implications for whale-watching.Crossref | GoogleScholarGoogle Scholar | 32539930PubMed |
Stack, S. H., McCordic, J. A., Machernis, A. F., Olson, G. L., and Currie, J. J. (2019). Preliminary report on the impacts of swim-with-whale tourism on humpback whale behaviour in Hervey Bay, Queensland, Australia. Paper SC/68A/WW/02 presented to the IWC Scientific Committee, Pacific Whale Foundation.
Stack, S. H., Currie, J. J., McCordic, J. A., Machernis, A. F., and Olson, G. L. (2020). Distribution patterns of east Australian humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland: a historical perspective. Australian Mammalogy 42, 16–27.
| Distribution patterns of east Australian humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland: a historical perspective.Crossref | GoogleScholarGoogle Scholar |
Stamation, K. A., Croft, D. B., Shaughnessy, P. D., Waples, K. A., and Briggs, S. V. (2010). Behavioral responses of humpback whales (Megaptera novaeangliae) to whale-watching vessels on the southeastern coast of Australia. Marine Mammal Science 26, 98–122.
| Behavioral responses of humpback whales (Megaptera novaeangliae) to whale-watching vessels on the southeastern coast of Australia.Crossref | GoogleScholarGoogle Scholar |
Strauss, D., Mirferendesk, H., and Tomlinson, R. (2007). Comparison of two wave models for Gold Coast, Australia. Journal of Coastal Research 50, 312–316.
Sullivan, M., and Cartwright, R. (2009). Associations with multiple male groups increase the energy expenditure of humpback whale (Megaptera novaeangliae) female and calf pairs on the breeding grounds. Behaviour 146, 1573–1600.
| Associations with multiple male groups increase the energy expenditure of humpback whale (Megaptera novaeangliae) female and calf pairs on the breeding grounds.Crossref | GoogleScholarGoogle Scholar |
Thums, M., Jenner, C., Waples, K., Salgado Kent, C., and Meekan, M. (2018) Humpback whale use of the Kimberley; understanding and monitoring spatial distribution. Report of Project 1.2.1 prepared for the Kimberley Marine Research Program, Perth, WA, Australia.
Tonachella, N., Nastasi, A., Kaufman, G., Maldini, D., and Rankin, R. W. (2012). Predicting trends in humpback whale (Megaptera novaeangliae) abundance using citizen science. Pacific Conservation Biology 18, 297–309.
| Predicting trends in humpback whale (Megaptera novaeangliae) abundance using citizen science.Crossref | GoogleScholarGoogle Scholar |
Torre-Williams, L., Martinez, E., Meynecke, J.-O., Reinke, J., and Stockin, K. (2019). Presence of newborn humpback whale (Megaptera novaeangliae) calves in Gold Coast Bay, Australia. Marine and Freshwater Behaviour and Physiology 52, 199–216.
| Presence of newborn humpback whale (Megaptera novaeangliae) calves in Gold Coast Bay, Australia.Crossref | GoogleScholarGoogle Scholar |
Trudelle, L., Cerchio, S., Zerbini, A. N., Geyer, Y., Mayer, F.-X., Jung, J.-L., Hervé, M. R., Pous, S., Sallée, J.-B., and Rosenbaum, H. C. (2016). Influence of environmental parameters on movements and habitat utilization of humpback whales (Megaptera novaeangliae) in the Madagascar breeding ground. Royal Society Open Science 3, 160616.
| Influence of environmental parameters on movements and habitat utilization of humpback whales (Megaptera novaeangliae) in the Madagascar breeding ground.Crossref | GoogleScholarGoogle Scholar | 28083104PubMed |
Tyack, P. (1981). Interactions between singing Hawaiian humpback whales and conspecifics nearby. Behavioral Ecology and Sociobiology 8, 105–116.
| Interactions between singing Hawaiian humpback whales and conspecifics nearby.Crossref | GoogleScholarGoogle Scholar |
Tyack, P., and Whitehead, H. (1983). Male competition in large groups of wintering humpback whales. Behaviour 83, 132–154.
| Male competition in large groups of wintering humpback whales.Crossref | GoogleScholarGoogle Scholar |
Valani, R., Meynecke, J.-O., and Olsen, M. T. (2020). Presence and movement of humpback whale (Megaptera novaeangliae) mother–calf pairs in the Gold Coast, Australia. Marine and Freshwater Behaviour and Physiology 53, 251–263.
| Presence and movement of humpback whale (Megaptera novaeangliae) mother–calf pairs in the Gold Coast, Australia.Crossref | GoogleScholarGoogle Scholar |
Vang, L. (2002) Distribution, abundance and biology of group V humpback whales Megaptera novaeangliae: a review. Queensland Government, Environmental Protection Agency, Brisbane, Qld, Australia.
Ward, E. J., Marshall, K. N., Ross, T., Sedgley, A., Hass, T., Pearson, S. F., Joyce, G., Hamel, N. J., Hodum, P. J., and Faucett, R. (2015). Using citizen-science data to identify local hotspots of seabird occurrence. PeerJ 3, e704.
| Using citizen-science data to identify local hotspots of seabird occurrence.Crossref | GoogleScholarGoogle Scholar | 25653898PubMed |
Whitehead, H. (1985). Humpback whale breaching. In ‘Investigations on Cetacea’. (Ed. G. Pilleri.) Vol. 17, pp. 117–155. (Hirnanatomisches Institut der Universität: Bern, Switzerland.)