Effects of temperature and ration on the otolith-to-somatic size relationship in juvenile Chinook salmon (Oncorhynchus tshawytscha): a test of the direct proportionality assumption
David G. Stormer A B and Francis Juanes AA Department of Biology, University of Victoria, Victoria, BC, V8P 5C2, Canada.
B Corresponding author. Email: dstormer@uvic.ca
Marine and Freshwater Research 67(7) 913-924 https://doi.org/10.1071/MF15206
Submitted: 25 May 2015 Accepted: 14 March 2016 Published: 24 May 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Fish otoliths are commonly used to estimate somatic growth rate, but this depends on the assumption that the otolith and body grow in direct proportion. Environmental conditions contribute to variability in somatic growth and can result in deviations from direct proportionality in the otolith-to-somatic size relationship. In the present study we examined the otolith-to-body size relationship for juvenile Chinook salmon (Oncorhynchus tshawytscha) subjected to simulated seasonal (summer, autumn and winter) water temperatures and feeding rations. The otolith-to-somatic size relationship became uncoupled during summer between fish subjected to the cool (15°C) and hot (21°C) water temperatures. A food ration effect was also observed during the summer, such that fish fed an unlimited ration had smaller otoliths than equivalently sized fish fed a limited ration. The effects of water temperature and ration disappeared by the end of autumn, indicating that a seasonal compensatory response occurred in the otolith-to-somatic size relationship after the extreme temperatures and food limitations were alleviated. In winter, this relationship became uncoupled again, but only between fish that were fed throughout the winter and fish that were starved during the 3-month experimental period. The effects of water temperature and rations on the otolith-to-somatic size relationship of juvenile Chinook salmon could have implications for accurately estimating somatic growth from otolith growth in natural populations and should be incorporated into back-calculation techniques.
Additional keywords: back-calculation, food ration, water temperature.
Introduction
For anadromous fish with complex early life history stages, stage-specific growth rates may vary considerably among individuals and are critically important in determining recruitment potential. Obtaining accurate estimates of early life stage-specific growth in fish collected from natural systems can be challenging because it requires calculating growth rates before collection. Pannella (1971) showed that daily bands were deposited on juvenile fish otoliths and these increments provided a record of individual growth rate. Otolith microstructure analysis has since become an effective tool in reconstructing past variability in somatic growth during the early life stages of fish. Further, the enumeration of daily increments on fish otoliths has been used to identify the timing of environmental transitions, and has revealed critically important periods in determining recruitment success during early life history (Beamish and Mahnken 2001).
Back-calculation techniques incorporate the relationship between otolith growth and somatic growth to estimate individual somatic growth rate before the collection of the fish (Francis 1990). Traditional back-calculation methods require the measurement of the otolith radius from the core to the edge along the longest axis of either the otolith length or width to estimate past somatic size and growth (Megalofonou 2006). The validity of these techniques depends on the assumption that otolith growth and somatic growth are constantly and directly proportional and is warranted by a strong correlation in size between the otolith and body as the fish grows (Campana and Neilson 1985). However, several examples of deviations in direct proportionality or uncoupling between otolith growth and somatic growth have been documented in larval and juvenile fish (Sogard 1991; Hare and Cowen 1995; Takasuka et al. 2008). Uncoupling can occur when somatic growth slows or ceases but the otolith continues to grow, resulting in slower-growing fish with larger otoliths than faster-growing fish of the same size and age (Campana 1990), or where the incremental deposition of calcium carbonate (CaCO3) on the otolith continues regardless of the somatic growth rate (Secor and Dean 1992). Violations in the assumptions of constant and direct proportionality between otolith development and somatic growth have been associated with changes in environmental factors, such as food availability and water temperature, in temperate fish (Mosegaard et al. 1988; Barber and Jenkins 2001).
Chinook salmon (Oncorhynchus tshawytscha) is a cool-water species that is economically and ecologically important throughout its range. The largest of all Pacific salmon species, Chinook salmon are valuable to commercial, recreational and First Nations fisheries. In North America, Chinook salmon exhibit highly variable life history strategies, especially with regard to downstream migration timing (Quinn 2005). In southern British Columbia (BC), Canada, ocean-type or sub-yearling Chinook salmon out-migrate from their natal streams to the coastal ocean in the spring and summer of their first year of life (Healey 1983). Consequently, rapid growth during this period is critical to avoid predation and attain an adequate size to survive the first marine winter (Beamish and Mahnken 2001).
During early marine life, juvenile ocean-type or sub-yearling Chinook salmon entering the Strait of Georgia (SOG) encounter a range of environmental conditions that drive first-year variability in growth and survival (Preikshot et al. 2013). However, long-term trends in SOG oceanographic conditions indicate that juvenile Chinook are experiencing regional-scale increasing water temperatures (Masson and Cummins 2007), particularly during summer. Moreover, the aforementioned trends in ocean temperatures have been shown to be associated with altered temporal and spatial secondary production (Mackas et al. 2007), including declines in zooplankton and forage fish abundances (El Sabaawi et al. 2009; Schweigert et al. 2013) in the SOG.
The deposition of daily growth increments on otoliths has been validated for juvenile Chinook salmon, with the ratio of otolith size to somatic size remaining constant for approximately 2 months across different photoperiods, suboptimal temperatures and feeding frequencies (Neilson and Geen 1982). However, the interactive effects of food quantity and superoptimal temperature regimens on the otolith-to-somatic size relationship have not been investigated and may have implications for Chinook salmon management, because otolith microstructure analysis has become a common technique for estimating critical life stage events, including size at marine entry and early marine growth rates (Marrin Jarrin and Miller 2013; Claiborne et al. 2014; Miller et al. 2014). Further, we know little about how this relationship is affected when extreme conditions, such as superoptimal water temperatures and food deprivation, are eased over a multiseasonal scale. The aim of the present study was to examine the relationship between otolith size and somatic size in juvenile ocean-type Chinook salmon subjected to a range of simulated seasonal water temperature and food ration experimental treatment combinations.
Materials and methods
Experimental design
Three experimental periods (summer, autumn and winter) were designed to simulate the first marine year seasonal conditions experienced by juvenile ocean-type Chinook salmon in the SOG. For each experimental period, three water temperature treatments were selected using long-term average monthly ocean temperature data recorded by the Canada Department of Fisheries and Oceans (DFO) Entrance Island recording station in the SOG to approximate historic (1937–75), current (1976–2012) and future (2050) seasonal ocean temperature conditions. The ‘historic’ water temperatures were selected to represent the time period before the multidecadal decline of southern BC Chinook salmon stocks (Tompkins et al. 2011) for which water temperature data were available (38 years). The ‘current’ water temperatures were selected to simulate ocean conditions during the period of BC Chinook salmon population declines, which spanned ~36 years through 2012. The ‘future’ water temperatures represented the projected water temperature 38 years into the future based on the rate of warming during the ‘current’ conditions time period. The ‘historic’ and ‘current’ environmental conditions were calculated as the mean water temperatures for each time period using the monthly mean water temperatures within each season. The ‘future’ seasonal water temperatures were calculated by extrapolating the simple linear regressions of the SOG water temperatures during the ‘current’ conditions time period (1976–2012) out to the year 2050 using the expression:
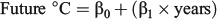
where β0 and β1 were the predicted 2012 water temperature and slope from the linear regressions of the preceding ‘current’ conditions time period respectively and the years equalled 38 for each season:
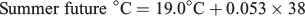
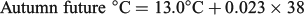
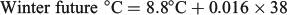
For all three seasonal experimental periods, the ‘historic’, ‘current’ and ‘future’ water temperatures will hereafter be defined as cool, warm and hot respectively.
During each seasonal experimental period, the three water temperature treatments were combined with a range of food rations. The food rations were based on percentage body weight (BW) per day (% BW day–1) of stomach contents and adjusted at the beginning of each experimental period to simulate the seasonal changes in feeding intensity described for ocean-type juvenile Chinook salmon in the coastal ocean (Schabetsberger et al. 2003). Commercial salmonid feed pellets (Bio-Oregon, Vancouver, BC, Canada) were administered manually twice a day to all treatments designated to receive food. Rations were dispensed 1–2 h after sunrise and within 1 h of sunset to simulate the bimodal diurnal feeding behaviour observed for juvenile Chinook salmon at the crepuscular periods (Benkwitt et al. 2009).
In May 2013, ocean-type Chinook salmon parr were obtained from the Nanaimo River Hatchery (Nanaimo, BC, Canada) and smolted in a 600-L holding tank at the University of Victoria Aquatic Research Facility (Victoria, BC, Canada). Post-smolts were acclimated to ambient marine conditions (salinity = 30.5 ppt; water temperature = 14.5°C) and reared at ambient conditions for ~1 month. On 14 June 2013, all fish were anaesthetised in a bath of 30 mg L–1 tricaine methanesulfonate (MS222), measured (±1.0-mm fork length, FL) and weighed (±0.01 g), and similar-sized individuals were transferred to the experimental aquaria. The water temperatures in all experimental aquaria were increased incrementally by 1.0°C per day until attaining the summer experimental period levels.
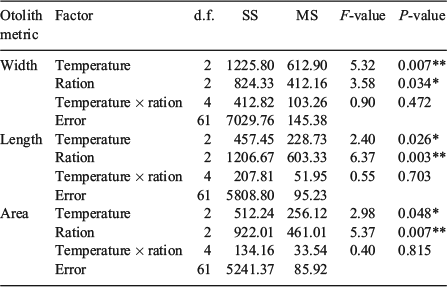
For the summer experimental period, each 240-L experimental aquarium received 17 juvenile Chinook salmon smolts. The cool, warm and hot water temperature treatments selected for the summer experimental period were 15.0°C (mean ± s.d., 14.9 ± 0.4°C), 18.0°C (18.1 ± 0.8°C) and 21.0°C (20.9 ± 0.7°C) respectively. Three food rations (high = 4.5% BW day–1; medium = 1.5% BW day–1; low = 0.5% BW day–1) were selected to encompass a range of food availability from ad libitum to food deprived. The food rations were adjusted for fish growth during the summer experimental period from the biweekly sampling of 10 fish per aquarium for weight. Single replicates of each three temperature × three food ration treatment combination resulted in a total of 18 experimental aquaria (Table 1). The summer experimental period lasted 92 days from 21 June 2013 to 21 September 2013.
The water temperatures in all experimental aquaria were reduced by ~1.0°C per day to acclimate the fish to the autumn experimental period, which began on 28 September 2013. The water temperatures selected to simulate the cool, warm and hot water temperature treatments during autumn were 10.0°C (mean ± s.d., 9.9 ± 0.5°C), 12.0°C (12.3 ± 0.6°C) and 14.0°C (14.0 ± 0.7°C) respectively. Two food rations (high = 2.0% BW day–1; low = 1.0% BW day–1) were selected to represent reduced feeding intensity from summer to autumn and adjusted for somatic growth from monthly sampling of all fish for weight. The high food ration was deemed ad libitum during the autumn experimental period because this ration exceeded satiation in all treatments. Fish densities (number of fish per aquarium) during autumn were reduced from the summer experimental period to n = 8 in the low ration treatments and n = 12 in the high ration treatments with single replicates of each treatment combination for a total of 120 fish and 12 experimental aquaria (Table 1). The autumn experimental period ended on 28 December 2013 (91 days).
The water temperatures in all aquaria were lowered by 0.5–1.0°C per day over a 7-day acclimation period until the winter experimental period water temperatures were attained: cool = 5.5°C (mean ± s.d., 5.7 ± 0.8°C), warm = 7.5°C (7.7 ± 0.5°C), hot = 9.5°C (9.7 ± 0.5°C). The food rations were reduced from autumn to winter and categorised as fed (1.0% BW day–1) and unfed (0.0% BW day–1). The rations were not adjusted for growth during the winter, but 1.0% BW day–1 exceeded satiation in all the fed treatments throughout this seasonal experimental period. In all, 30 fish were equally distributed among the unfed–temperature treatment aquaria with single replicates of each treatment combination, whereas the fed treatment haphazardly received either seven or eight fish per replicate aquarium (Table 1). The winter experimental period lasted 95 days from 5 January to 10 April 2014.
Fish that were subjected to the cool, warm and hot water temperature treatments during the summer experimental period were maintained in these respective temperatures during the autumn and winter experimental periods. At the end of each experimental period, a subsample of fish (summer, n = 70; autumn, n = 21; winter, n = 60) were killed with a lethal overdose of MS222, measured, weighed and preserved frozen (–20°C) for otolith microstructure analysis. The experimental design was approved by the University of Victoria Institutional Animal Care and Use Committee (AUP# 2012–021) and the study was conducted in compliance with the Canadian Council on Animal Care standards and policies for biological research with vertebrates.
Both sagittal otoliths were extracted from each of the subsampled fish, cleaned of adhering tissue with distilled water and stored dry in individually labelled plastic vials. All otoliths broken during dissection were discarded, but at least one unbroken otolith was dissected from all of the subsampled fish. The otoliths were mounted on glass microscope slides and digitised in whole view under transmitted light at magnifications of 40–100× with a digital camera (Model DP26; Olympus Canada, Richmond, Canada) connected to a stereomicroscope controlled by a desktop computer. Maximum otolith length (OL) was defined as the longest axis between the anterior and posterior edge, whereas maximum otolith width (OW) was defined as the maximum distance from dorsal to ventral edge perpendicular to the length through the core. The length and width of each otolith were measured to the nearest 0.01 mm along the sagittal plane. The images of each otolith were converted from grey scale to the binary form and otolith area (OA; ±0.01 mm2) was calculated by binary segmentation from a preselected threshold value. All measurements and thresholding were performed using CellSens image analysis software (Olympus).
Statistical analysis
One-way analysis of variance (ANOVA) was used to evaluate the treatment effects on the sizes (FL) of the juvenile Chinook salmon at the beginning and end of each seasonal experimental period. We tested the assumption of constant proportionality between otolith size to juvenile Chinook somatic size by examining the relationship between the otolith metrics (OL, OW, OA) and FL with simple linear regression. The coefficients of determination were used to identify the otolith metric that explained the most variability in somatic size during each experimental period. We tested for morphological anomalies between the left and right otoliths using analysis of covariance (ANCOVA), with otolith size (mm) as the covariate and otolith (left v. right) as the explanatory variable; no morphological anomalies were found (P > 0.10), so one otolith was randomly selected from each fish for subsequent analyses.
To evaluate the assumption of direct proportionality in the otolith-to-body size relationship related to temperature and food rations, the residuals (observed FL – predicted FL) of the above-described full linear regressions were identified and grouped by each treatment level and compared with a general linear model (GLM). Under the assumption of direct proportionality, the mean residual values should have been similar among all the treatments. For the summer and winter experimental periods, the interaction between water temperature and food ration effects on the residuals of the otolith-to-body size linear regression was tested with a two-way ANOVA, with temperature and ration as the main effects. The low sample size of fish from the hot water temperature + low food ration treatment combination at the end of the autumn experimental period precluded the evaluation of the interaction between temperature and food ration effects (Table 1). Consequently, a one-way ANOVA and two-sample t-test were used to compare the effects of the three water temperatures and two food rations on the residuals of the otolith-to-body size relationship respectively. All significant ANOVAs were followed by Tukey’s honestly significant difference (HSD) multiple comparison tests to determine differences between treatments. For each test of significance, the treatment replicate effects, main effects and interactions were considered significant at a probability level of two-tailed P < 0.05. We found no effect of the treatment replicates on any of the response variables during all three seasons (P > 0.10), so the samples from the replicate aquaria were pooled in subsequent analyses. All analyses were performed in SIGMAPLOT 11.0 (Systat Software, San Jose, CA, USA).
Results
Summer experimental period
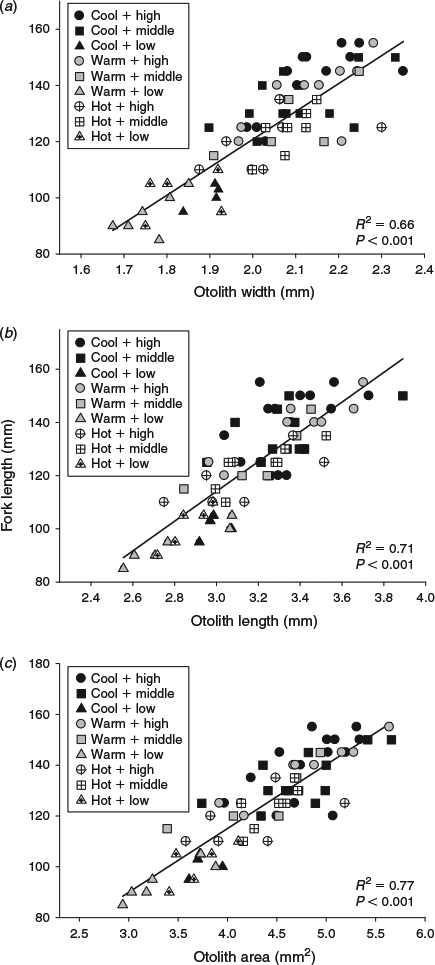
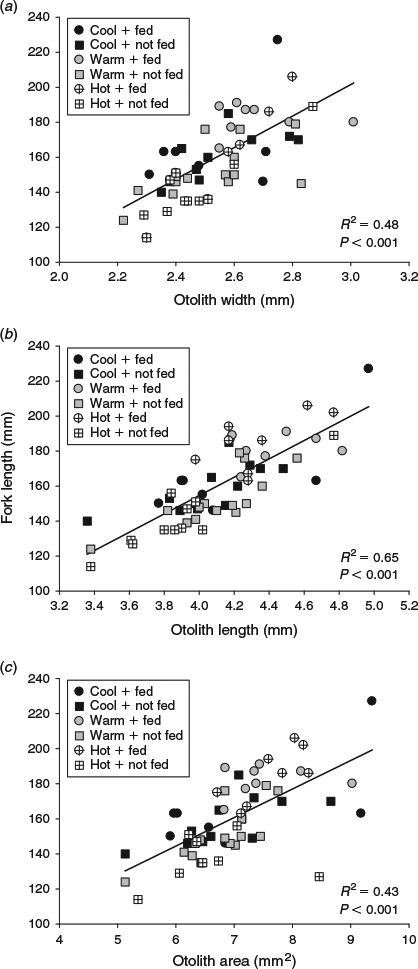
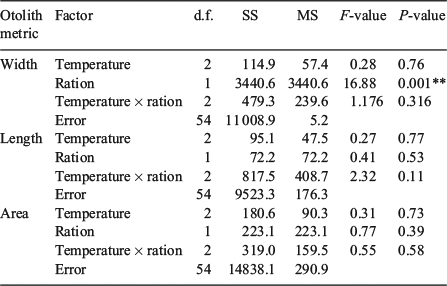
The sizes of the juvenile Chinook salmon were similar at the beginning of the summer experimental period, but varied across both water temperature and food ration treatments by the end of the season (F8,69 = 18.9, P < 0.001). Temperature affected somatic size such that fish in the cool temperature + high ration treatment combination were larger than fish subjected to the hot water temperatures regardless of ration, whereas FL was similar among fish fed the middle and low rations across all three temperatures (Table 1). All three otolith metrics (OW, OL, OA) were positively related to somatic size (P < 0.001). Of the three otolith metrics, OA explained the most variability in body size (R2 = 0.77; Fig. 1). Water temperature and food ration had significant effects on the otolith-to-somatic size relationship, but no interaction between the main effects was evident (Table 2). Tukey’s HSD showed that the residuals of the otolith-to-somatic size regression for fish inhabiting the cool water temperature treatments were greater than for fish in hot temperatures (P < 0.05), whereas the regression residuals between fish in cool and warm temperatures, and warm and hot temperatures, were similar across all three otolith metrics (Table 2). The regression residuals of fish in the high and medium food rations were similar (P > 0.10) and greater than for fish in the low rations across all three otolith metrics (P < 0.05; Fig. 2). That is, the full linear regression between otolith size and somatic size underestimated juvenile Chinook FL in the cool temperatures and high rations by an average of 4.1 and 3.8 mm respectively, and overestimated the somatic size of fish in the hot temperatures and low rations by an average of 5.1 and 7.9 mm respectively. Subtracting the underestimations of 4.1 and 3.8 mm from the average FL of fish in the cool (mean FL = 125 mm) and high (mean FL = 133 mm) treatments equalled percentage errors in the regression-derived somatic size estimates of 3.3 and 2.9% respectively. Adding the overestimations of 5.1 and 7.9 mm to the hot (mean FL = 115 mm) and low (mean FL = 98 mm) treatments yielded percentage errors in the regression-derived somatic size estimates of 4.4 and 8.1% respectively.
|
|
Autumn experimental period
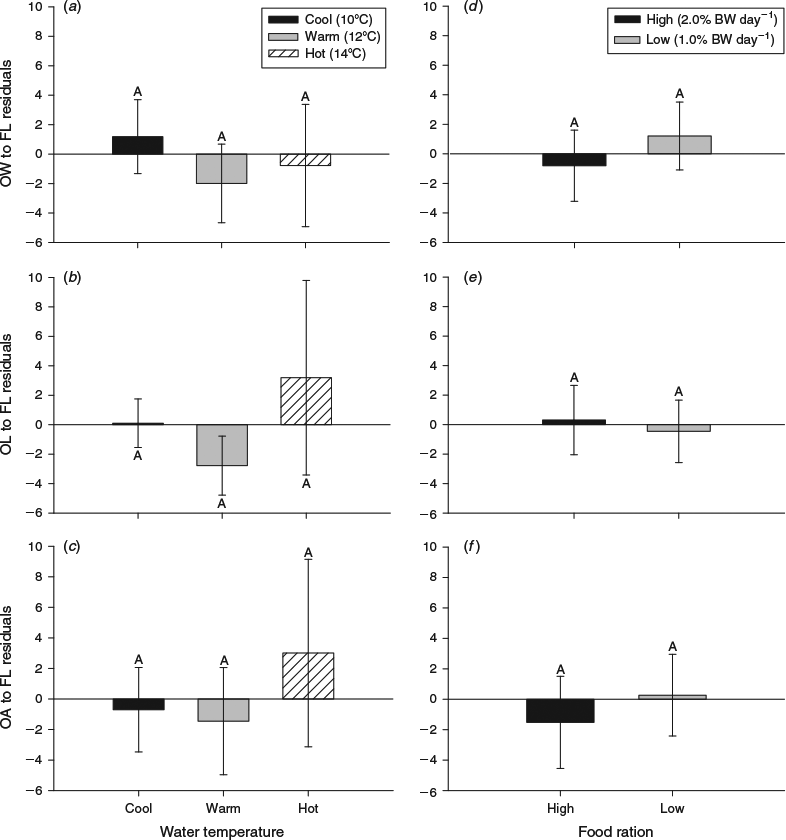
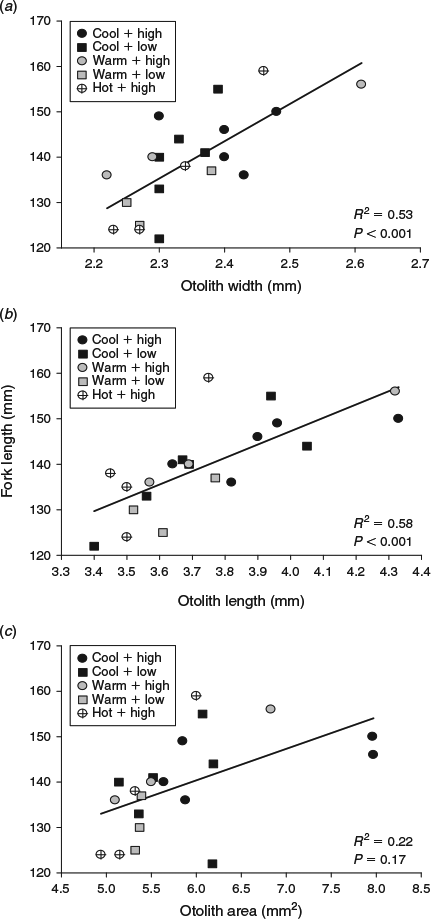
The cool, warm and hot water temperatures were decreased by 5.0, 6.0 and 7.0°C respectively from summer to autumn. Somatic size varied at the beginning of the autumn experimental period (F5,119 = 6.1, P < 0.001), but only between fish in the cool and hot water temperature treatments (Table 1). By the end of autumn, compensatory growth was evident in fish subjected to the hot water temperature and fed the high ration because somatic size was similar among all the fish killed for otolith microstructure analysis (Table 1). However, the coefficients of determination of the otolith-to-somatic size relationships declined from summer to autumn for all three otolith metrics (Fig. 3). OA, which was the best predictor of somatic size during summer, performed the worst of the three otolith metrics during autumn (R2 = 0.22, P = 0.22), indicating a multidimensional developmental response in the otoliths to the lowered water temperatures and increased food availability. OL outperformed OW and OA in explaining the variability in somatic size (Fig. 3). A compensatory response in the effects of temperature and ration on the otolith-to-somatic size relationship was also evident during autumn because the one-way ANOVAs of the otolith-to-body size regression residuals showed no differences among the three temperatures for OW (P = 0.77), OL (P = 0.49) and OA (P = 0.75). The regression residuals were also similar between the high and low rations across the three otolith metrics (Fig. 4).
|
Winter experimental period
At the beginning of the winter experimental period, fish in the warm temperature + fed treatment combination were larger than fish in the hot temperature + unfed treatment combination, but somatic size was similar among all other treatment combinations (Table 1). Both water temperature and ration influenced the size of juvenile Chinook salmon by the end of the winter experimental period (F5,59 = 14.8, P < 0.001). The effects of temperature and food ration on the growth rates of juvenile Chinook salmon were most evident in fish subjected to the hot water temperature, wherein growth was nil in unfed fish and greatest in fed fish across all the treatments (Table 1). Similar to the summer experimental period, in winter all three otolith metrics (OW, OL, OA) were positively related to somatic size (P < 0.001). During winter, as in autumn, of the three otolith metrics, OL explained the highest variability (R2 = 0.65, P < 0.001) in somatic size. The explanatory power in predicting somatic size increased for OL and OA, and decreased for OW, from the previous season (Fig. 5). The two-way ANOVA revealed that ration only had an effect on the otolith-to-somatic size linear regression (Table 3). However, the effect of ration was only evident for OW, wherein the regression residuals were greater for fed fish (F1,59 = 16.9, P < 0.001) than unfed fish (Fig. 6). Subtracting the underestimation of 9.6 mm from the average FL of fed fish (mean FL = 190 mm) and adding the overestimation of 6.0 mm to the average FL of unfed fish (mean FL = 161 mm) yielded percentage errors in the regression-derived somatic size estimates of 5.1 and 3.8% for the fed and unfed fish respectively.
|
|
Discussion
The experimental treatments that were selected for the present study produced positive relationships between all otolith metrics and juvenile Chinook salmon somatic size in all seasonal periods, except for OA during autumn, indicating general agreement with the constant proportionality assumption. The positive relationships between otolith size and FL were not unexpected because some calcium carbonate accretes on fish otoliths whether or not the fish grows in size, and organic material is deposited on the otolith even when somatic growth is minimal (Marshall and Parker 1982). However, the discrepancy in the explanatory power of the different otolith metrics in the different seasons suggests that the juvenile Chinook otoliths grew at different rates along the OL and OW axes. This is an interesting finding because daily growth increments are known to be deposited in concentric rings on the fish otoliths (Tanaka et al. 1981; Neilson and Geen 1982), thus we expected that the coefficients of determination for OL and OW would have been equivalent within each season. Coefficients of determination between otolith growth and somatic growth much greater than observed here have been reported for juveniles of other fish (Waessle et al. 2003), even over a range of environmental conditions (Otterlei et al. 2002). We showed that the modest relationships between the otolith metrics and somatic size in the present study were due to the extreme treatment combination of the hot water temperature and low food ration during summer and complete food deprivation during winter. Further, the uncoupling, or non-significant slope (Wilson et al. 2009), between OA and FL during autumn indicated that the otoliths responded to the reduction in water temperature in a manner that was not revealed in the coordinate plane measurement approach.
Fish can experience environmental conditions that lead to asynchronous changes in the relationship between otolith growth and somatic growth (Secor and Dean 1989). Such deviations from direct proportionality in the otolith-to-somatic size relationship can be driven by variation in growth rate, leading to similar-sized fish with different-sized otoliths (growth effect; Reznick et al. 1989). For example, the cessation of somatic growth could occur during periods of food deprivation (Wright et al. 1990) or at extreme water temperatures (Otterlei et al. 2002), but the accretion of CaCO3 continues, causing an increase in the size of the otolith without a corresponding increase in fish size. Alternatively, faster-growing fish that have smaller otoliths may be younger (age effect) than similar-sized slower-growing fish with larger otoliths (Secor and Dean 1992). Although we found considerable variation in somatic sizes across treatments by the end of the summer season, all the fish used in this experiment were hatched on the same day. Therefore, the deviations from direct proportionality between the otolith metrics and FL produced by the food-limited (0.5% BW day–1) and hot temperature (21°C) treatments during the summer experimental period and complete food deprivation during the winter period can be attributed to the growth effect. However, an additional approach that used the residuals of the linear regressions between the otolith metrics and FL was required to quantify the growth effects of the treatments on the otolith-to-somatic size relationship.
Under the direct proportionality assumption, somatic growth variation should be revealed in the otolith microstructure within discrete life history stages of fish (Campana and Neilson 1985). We extended upon this assumption in the present study, wherein proportionately less otolith growth should have occurred in fish from the low ration + suboptimal temperature treatments and vice versa for the fish fed the high ration and subjected to optimal temperatures. Consequently, the distribution of residuals from the otolith-to-somatic size linear regression should have been similar across treatments. The results indicated that the juvenile Chinook salmon otoliths did not reflect the true variation in somatic growth, thus we rejected the direct proportionality assumption. Mosegaard et al. (1988) demonstrated uncoupling between otolith and somatic growth in Arctic char (Salvelinus alpinus) at temperatures ranging from optimal to superoptimal. The optimum temperature for juvenile Chinook salmon growth fed at maximum ration is 19°C, above which growth rates decline (Brett et al. 1982). In the present study, the hot (21°C) water temperature treatment during summer surpassed the optimum temperature for growth even for the juvenile Chinook fed the high (exceeded satiation) ration, representing a plausible explanation for the uncoupling in the otolith size-to-FL relationship between the cool and hot temperature treatments. Food deprivation has been shown to influence the otolith-to-somatic size relationship in other fish as a result of continued otolith development during periods when somatic growth is halted (Baumann et al. 2005; Starrs et al. 2013). Although the effect of prolonged starvation on increment formation has not been examined in post-smolt juvenile Chinook salmon, Neilson and Geen (1985) showed that alevins and fry produced at least one otolith increment per day across multiple rations, indicating some level of endogenously driven daily incremental deposition of CaCO3. We found that the full linear regression between otolith size and somatic size underestimated juvenile Chinook FL in the high rations by 3.7–4.2 mm and overestimated somatic size in the low rations by 7.4–8.7 mm across the otolith metrics. In ecological terms, the average FL of juvenile Chinook fed the low ration was 98 mm at the end of the summer, so an overestimation in this range would produce considerable error when using the otolith radius at the time of capture in back-calculation techniques.
At the end of the summer experimental period, residuals analysis showed that fish subjected to the cool temperature treatments had smaller otoliths for their size than fish in the hot temperature treatments. We believe our approach to be novel because it accounted for the ontogenetic linkage in growth between the body and otolith (Secor and Dean 1989) and quantified the magnitude of error in the predicted size of the fish from the otolith to somatic size linear regression. Zhang and Beamish (2000) used the back-calculation of juvenile Chinook salmon otolith increments to estimate early marine daily somatic growth rates and identified the critical period during which growth rates needed to be maximised in order to survive the first marine winter. The results of the present study showed that juvenile Chinook salmon otoliths and bodies grew disproportionately over a range of environmental conditions, leading to erroneous estimates of otolith-predicted somatic growth. Although the percentage errors in the regression-derived somatic sizes of the fish in the present study appear modest, converting the errors in sizes to percentage daily growth rates could produce misleading estimates of growth during the critical period of early marine life. For example, Tomaro et al. (2012) showed that the average annual adult return of yearling Chinook salmon was more than three-fold greater when the back-calculated daily growth rates (% body length (BL) day–1) were above 0.5% BL day–1 during early summer than in years when early marine daily growth rates were below this threshold. In the present study, the observed daily growth rate (% FL day–1 = end FL – start FL day–1 of seasonal experiment) of fish from the cool temperature + high ration treatment combination during the summer was 0.53% FL day–1, but the growth rate produced from the OW-underestimated size of fish in this treatment combination declined below this threshold to 0.43% FL day–1. Further, the estimated daily growth rate of fish subjected to the low ration during summer doubled from 0.08 to 1.6% FL day–1 when using the OW-overestimated sizes v. the observed FL, indicating the potential for spurious interpretations of critical size and growth from subtle errors in back-calculated growth estimates.
During the autumn experimental period, the elimination of the conditions that have been shown to constrain growth (e.g. hot temperature and low ration) in juvenile Chinook salmon (Brett et al. 1982) resulted in the removal of the uncoupling effects of temperature and ration on the otolith-to-somatic size relationship observed during the previous summer (i.e. the regression residuals were similar across all temperature and ration treatments). The capacity for compensatory somatic growth was documented here and has been exhibited by juvenile Chinook salmon over a range of environmental conditions (Triebenbach et al. 2009), but the present study represented a unique example of a compensatory response in the effects of temperature and ration on the otolith growth-to-somatic growth relationship over multiple seasons.
The disparity in the residuals between the fed and unfed groups (15.5 mm) for OW during the winter was the largest difference between any treatments (temperature or ration) across all three otolith metrics and all three seasons. It was expected that somatic growth would be negligible by the end of winter because fish in the unfed treatments were completely food deprived for the entire 105-day winter experimental period. The feeding habits of juvenile ocean-type Chinook salmon during their first marine winter in the wild are unknown, but other life history types of this species, as well as other salmonids, are known to undergo and withstand extensive periods of fasting during winter (Reimers et al. 1993; Larsen et al. 2001; Jorgensen et al. 2013). Feeding frequency has been shown to affect the formation of daily rings in juvenile Chinook salmon such that fish fed multiple times produced more increments than fish fed only once per day (Neilson and Geen 1982). Interestingly, we found the effect of overwinter starvation on the otolith-to-somatic size relationship to be significant only for OW. In contrast, the regression residuals between fed and unfed fish were similar during winter for OL and OA, indicating that the continual accretion of the otoliths during the prolonged period of starvation only occurred along the OW axis. To our knowledge, this divergence in otolith development has not been observed previously and warrants further investigation.
Management implications
In all three seasonal experimental periods, OL explained more variability in the somatic size of juvenile ocean-type Chinook salmon than OW, and OW was the only otolith metric that was affected by food deprivation during the winter. Consequently, otolith-at-capture measurements along the length axis may provide more accurate estimates of back-calculated size and growth than measurements along the OW axis, particularly for fish collected from environmental conditions similar to those simulated in the present study. The results revealed a clear compensatory response in the otolith-to-somatic size relationship after conditions were changed from superoptimal water temperatures and a food-limited environment during summer to optimal water temperatures and unconstrained food rations in autumn. However, the coefficients of determination declined from summer to autumn across all otolith metrics, indicating that this relationship was not just dependent on current water temperatures and feeding conditions, but also on previous conditions. When conducting life stage-specific growth rate estimations, we suggest that fisheries managers responsible for evaluating the recruitment dynamics of juvenile Chinook salmon in the nearshore SOG include the seasonal environmental conditions that fish likely experienced before the winter and age-1 spring surveys. Finally, the effects of temperature and ration on the otolith-to-somatic size relationship during summer in natural systems similar to those simulated here could have implications for efforts to accurately estimate somatic growth from otolith growth, especially for fish encountering variable summer conditions. We have provided preliminary calculations of treatment-specific error in predicting somatic size from the otolith-to-somatic size linear regression that could be used to inform new back-calculation techniques in estimating juvenile Chinook salmon growth in the future.
Acknowledgements
This paper was presented at the 5th International Otolith Symposium in Mallorca, Spain, October 2014. Funding of the experiment was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC), the University of Victoria (UVic) and the Liber Ero Foundation. The authors are grateful to the staff of the UVic aquatic research facility for their assistance in maintaining the experimental conditions. Invaluable assistance in fish processing and otolith measurements was provided by UVic students Kelcy Tousignant and Paige Borrett.
References
Barber, M., and Jenkins, P. (2001). Differential effects of food and temperature lead to decoupling of short-term otolith and somatic growth rates in juvenile King George whiting. Journal of Fish Biology 58, 1320–1330.| Differential effects of food and temperature lead to decoupling of short-term otolith and somatic growth rates in juvenile King George whiting.Crossref | GoogleScholarGoogle Scholar |
Baumann, H., Peck, M. A., and Herrmann, J. (2005). Short-term decoupling of otolith and somatic growth induced by food level changes in postlarval Baltic sprat, Sprattus sprattus. Marine and Freshwater Research 56, 539–547.
| Short-term decoupling of otolith and somatic growth induced by food level changes in postlarval Baltic sprat, Sprattus sprattus.Crossref | GoogleScholarGoogle Scholar |
Beamish, R. J., and Mahnken, C. (2001). A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Progress in Oceanography 49, 423–437.
| A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change.Crossref | GoogleScholarGoogle Scholar |
Benkwitt, C. E., Brodeur, R. D., Hurst, T. P., and Daly, E. A. (2009). Diel feeding chronology, gastric evacuation, and daily food consumption of juvenile Chinook salmon in Oregon coastal waters. Transactions of the American Fisheries Society 138, 111–120.
| Diel feeding chronology, gastric evacuation, and daily food consumption of juvenile Chinook salmon in Oregon coastal waters.Crossref | GoogleScholarGoogle Scholar |
Brett, J. R., Clarke, W. C., and Shelbourn, J. E. (1982). Experiments on thermal requirements for growth and food conversion efficiency of juvenile Chinook salmon, Oncorhynchus tshawytscha. Canadian Technical Report of Fisheries Aquatic Sciences Number 1127, Nanaimo, BC, Canada.
Campana, S. (1990). How reliable are growth back-calculations based on otoliths? Canadian Journal of Fisheries and Aquatic Sciences 47, 2219–2227.
| How reliable are growth back-calculations based on otoliths?Crossref | GoogleScholarGoogle Scholar |
Campana, S., and Neilson, J. D. (1985). Microstructure of fish otoliths. Canadian Journal of Fisheries and Aquatic Sciences 42, 1014–1032.
| Microstructure of fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Claiborne, A. M., Miller, J. A., Weitkamp, L. A., Teel, D. J., and Emmett, R. L. (2014). Evidence for selective mortality in marine environments: the role of fish migration size, timing, and production type. Marine Ecology Progress Series 515, 187–202.
| Evidence for selective mortality in marine environments: the role of fish migration size, timing, and production type.Crossref | GoogleScholarGoogle Scholar |
El-Sabaawi, R., Dower, J. F., Kainz, M., and Mazumder, A. (2009). Interannual variability in fatty acid composition of the copepod Neocalanus plumchrus in the Strait of Georgia, British Columbia. Marine Ecology Progress Series 382, 151–161.
| Interannual variability in fatty acid composition of the copepod Neocalanus plumchrus in the Strait of Georgia, British Columbia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvFaisrc%3D&md5=37e3cfe58060d3e151642d1ae59a2de5CAS |
Francis, R. I. C. C. (1990). Back-calculation of fish length: a critical review. Journal of Fish Biology 36, 883–902.
| Back-calculation of fish length: a critical review.Crossref | GoogleScholarGoogle Scholar |
Hare, J. A., and Cowen, R. K. (1995). Effect of age, growth rate, and ontogeny on the otolith size–fish size relationship in bluefish, Pomatomus saltatrix, and the implications for back-calculation of size in fish early life history stages. Canadian Journal of Fisheries and Aquatic Sciences 52, 1909–1922.
| Effect of age, growth rate, and ontogeny on the otolith size–fish size relationship in bluefish, Pomatomus saltatrix, and the implications for back-calculation of size in fish early life history stages.Crossref | GoogleScholarGoogle Scholar |
Healey, M. C. (1983). Coastwide distribution and ocean migration patterns of stream- and ocean-type Chinook salmon, Oncorhynchus tshawytscha. Canadian Field Naturalist 97, 427–433.
Jorgensen, E. H., Martinsen, M., Strom, V., Hansen, K. E. R., Ravuri, C. S., Gong, N., and Jobling, M. (2013). Long-term fasting in the anadromous Arctic charr is associated with downregulation of metabolic enzyme activity and upregulation of leptin A1 and SOCS expression in the liver. Journal of Experimental Biology 216, 3222–3230.
| Long-term fasting in the anadromous Arctic charr is associated with downregulation of metabolic enzyme activity and upregulation of leptin A1 and SOCS expression in the liver.Crossref | GoogleScholarGoogle Scholar | 23685975PubMed |
Larsen, D. A., Beckman, B. R., and Dickhoff, W. W. (2001). The effect of low temperature and fasting during the winter on growth and smoltification of coho salmon. North American Journal of Aquaculture 63, 1–10.
| The effect of low temperature and fasting during the winter on growth and smoltification of coho salmon.Crossref | GoogleScholarGoogle Scholar |
Mackas, D. L., Batten, S., and Trudel, M. (2007). Effects on zooplankton of a warmer ocean: recent evidence from the northeast Pacific. Progress in Oceanography 75, 223–252.
| Effects on zooplankton of a warmer ocean: recent evidence from the northeast Pacific.Crossref | GoogleScholarGoogle Scholar |
Marin Jarrin, J. R. M., and Miller, J. A. (2013). Sandy beach surf zones: an alternative nursery habitat for 0-age Chinook salmon. Estuarine, Coastal and Shelf Science 135, 220–230.
| Sandy beach surf zones: an alternative nursery habitat for 0-age Chinook salmon.Crossref | GoogleScholarGoogle Scholar |
Marshall, S. L., and Parker, S. S. (1982). Pattern identification in the microstructure of sockeye salmon (Oncorhynchus nerka) otoliths. Canadian Journal of Fisheries and Aquatic Sciences 39, 542–547.
| Pattern identification in the microstructure of sockeye salmon (Oncorhynchus nerka) otoliths.Crossref | GoogleScholarGoogle Scholar |
Masson, D., and Cummins, P. F. (2007). Temperature trends and interannual variability in the Strait of Georgia, British Columbia. Continental Shelf Research 27, 634–649.
| Temperature trends and interannual variability in the Strait of Georgia, British Columbia.Crossref | GoogleScholarGoogle Scholar |
Megalofonou, P. (2006). Comparison of otolith growth and morphology with somatic growth and age in young-of-the-year bluefin tuna. Journal of Fish Biology 68, 1867–1878.
| Comparison of otolith growth and morphology with somatic growth and age in young-of-the-year bluefin tuna.Crossref | GoogleScholarGoogle Scholar |
Miller, J. A., Teel, D. J., Peterson, W. T., and Baptista, A. M. (2014). Assessing the relative importance of local and regional processes on the survival of a threatened salmon population. PLoS One 9, e99814.
| Assessing the relative importance of local and regional processes on the survival of a threatened salmon population.Crossref | GoogleScholarGoogle Scholar | 24924741PubMed |
Mosegaard, H., Svedang, H., and Taberman, K. (1988). Uncoupling of somatic and otolith growth rates in Arctic char (Salvelinus alpinus) as an effect of differences in temperature response. Canadian Journal of Fisheries and Aquatic Sciences 45, 1514–1524.
| Uncoupling of somatic and otolith growth rates in Arctic char (Salvelinus alpinus) as an effect of differences in temperature response.Crossref | GoogleScholarGoogle Scholar |
Neilson, J. D., and Geen, G. H. (1982). Otoliths of Chinook salmon (Oncorhynchus tskwytsch): daily growth increments and factors influencing their production. Canadian Journal of Fisheries and Aquatic Sciences 39, 1340–1347.
| Otoliths of Chinook salmon (Oncorhynchus tskwytsch): daily growth increments and factors influencing their production.Crossref | GoogleScholarGoogle Scholar |
Neilson, J. D., and Geen, G. H. (1985). Effects of feeding regimes and diel temperature cycles on otolith increment formation in juvenile Chinook salmon, Oncorhynchus tshawytscha. Fishery Bulletin 83, 91–101.
Otterlei, E., Folkvord, A., and Nyhammer, G. (2002). Temperature dependent otolith growth of larval and early juvenile Atlantic cod (Gadus morhua). ICES Journal of Marine Science 59, 401–410.
| Temperature dependent otolith growth of larval and early juvenile Atlantic cod (Gadus morhua).Crossref | GoogleScholarGoogle Scholar |
Pannella, G. (1971). Fish otoliths: daily growth layers and periodical patterns. Science 173, 1124–1127.
| Fish otoliths: daily growth layers and periodical patterns.Crossref | GoogleScholarGoogle Scholar |
Preikshot, D., Beamish, R. J., and Neville, C. M. (2013). A dynamic model describing ecosystem-level changes in the Strait of Georgia from 1960 to 2010. Progress in Oceanography 115, 28–40.
| A dynamic model describing ecosystem-level changes in the Strait of Georgia from 1960 to 2010.Crossref | GoogleScholarGoogle Scholar |
Quinn, T. P. (2005). ‘The Behavior and Ecology of Pacific Salmon and Trout.’ (University Press: Seattle, WA, USA.)
Reimers, A., Kjorrefjord, G., and Stavostrand, S. M. (1993). Compensatory growth and reduced maturation in second sea winter farmed Atlantic salmon following starvation in February and March. Journal of Fish Biology 43, 805–810.
| Compensatory growth and reduced maturation in second sea winter farmed Atlantic salmon following starvation in February and March.Crossref | GoogleScholarGoogle Scholar |
Reznick, D., Lindbeck, E., and Bryga, H. (1989). Slower growth results in larger otoliths: an experimental test with guppies (Poecilia reticulata). Canadian Journal of Fisheries and Aquatic Sciences 46, 108–112.
| Slower growth results in larger otoliths: an experimental test with guppies (Poecilia reticulata).Crossref | GoogleScholarGoogle Scholar |
Schabetsberger, R., Morgan, C. A., Brodeur, R. D., Potts, C. L., Peterson, W. T., and Emmett, R. L. (2003). Prey selectivity and diel feeding chronology of juvenile Chinook (Oncorhynchus tshawytscha) and coho (O. kisutch) salmon in the Columbia River plume. Fisheries Oceanography 12, 523–540.
| Prey selectivity and diel feeding chronology of juvenile Chinook (Oncorhynchus tshawytscha) and coho (O. kisutch) salmon in the Columbia River plume.Crossref | GoogleScholarGoogle Scholar |
Schweigert, J. F., Thompson, M., Fort, C., Hay, D. E., Therriault, T. W., and Brown, L. N. (2013). Factors linking Pacific herring (Clupea pallasi) productivity and the spring plankton bloom in the Strait of Georgia, British Columbia, Canada. Progress in Oceanography 115, 103–110.
| Factors linking Pacific herring (Clupea pallasi) productivity and the spring plankton bloom in the Strait of Georgia, British Columbia, Canada.Crossref | GoogleScholarGoogle Scholar |
Secor, D. H., and Dean, J. M. (1989). Somatic growth effects on the otolith–fish size relationship in young pond-reared striped bass, Morone saxatilis. Canadian Journal of Fisheries and Aquatic Sciences 46, 113–121.
| Somatic growth effects on the otolith–fish size relationship in young pond-reared striped bass, Morone saxatilis.Crossref | GoogleScholarGoogle Scholar |
Secor, D. H., and Dean, J. M. (1992). Comparison of otolith-based back-calculation methods to determine individual growth histories of larval striped bass, Morone saxatilis. Canadian Journal of Fisheries and Aquatic Sciences 49, 1439–1454.
| Comparison of otolith-based back-calculation methods to determine individual growth histories of larval striped bass, Morone saxatilis.Crossref | GoogleScholarGoogle Scholar |
Sogard, S. M. (1991). Interpretation of otolith microstructure in juvenile winter flounder (Pseudopleuronectes americanus): ontogenetic development, daily increment validation, and somatic growth relationships. Canadian Journal of Fisheries and Aquatic Sciences 48, 1862–1871.
| Interpretation of otolith microstructure in juvenile winter flounder (Pseudopleuronectes americanus): ontogenetic development, daily increment validation, and somatic growth relationships.Crossref | GoogleScholarGoogle Scholar |
Starrs, D., Ebner, B. C., and Fulton, C. J. (2013). Can backcalculation models unravel complex larval growth histories in a tropical freshwater fish? Journal of Fish Biology 83, 96–110.
| Can backcalculation models unravel complex larval growth histories in a tropical freshwater fish?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sjmsVWqug%3D%3D&md5=54f9de95884bcf460988d267458d4286CAS | 23808694PubMed |
Takasuka, A., Oozeki, Y., Aoki, I., Kimura, R., Kubota, H., Sugisaki, H., and Akamine, T. (2008). Growth effect on the otolith and somatic size relationship in Japanese anchovy and sardine larvae. Fisheries Science 74, 308–313.
| Growth effect on the otolith and somatic size relationship in Japanese anchovy and sardine larvae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltFSku7s%3D&md5=041172745ed29e56116bb49724d4969eCAS |
Tanaka, K., Mugiya, Y., and Yamada, J. (1981). Effects of photoperiod and feeding on daily growth patterns in otoliths of juvenile Tilapia nilotica. Fishery Bulletin 79, 459–466.
Tomaro, L. M., Teel, D. J., Peterson, W. T., and Miller, J. A. (2012). When is bigger better? Early marine residence of middle and upper Columbia River spring Chinook salmon. Marine Ecology Progress Series 452, 237–252.
| When is bigger better? Early marine residence of middle and upper Columbia River spring Chinook salmon.Crossref | GoogleScholarGoogle Scholar |
Tompkins, A., Brown, G., and Thiess, M. (2011). Temporal patterns in productivity of North American sockeye and Chinook salmon. NPAFC Doc. 1356, Fisheries and Oceans Canada.
Triebenbach, S. B., Smoker, W. W., Beckman, B. R., and Focht, R. (2009). Compensatory growth after winter food deprivation in hatchery-produced coho salmon and Chinook salmon smolts. North American Journal of Aquaculture 71, 384–399.
| Compensatory growth after winter food deprivation in hatchery-produced coho salmon and Chinook salmon smolts.Crossref | GoogleScholarGoogle Scholar |
Waessle, J. A., Lasta, C. A., and Favero, M. (2003). Otolith morphology and body size relationships for juvenile Sciaenidae in the Rio de la Plata Estuary (35–36°S). Scientia Marina 67, 233–240.
Wilson, J. A., Vigliola, L., and Meekan, M. G. (2009). The back-calculation of size and growth from otoliths: validation and comparison of models at an individual level. Journal of Experimental Marine Biology and Ecology 368, 9–21.
| The back-calculation of size and growth from otoliths: validation and comparison of models at an individual level.Crossref | GoogleScholarGoogle Scholar |
Wright, P. J., Metcalfe, N. B., and Thorpe, J. E. (1990). Otolith and somatic growth rates in Atlantic salmon parr, Salmo salar L: evidence against coupling. Journal of Fish Biology 36, 241–249.
| Otolith and somatic growth rates in Atlantic salmon parr, Salmo salar L: evidence against coupling.Crossref | GoogleScholarGoogle Scholar |
Zhang, Z., and Beamish, R. J. (2000). Use of otolith microstructure to study life history of juvenile Chinook salmon in the Strait of Georgia in 1995 and 1996. Fisheries Research 46, 239–250.
| Use of otolith microstructure to study life history of juvenile Chinook salmon in the Strait of Georgia in 1995 and 1996.Crossref | GoogleScholarGoogle Scholar |