Otoliths as individual indicators: a reappraisal of the link between fish physiology and otolith characteristics
Peter GrønkjærDepartment of Bioscience, Aquatic Biology, Aarhus University, Ole Worms Allé 1, DK-8000 Aarhus C, Denmark. Email: peter.groenkjaer@biology.au.dk
Marine and Freshwater Research 67(7) 881-888 https://doi.org/10.1071/MF15155
Submitted: 16 April 2015 Accepted: 23 December 2015 Published: 18 March 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Otoliths are remarkable recorders that store visual and chemical information that can be interpreted with regard to individual fish phenotype trajectory, life history events and environment. However, the information stored in the otoliths must be interpreted with the knowledge that the otolith is an integral part of fish sensory systems. This means that the environmental signals recorded in the otoliths will be regulated by the homeostatic apparatus of the individual fish – its physiology and ultimately its genetic make-up. Although this may complicate interpretation of environmental signals, it also opens up avenues for new research into the physiology and life history of individual fish. This review focuses on research areas where the coupling between otolith characteristics and fish physiology may yield new insights. Most of the research ideas are by no means new, but rather represent largely forgotten or less-explored research areas. Examples of questions that are fundamental, unanswered and with the potential to yield significant new insights are those related to the coupling of otolith and fish growth through metabolism, and the formation of opaque and translucent growth zones in relation to the physiology of the individual. An integration of visual and chemical data with bioenergetic modelling may yield some of the answers.
Additional keywords: environment, genotype, metabolism, opaque and translucent zones, stable isotopes, viability.
Introduction
Otoliths are remarkable recorders of fish life histories and the environment, and the flight recorder analogy is well deserved. Otolith research based on the premise that otoliths are unbiased recorders of age, growth and other life history events has significantly advanced ecological and environmental research and is a key pillar of fisheries management. However, there is one important difference to the flight recorder: many of the signals recorded in the otolith are influenced by the state of the individual fish – its physiology and ultimately its genetic make-up. This means that the same environmental signal may be recorded differently in the otoliths of two individual fish, but also that otolith characteristics may reveal insights into the physiology of the individual and the ecological effects of pheno- and genotypic variation among individuals.
The present treatise is not in any way a comprehensive overview of the multitude of ways in which otoliths may be used as individual indicators, but rather a look at the forgotten, the less-explored or the completely new ways in which otoliths may contribute to knowledge on how individual fish thrive and survive in a changing environment.
Otolith growth
The main assumption behind back-calculation of fish lengths using otoliths, namely the tight coupling and resulting proportionality between fish growth and otolith growth, was challenged in the late 1980s by a series of studies showing decoupling between fish and otolith growth when fish were exposed to varying temperatures or food regimens (Mosegaard et al. 1988; Reznick et al. 1989). Studies examining the relationship between otolith increment widths and metabolic rates eventually led to the conclusion that the proportionality observed in most studies was due to a common dependence on metabolic processes (Wright et al. 1991; Yamamoto et al. 1998; Armstrong et al. 2004; Bang and Grønkjær 2005). In short, processes related to the metabolism of fish govern both fish and otolith growth and give rise to the observed proportionality of otolith and fish size.
It remains an open question as to how the different components of fish metabolism influence otolith growth. Early studies found a correlation between otolith growth and temperature (Mosegaard et al. 1988), which indicated a relationship between fish metabolism and otolith growth. This idea was substantiated by studies finding a correlation between standard or resting metabolic rate and otolith growth (Wright 1991; Yamamoto et al. 1998; Neat et al. 2008). However, Wright et al. (2001) did not find a simple proportionality between resting metabolic rate and otolith growth, suggesting that additional factors are important. Moreover, this mechanism does not link changes in fish growth driven by variation in ingestion to otolith growth, because standard metabolic rate (SMR) should be unaffected by ingestion rate. This link was provided by studies showing that the specific dynamic action (SDA; the postprandial increase in metabolism) correlates with otolith growth (Armstrong et al. 2004). Other studies again document otolith growth even after longer periods of starvation, suggesting that a simple relationship between the magnitude and duration of SDA and otolith growth is not a complete explanation. A model including the effect of both SMR and SDA may explain both continued otolith growth during starvation and the effect of increased ingestion on otolith growth. Alternatively, an apparent reconciliation of the two lines of evidence can be found in a recent study (Van Leeuwen et al. 2012) suggesting that the quantity of food consumed may affect SMR independent of specific dynamic action. Despite the uncertainty regarding the mechanisms, the hypothesis that otolith growth reflects metabolism has fuelled a very successful line of ecological research into the early life stages of fish.
The possibility of using otolith growth and size as an indicator of physiological performance was introduced by Mosegaard (1990) and further developed by, among others, Titus and Mosegaard (1991), Metcalfe et al. (1992) and Yamamoto et al. (1998). These works linked life history traits such as growth potential and dominance status in salmonids to the size of their otoliths at emergence. It was argued that this was rooted in inherent differences in the metabolic rates that determined both otolith growth and competitive abilities (Metcalfe 1998; Millidine et al. 2009) and the potential of otoliths to reveal physiological processes and track the fate of individuals with specific physiological traits was convincingly demonstrated.
In the marine environment, similar insights were gained from studies of Atlantic cod larvae and juveniles. Meekan and Fortier (1996) showed evidence for superior survival of fast-growing cod larvae and juveniles on the Scotian shelf, and further showed that these fast-growing individuals could be identified by the larger size of their otoliths at hatch. Even though significantly larger otoliths at hatch were only found in a year with strong size-selective survival, the conclusion was that traits enabling individual cod to grow fast were present at hatch. In the brackish Baltic Sea, the first-feeding cod larvae have to migrate from a hatching depth at 50–60 m through the pycnocline and into the low saline (~7‰) surface water to feed (Grønkjær and Wieland 1997). Grønkjær and Schytte (1999) showed that the more viable larvae that completed this migration and maintained themselves in the surface waters were larvae with larger than average otoliths sizes at hatch. Moreover, there was a significant correlation between the hatch check size and protein growth rates as estimated from RNA : DNA ratios and ambient temperatures, suggesting that the protein synthesising capacity was higher in individuals with larger otoliths at hatch.
It is clear from the above studies that physiological determinants of life histories may be imprinted in otoliths from birth or reflected in otolith characteristics at later stages; however, the ecological effects of the physiological traits reflected in otolith size at these life history transition points will be strongly context dependent. This is evident from a series of studies with recruiting coral reef fish (Gagliano et al. 2007; McCormick and Meekan 2010), where selection on otolith traits related to fish metabolism, growth and behaviour change direction during early ontogeny. For example, Gagliano et al. (2007) found that the shape of fitness curves relating relative survival to otolith growth rate differed significantly between three different time intervals from hatching to 3 weeks after settlement. Survival from hatching to settlement showed a negative relationship with otolith growth, whereas growth during the first 2 weeks after settlement was positively related to survival. During the final period, survival was again negatively related to otolith growth. The change in the direction of selective pressure may be explained by ontogenetic changes in feeding intensity and behaviour that determine both growth rates and predation risk (McCormick and Meekan 2010), so that high feeding intensity, and associated growth, may be correlated with increased risk of mortality during specific periods in the ontogeny or in specific habitats.
In addition, prey availability may significantly affect the fitness related to a trait such as metabolism. The capacity to process food and grow fast, or maintain a dominant social status endowed by a high SMR (Millidine et al. 2009), may be a disadvantage during food shortage (Metcalfe 1986; Burton et al. 2011), when these individuals are likely to lose energy reserves faster than low-SMR individuals. Especially for larval fish with high weight-specific metabolic rates and low energy reserves, the interaction between metabolism and environmental conditions could be an important factor determining survival. This was demonstrated by Bochdansky et al. (2005), who showed that longevity of larval radiated shanny (Ulvaria subbifurcata) was negatively correlated with otolith size at hatch when food density was lower than the density needed for maximum food intake. Moreover, the slope of the relationship between longevity and otolith hatch check size became increasingly more negative with decreasing food density.
The above studies are good examples of responses that are likely to reflect differences in genotypes. Ultimately, the physiological response, and hence the observed otolith characteristics, of individual fish to environmental factors such as feeding levels will be modulated by the genotype of that individual (De-Santis and Jerry 2007; Martens et al. 2014). This is the fact that underlies the enormous success of aquaculture breeding programs, where large increases in growth rates and growth efficiencies have been achieved by selecting the optimal genotypes. Consequently, the importance of the genotype with regard to shaping both the physiological and the otolith response to natural environmental variability should not be underestimated (Nielsen et al. 2009). Recently, the implicit assumption of many otolith shape studies, namely a population-specific genetic component to otolith shape, has been substantiated (Vignon and Morat 2010; Annabi et al. 2013), adding further to the link between genetic make-up and otolith characteristics of populations and individuals.
Research efforts directed towards documenting the relationship between otolith growth and fish physiology have declined since the surge of papers in the 1980s and 1990s. In the meantime, new molecular technologies (Narum et al. 2013) and increased research into the ecophysiology of fish, their individual adaptations and responses to changing environmental conditions (Jørgensen et al. 2012) has brought about new insights that beg revisiting of the question: what is reflected in otolith growth? An improved understanding of this is the key to progress otolith-based research and answer fundamental questions related to the performance and viability of fish in a changing environment. The unique otolith traits are still the best tools to track individual life histories through time in nature, and link growth and survival to environmental conditions. However, they also allow us to also take one step further and examine environmental effects on individual fish with different capabilities endowed by their genetic make-up and parental contributions. Combining the power of population genomics to identify genotypes, candidate genes and their expression with the possibility to back-track the life history of the individual through its lifetime will be a big step towards understanding and solving fundamental questions related to fish recruitment and population dynamics in the face of environmental change.
Otolith opacity
A fundamental characteristic of otoliths is the regular change in otolith opacity recognised as daily and annual increments, as well as checks laid down during fish life history transitions and in response to rapidly changing environmental conditions. The classic ecophysiological interpretation of the annual otolith zones is that the opaque zones represent fast ‘summer growth’ fuelled by high temperatures and food intake, whereas the translucent zone is laid down in winter in response to decreased feeding and low temperatures (Beckman and Wilson 1995).
Two lines of research have dominated the research into otolith increment formation, one driven by studies of the chemical properties of the endolymph, its relationship with the crystallisation of aragonite and dependence on exogenous factors (Morales-Nin 2000; Guibbolini et al. 2006), which have shown that changes in opacity represent variations in the relative fractions of mineral and organic matter in the otolith zones (Mugiya 1984; Morales-Nin 2000; Hüssy et al. 2004), and the other focussed on bioenergetic modelling of the link between otolith growth, opacity and the metabolism of the individual (Hüssy and Mosegaard 2004; Fablet et al. 2011; Pecquerie et al. 2012). In addition to these approaches, numerous field and laboratory experiments have investigated the width and frequency of increment formation under different conditions and provided empirical data for the modelling. Despite the considerable research effort laid into resolving what exogenous or endogenous drivers causes the change from opaque to translucent zones, there is still no consensus.
One prominent feature that reflects the apparent complexity of increment formation is their universal occurrence. Annual increments are found in species living at depths where light and temperature are unlikely to function as zeitgebers for an endogenous circadian rhythm (Stewart et al. 1995; Allain and Lorance 2000; Morales-Nin and Panfili 2005) and daily increment formation has been observed in laboratory experiments without cyclical variation in feeding, light and temperature (Wright et al. 1991). Neat et al. (2008) summarised the processes proposed to be responsible for the formation of otolith zones as follows: (1) physiological changes related to growth and reproduction; (2) environmental change that acts independently of somatic and reproductive processes; and (3) an endogenous biological rhythm. These processes are not mutually exclusive.
There is no doubt that temperature overall plays an important role with regard to the cyclic pattern of otolith opacity and several studies have explored the role of seasonal temperature variations in generating translucent and opaque growth zones (Ewing et al. 2003; Newman and Dunk 2003; Pilling et al. 2007; Borthagaray et al. 2011; Gjøsæter and Danielssen 2011). This has primarily been through studies of seasonality of marginal increment opacity from which temperature and seasonal effects have been inferred. These studies are often confounded by the fact that the temperature experienced by the individual fish is unknown and that seasonal phenomena, such as spawning, starvation and migrations, are difficult to disentangle from pure temperature effects. Studies that have directly manipulated temperatures or measured the temperature experienced by the fish during the formation of otolith growth zones are few, but highlight changes in temperature as a major driver of otolith opacity variations. Neat et al. (2008) found that translucent zones were formed at temperatures above 9°C in 1- to 2-year-old laboratory-reared Atlantic cod, whereas at lower temperatures opaque zones were formed. Opacity was inversely related to otolith increment width, but not related to somatic growth of the fish, suggesting an effect that is unrelated to growth. Translucent zone formation at temperatures higher than the optimal range for growth was found by Høie and Folkvord (2006), who used otolith stable oxygen isotopes to deduce the temperatures experienced by pen-reared Atlantic cod. Similar conclusions were reached by Millner et al. (2011), who found that over a period of 20 years the annual timing of translucent zone formation was related to temperature, such that in warm years the formation started earlier than in cold years.
So, although opaque summer growth zones and translucent winter growth may reflect the situation in a range of temperate populations, this pattern is not universal. Moreover, the seasonality of zone formation may vary across the distribution range of a species and with age within a population. A very good example of this, which also serves as a basis for exploring the physiological background for zone formation, is the study of Høie et al. (2009), in which zone formation was compared between southern North Sea (approximately 52°N, 3°E) and Barents Sea (72°N, 25°E) cod. In the southern North Sea, the translucent zone forms during July–November, the warmest period of the year, whereas the translucent zone is formed in December–April in the Barents Sea. The southern North Sea cod experience temperatures close to the upper thermal limit of cod during the period July–October (Righton et al. 2010) and, despite population-specific adaptation to these temperatures, there were indications of reduced growth at temperatures above 16°C. These temperatures temporally coincide with the formation of translucent zones. Because the translucent zone is formed at much lower temperatures in other more northerly cod populations compared to southern populations, it is not clear to what extent temperatures per se (i.e. solely through the kinetic effects on mineralisation and synthesis of otolith protein) control the formation of the different zones. Temperature signals are likely modulated by population-specific adaptations and the physiology of the individual (Hüssy et al. 2009). However, this should be investigated further by subjecting cod from different populations to a range of temperatures in a common garden set-up. This will allow for a test of whether translucent zone formation is initiated when temperatures reach beyond a population-specific optimal range. A further elaboration of this study would be to manipulate food levels to also address the more complex hypothesis that translucent zone formation is initiated when the individual experiences energetic stress, which could be, but is not necessarily, reflected in reduced growth. Moreover, translucent zones deposited during warm and cold periods may be the result of different processes. In white anglerfish (Lophius piscatorius) two types of translucent zones were found, a wide and a narrow zone, which, on the microstructural level, were preceded by a gradually and an abrupt decreasing increment width respectively (Wright et al. 2002). It was suggested that the narrow translucent zones are laid down in response to short-term poor feeding conditions that do not immediately affect otolith growth but change the ratio between the organic and mineral fractions, and hence opacity, of the otolith.
A very simple conceptual model relating opacity to fish energetic status is based on the work by (Jobling 1997; Fig. 1a). Here, the scope for growth (a measure of surplus energy beyond that needed for maintenance) was shown as the difference between energy acquisition (ingestion) and metabolism over the temperature range experienced by the fish. The surplus energy peaks at intermediate temperatures and decreases towards the upper and lower thermal limits of the fish. Assuming that translucent zones are formed when the surplus energy is lower than a certain threshold (e.g. Pilling et al. 2007), this model could explain the formation of translucent zones at high as well as low temperatures (Fig. 1b) and serve as a starting point for exploring population and individual differences in the timing of zone formation. If food availability is limited, this would reduce the range of temperatures at which surplus energy is above the threshold (Fig. 1b; Høie et al. 2008). This will be most marked at high temperatures, where energy needs for maintenance are highest. We could also imagine two populations with different adaptations to temperature. The curve showing surplus energy of the cold-adapted population would likely be shifted towards lower temperatures, meaning that they would be above the threshold at lower temperatures than a warm-adapted population (Fig. 1c).
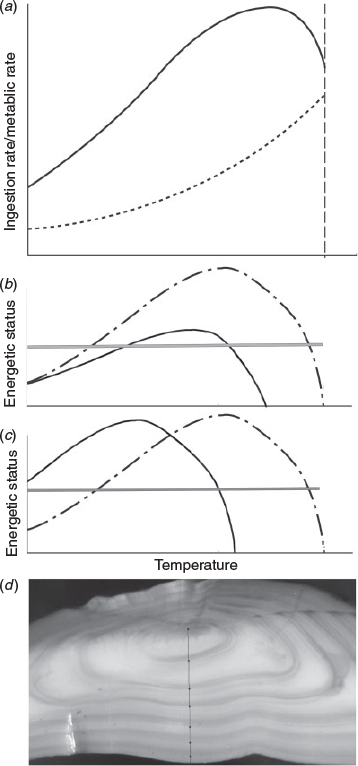
|
The differences may not only be found among different populations, but also between genotypes within a population. A classic example is the different haemoglobin genotypes in Atlantic cod (Ross et al. 2013). Temperature preference has been shown to vary between haemoglobin genotypes and relate to differences in oxygen affinity between genotypes. In cod from Kattegat (Denmark), the temperature preference of the cold-adapted genotype (HbI-2/2) was 8.2°C ± 1.5 s.d., whereas the warm adapted (HbI-1/1) preferred temperatures as high as 15.4°C ± 1.1 during normoxia (Petersen and Steffensen 2003). Hence, there could be plenty of variability in otolith zone formation even among fish from a single population living under similar conditions because each fish may respond a little differently to the conditions based on energetic status, physiology and genotype.
A promising approach to further explore the links between environment, physiology and otolith characteristics is the use of dynamic energy budget (DEB) models to simulate otolith growth and opacity (Fablet et al. 2011; Pecquerie et al. 2012). Within this framework the mineral and organic fractions of the otolith are regarded as individual metabolic products involving contributions from somatic growth and maintenance DEB fluxes and so, to some extent, combine hypotheses of standard metabolism v. SDA as drivers of otolith growth. The change in opacity is due to variations in the ratio between the mineral and organic fractions. Moreover, otolith growth and opacity can be regarded as functions of the state of the individual (reserves and size) and of its environment (temperature and food availability). So far, this approach has been successful in simulating otolith growth and opacity patterns for larval and adult fish. In particular, the inverse opacity patterns exhibited by Barents Sea (translucent zones during cold months) and southern North Sea (translucent zones during warm months) cod has been reconstructed, which shows that the techniques holds promise with regard to being able to explore not only population-specific responses, but potentially also individual or genotype responses (Fablet et al. 2011). The models can also be run ‘backwards’ using ambient temperature and observed otolith opacity patterns to reconstruct individual growth and food intake (Pecquerie et al. 2012).
We still lack a thorough understanding of what triggers daily and annual growth zones to be formed despite the fact that analysis and interpretation of these zones is the most widespread use of otoliths and a cornerstone of fisheries research and management. The complexity in individual responses to environmental drivers suggests that the key to this insight lies in understanding the coupling between fish physiology and otolith formation.
Otolith chemistry
So far this review has only considered the visual properties of otoliths, but the chemical composition of otoliths may also be significantly related to individual physiological factors and not only to the environmental signals (e.g. temperature, salinity, food) they are thought to reflect (Radtke and Shafer 1992; Walther et al. 2010; Barnes and Gillanders 2013; Sturrock et al. 2014).
Despite the concerns regarding intrinsic factors and their influence, otolith microchemistry has significantly advanced our understanding of fish habitat use, migrations and population connectivity (Campana 1999). This development has to a large extent been driven by research into estuarine or diadromous fishes that inhabit or migrate across large gradients in water chemistry, and it has been backed up by laboratory studies that, in general, have supported a predictable relationship between ambient environmental conditions and the chemical signal recorded in the otolith (Secor and Rooker 2000; Gillanders 2005). Typically, the laboratory validation studies have been conducted with fast-growing juvenile fish exposed to a very wide range of temperatures and salinities (Martin and Wuenschel 2006; Panfili et al. 2015). However, this use of extreme ranges of environmental conditions and juvenile fish may have masked the effect of non-environmental factors that influence the uptake and incorporation of specific elements in the otolith (Walther et al. 2010; Sturrock et al. 2012, 2014).
Kalish (1991b) was among the first to argue that physiological, in addition to environmental, factors influence the chemical composition of the otolith. He suggested that temperature does not directly influence the incorporation of elements such as Sr, Na, K and S into the otolith. Instead, he noted that changes in blood plasma composition, in particular the composition and concentration of ion-binding proteins, during gonad development appeared to be important and that the apparent relationship between temperature and otolith elemental composition was due to the effect of temperature on gonad development.
Strontium has been particularly important in the ecological application of otolith microchemistry. Numerous studies have used Sr to track migrations of fish and reconstruct birth places and nurseries (for a review, see Secor and Rooker 2000). Owing to the successful use of elements such as strontium in freshwater and estuarine settings, they have been applied to marine environments and species, where the results have been ambiguous or contradictory. In a thorough treatise, Brown and Severin (2009) ended up concluding that the water Sr : Ca ratio is the primary factor affecting otolith Sr : Ca for freshwater and estuarine fish, but not marine fish. Even though lifetime Sr : Ca profiles of the marine fish analysed varied as much as those of estuarine fish, there was no apparent relationship with water chemistry, suggesting that other factors are able to introduce significant variability in Sr : Ca.
This conclusion has recently been explored further in an elaborate rearing experiment that allowed Sturrock et al. (2014, 2015) to test of the effect of physiological factors on the otolith chemistry of adult marine fish. Blood samples, physiological data and water chemistry were sampled over the course of a year and related to temporally matched profiles of otolith element : calcium ratios. Physiological influences were especially strong for thiophilic elements such a Mn, Cu, Zn, Se and Pb, as well as Sr and Ca, and were stronger than seasonal variations in water chemistry. Significant relationships were observed between element : calcium ratios and physiological factors such as length, sex, growth rate, condition and spawning. In Sturrock et al. (2014), as in other studies (Secor and Rooker 2000; Stanley et al. 2015), growth rate appeared to be prominent among the physiological factors that showed a relationship with otolith elemental composition. Secor and Rooker (2000) reviewed the literature on strontium and found that growth was commonly identified as a significant influence on otolith Sr : Ca. This has recently been confirmed by Stanley et al. (2015), who also noted a relationship between Mn : Ca and growth. Growth is a complex physiological phenomenon involving transformation of food into tissue and the transport of proteins, amino acids, lipids and other building blocks in the blood. Considering the increase in the concentration of these substances in the blood during growth, and that many of these circulating substances are able to bind some of the most commonly investigated elements, it is likely that the growth process is associated with a change the availability of these elements. The finding that the examined element : calcium ratios are related to different sets of physiological variables possibly reflects different roles of the elements in fish physiology and homeostasis, as well as their chemical properties and affinities for binding to plasma proteins. Despite the few studies that have investigated physiological effects on elemental composition in the blood, it seems that plasma protein concentrations are key to understanding ion availability in the endolymph and constitute the link between fish physiology and otolith chemistry.
Recently, studies have added a new level of complexity to the interpretation of otolith element composition in an ecological context by pointing to genetics and population-specific responses to changes in environmental conditions (Clarke et al. 2011; Barnes and Gillanders 2013; Chang and Geffen 2013). This is fully in line with the growing evidence of physiological effects on otolith microchemistry.
The above studies clearly document the importance of physiological factors in controlling the elemental composition of fish otoliths. Although this may complicate research that uses otolith chemistry to study habitat associations, migration patterns and stock structure (Chang and Geffen 2013), it also opens up avenues for exciting new research into otolith chemical markers for important life history events, such as metamorphosis, maturity and spawning.
Although the focus on the physiological effect with regard to the incorporation of elements in the otoliths is new, the link between the otolith isotopic ratios of, for example, carbon and the physiology of the fish has been investigated since the late 1980s (Radtke et al. 1987; Kalish 1991a).
The carbon isotopic composition of the otolith aragonite is given by the following relationship (Jamieson et al. 2004):
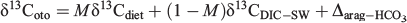
where δ13Cdiet is the average δ13C value of the diet, δ13CDIC–SW is the δ13C value of dissolved inorganic carbon (DIC) in seawater, Δarag–HCO3 is the isotopic fractionation between aragonite and bicarbonate (~2.7‰; Romanek et al. 1992) and M is the fraction of DIC in plasma derived from metabolism and, as such, a relative measure of size-specific metabolism. This means that the otolith contains an isotopic signature that may be used to infer time-resolved metabolic rates under natural conditions assuming that the other factors can be measured with sufficient precision. Although δ13Coto is routinely measured with great precision and δ13CDIC–SW can be measured directly or extracted from isoscapes (McMahon et al. 2013), the problem has been to estimate the diet isotopic composition. Progress in the analysis of carbon isotopic signatures from the otolith organic matrix, a proxy for δ13Cdiet, brings confidence that M can now be estimated with reasonable certainty (McMahon et al. 2011; Grønkjær et al. 2013).
The possibility of estimating field metabolic rates using otolith carbon isotopes will add a powerful tool to the otolith toolbox. It will help solve the fundamental questions related to what determines otolith growth and the formation of annual growth zones, but, even more importantly, it can be put to use in research directed towards understanding the effect of climate variability on the physiology, behaviour and productivity of fish populations and individuals.
Conclusion
The variability in life history traits among individuals and the importance of this variability in a fluctuating and changing environment has recently gained a lot of interest in ecology and physiology, but has not received the same attention in fish ecology and otolith research. This is unfortunate because fish are perfect model organisms to study impact of, for example, environmental change and otoliths are true individual indicators that, given proper analysis, yield unique time-resolved information on the developing phenotype, its physiology and the environmental conditions that the individual has experienced. The recent methodological developments and insights into the coupling between otolith growth, zone formation, chemical composition and the physiology of the individual hint that the otolith flight recorder still has some secrets to reveal.
Acknowledgements
The author thanks Beatriz Morales-Nin and Audrey Geffen, the conveners of the 5th International Otolith Symposium held during 20–24 October 2014 in Mallorca, Balearic Islands, Spain, for inviting me to present these ideas at the symposium.
References
Allain, V., and Lorance, P. (2000). Age estimation and growth of some deep-sea fish from the northeast Atlantic ocean. Cybium 24, 7–16.Annabi, A., Said, K., and Reichenbacher, B. (2013). Inter-population differences in otolith morphology are genetically encoded in the killifish Aphanius fasciatus (Cyprinodontiformes). Scientia Marina 77, 269–279.
| Inter-population differences in otolith morphology are genetically encoded in the killifish Aphanius fasciatus (Cyprinodontiformes).Crossref | GoogleScholarGoogle Scholar |
Armstrong, J. D., Fallon-Cousins, P. S., and Wright, P. J. (2004). The relationship between specific dynamic action and otolith growth in pike. Journal of Fish Biology 64, 739–749.
| The relationship between specific dynamic action and otolith growth in pike.Crossref | GoogleScholarGoogle Scholar |
Bang, A., and Grønkjær, P. (2005). Otolith size-at-hatch reveals embryonic oxygen consumption in the zebrafish, Danio rerio. Marine Biology 147, 1419–1423.
| Otolith size-at-hatch reveals embryonic oxygen consumption in the zebrafish, Danio rerio.Crossref | GoogleScholarGoogle Scholar |
Barnes, T. C., and Gillanders, B. M. (2013). Combined effects of extrinsic and intrinsic factors on otolith chemistry: implications for environmental reconstructions. Canadian Journal of Fisheries and Aquatic Sciences 70, 1159–1166.
| Combined effects of extrinsic and intrinsic factors on otolith chemistry: implications for environmental reconstructions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFCisb3J&md5=acf318b02a121fb570d2d192d002121cCAS |
Beckman, D., and Wilson, C. A. (1995). Seasonal timing of opaque zone formation in fish otoliths. In ‘Recent Developments in Fish Otolith Research’, 23–27 January 1993, Hilton Head, SC, USA. (Eds D. H. Secor, J. M. Dean, and S. E. Campana.) pp. 27–43. (University of South Carolina Press: Columbia, SC, USA.)
Bochdansky, A. B., Grønkjær, P., Herra, T. P., and Leggett, W. C. (2005). Experimental evidence for selection against fish larvae with high metabolic rates in a food limited environment. Marine Biology 147, 1413–1417.
| Experimental evidence for selection against fish larvae with high metabolic rates in a food limited environment.Crossref | GoogleScholarGoogle Scholar |
Borthagaray, A. I., Verocai, J., and Norbis, W. (2011). Age validation and growth of Micropogonias furnieri (Pisces – Sciaenidae) in a temporally open coastal lagoon (South-western Atlantic – Rocha – Uruguay) based on otolith analysis. Journal of Applied Ichthyology 27, 1212–1217.
| Age validation and growth of Micropogonias furnieri (Pisces – Sciaenidae) in a temporally open coastal lagoon (South-western Atlantic – Rocha – Uruguay) based on otolith analysis.Crossref | GoogleScholarGoogle Scholar |
Brown, R. J., and Severin, K. P. (2009). Otolith chemistry analyses indicate that water Sr : Ca is the primary factor influencing otolith Sr : Ca for freshwater and diadromous fish but not for marine fish. Canadian Journal of Fisheries and Aquatic Sciences 66, 1790–1808.
| Otolith chemistry analyses indicate that water Sr : Ca is the primary factor influencing otolith Sr : Ca for freshwater and diadromous fish but not for marine fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlWktrbN&md5=87d81843029be8f04b77afa4c0c13805CAS |
Burton, T., Killen, S. S., Armstrong, J. D., and Metcalfe, N. B. (2011). What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proceedings of the Royal Society – B. Biological Sciences 278, 3465–3473.
| What causes intraspecific variation in resting metabolic rate and what are its ecological consequences?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbkt1Wgtg%3D%3D&md5=786107e9eb7b1b49a48e5be68349a1c7CAS | 21957133PubMed |
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188, 263–297.
| Chemistry and composition of fish otoliths: pathways, mechanisms and applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtFKmtA%3D%3D&md5=1c1a0b19f44f50ec78fd6669fd952b22CAS |
Chang, M. Y., and Geffen, A. J. (2013). Taxonomic and geographic influences on fish otolith microchemistry. Fish and Fisheries 14, 458–492.
| Taxonomic and geographic influences on fish otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Clarke, L. M., Thorrold, S. R., and Conover, D. O. (2011). Population differences in otolith chemistry have a genetic basis in Menidia menidia. Canadian Journal of Fisheries and Aquatic Sciences 68, 105–114.
| Population differences in otolith chemistry have a genetic basis in Menidia menidia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtFKitA%3D%3D&md5=0bcd671d926fee413536ad95b5b62ec2CAS |
De-Santis, C., and Jerry, D. R. (2007). Candidate growth genes in finfish: where should we be looking? Aquaculture 272, 22–38.
| Candidate growth genes in finfish: where should we be looking?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1yku7%2FF&md5=466badb060907d75ca942670b08e4580CAS |
Ewing, G. P., Welsford, D. C., Jordan, A. R., and Buxton, C. (2003). Validation of age and growth estimates using thin otolith sections from the purple wrasse, Notolabrus fucicola. Marine and Freshwater Research 54, 985–993.
| Validation of age and growth estimates using thin otolith sections from the purple wrasse, Notolabrus fucicola.Crossref | GoogleScholarGoogle Scholar |
Fablet, R., Pecquerie, L., de Pontual, H., Hoie, H., Millner, R., Mosegaard, H., and Kooijman, S. A. L. M. (2011). Shedding light on fish otolith biomineralization using a bioenergetic approach. PLoS One 6, e27055.
| Shedding light on fish otolith biomineralization using a bioenergetic approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFyntbvP&md5=3a4e29841c9e26e81d5b74e3158e8654CAS | 22110601PubMed |
Gagliano, M., McCormick, M. I., and Meekan, M. G. (2007). Survival against the odds: ontogenetic changes in selective pressure mediate growth-mortality trade-offs in a marine fish. Proceedings. Biological Sciences 274, 1575–1582.
| Survival against the odds: ontogenetic changes in selective pressure mediate growth-mortality trade-offs in a marine fish.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M. (2005). Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats. Estuarine, Coastal and Shelf Science 64, 47–57.
| Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats.Crossref | GoogleScholarGoogle Scholar |
Gjøsæter, J., and Danielssen, D. S. (2011). Age, growth and otolith annulus formation of cod (Gadus morhua) in the Risor area on the Norwegian Skagerrak coast during 1986–1996. Marine Biology Research 7, 281–288.
| Age, growth and otolith annulus formation of cod (Gadus morhua) in the Risor area on the Norwegian Skagerrak coast during 1986–1996.Crossref | GoogleScholarGoogle Scholar |
Grønkjær, P., and Schytte, M. (1999). Non-random mortality of Baltic cod larvae inferred from otolith hatch-check sizes. Marine Ecology Progress Series 181, 53–59.
| Non-random mortality of Baltic cod larvae inferred from otolith hatch-check sizes.Crossref | GoogleScholarGoogle Scholar |
Grønkjær, P., and Wieland, K. (1997). Ontogenetic and environmental effects on vertical distribution of cod larvae in the Bornholm basin, Baltic sea. Marine Ecology Progress Series 154, 91–105.
| Ontogenetic and environmental effects on vertical distribution of cod larvae in the Bornholm basin, Baltic sea.Crossref | GoogleScholarGoogle Scholar |
Grønkjær, P., Pedersen, J. B., Ankjaero, T. T., Kjeldsen, H., Heinemeier, J., Steingrund, P., Nielsen, J. M., and Christensen, J. T. (2013). Stable N and C isotopes in the organic matrix of fish otoliths: validation of a new approach for studying spatial and temporal changes in the trophic structure of aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences 70, 143–146.
| Stable N and C isotopes in the organic matrix of fish otoliths: validation of a new approach for studying spatial and temporal changes in the trophic structure of aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar |
Guibbolini, M., Borelli, G., Mayer-Gostan, N., Priouzeau, F., De Pontual, H., Allemand, D., and Payan, P. (2006). Characterization and variations of organic parameters in teleost fish endolymph during day–night cycle, starvation and stress conditions. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 145, 99–107.
| Characterization and variations of organic parameters in teleost fish endolymph during day–night cycle, starvation and stress conditions.Crossref | GoogleScholarGoogle Scholar |
Høie, H., and Folkvord, A. (2006). Estimating the timing of growth rings in Atlantic cod otoliths using stable oxygen isotopes. Journal of Fish Biology 68, 826–837.
| Estimating the timing of growth rings in Atlantic cod otoliths using stable oxygen isotopes.Crossref | GoogleScholarGoogle Scholar |
Høie, H., Folkvord, A., Mosegaard, H., Li, L., Clausen, L. A. W., Norberg, B., and Geffen, A. J. (2008). Restricted fish feeding reduces cod otolith opacity. Journal of Applied Ichthyology 24, 138–143.
| Restricted fish feeding reduces cod otolith opacity.Crossref | GoogleScholarGoogle Scholar |
Høie, H., Millner, R. S., McCully, S., Nedreaas, K. H., Pilling, G. M., and Skadal, J. (2009). Latitudinal differences in the timing of otolith growth: a comparison between the Barents Sea and southern North Sea. Fisheries Research 96, 319–322.
| Latitudinal differences in the timing of otolith growth: a comparison between the Barents Sea and southern North Sea.Crossref | GoogleScholarGoogle Scholar |
Hüssy, K., and Mosegaard, H. (2004). Atlantic cod (Gadus morhua) growth and otolith accretion characteristics modelled in a bioenergetics context. Canadian Journal of Fisheries and Aquatic Sciences 61, 1021–1031.
| Atlantic cod (Gadus morhua) growth and otolith accretion characteristics modelled in a bioenergetics context.Crossref | GoogleScholarGoogle Scholar |
Hüssy, K., Mosegaard, H., and Jessen, F. (2004). Effect of age and temperature on amino acid composition and the content of different protein types of juvenile Atlantic cod (Gadus morhua) otoliths. Canadian Journal of Fisheries and Aquatic Sciences 61, 1012–1020.
| Effect of age and temperature on amino acid composition and the content of different protein types of juvenile Atlantic cod (Gadus morhua) otoliths.Crossref | GoogleScholarGoogle Scholar |
Hüssy, K., Nielsen, B., Mosegaard, H., and Clausen, L. W. (2009). Using data storage tags to link otolith macrostructure in Baltic cod Gadus morhua with environmental conditions. Marine Ecology Progress Series 378, 161–170.
| Using data storage tags to link otolith macrostructure in Baltic cod Gadus morhua with environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Jamieson, R. E., Schwarcz, H. P., and Brattey, J. (2004). Carbon isotopic records from the otoliths of Atlantic cod (Gadus morhua) from eastern Newfoundland, Canada. Fisheries Research 68, 83–97.
| Carbon isotopic records from the otoliths of Atlantic cod (Gadus morhua) from eastern Newfoundland, Canada.Crossref | GoogleScholarGoogle Scholar |
Jobling, M. (1997). Temperature and growth: modulation of growth rate via temperature change. In ‘Global Warming: Implications for Freshwater and Marine Fish’. (Eds C. M. Wood and D. G. McDonald.) pp. 225–253. (Cambridge University Press: Cambridge, UK.)
Jørgensen, C., Peck, M. A., Antognarelli, F., Azzurro, E., Burrows, M. T., Cheung, W. W. L., Cucco, A., Holt, R. E., Huebert, K. B., Marras, S., McKenzie, D., Metcalfe, J., Perez-Ruzafa, A., Sinerchia, M., Steffensen, J. F., Teal, L. R., and Domenici, P. (2012). Conservation physiology of marine fishes: advancing the predictive capacity of models. Biology Letters 8, 900–903.
| Conservation physiology of marine fishes: advancing the predictive capacity of models.Crossref | GoogleScholarGoogle Scholar | 22859560PubMed |
Kalish, J. M. (1991a). 13C and 18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects. Marine Ecology Progress Series 75, 191–203.
| 13C and 18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects.Crossref | GoogleScholarGoogle Scholar |
Kalish, J. M. (1991b). Determinants of otolith chemistry: seasonal variation in the composition of blood plasma, endolymph and otoliths of bearded rock cod Pseudophycis barbatus. Marine Ecology Progress Series 74, 137–159.
| Determinants of otolith chemistry: seasonal variation in the composition of blood plasma, endolymph and otoliths of bearded rock cod Pseudophycis barbatus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmslKhtrw%3D&md5=242012cf7eb6dc2135c085c46b75c666CAS |
Martens, M. T., Wall, A. J., Pyle, G. G., Wasylenko, B. A., Dew, W. A., Devlin, R. H., and Blanchfield, P. J. (2014). Growth and feeding efficiency of wild and aquaculture genotypes of rainbow trout (Oncorhynchus mykiss) common to Lake Huron, Canada. Journal of Great Lakes Research 40, 377–384.
| Growth and feeding efficiency of wild and aquaculture genotypes of rainbow trout (Oncorhynchus mykiss) common to Lake Huron, Canada.Crossref | GoogleScholarGoogle Scholar |
Martin, G. B., and Wuenschel, M. J. (2006). Effect of temperature and salinity on otolith element incorporation in juvenile gray snapper Lutjanus griseus. Marine Ecology Progress Series 324, 229–239.
| Effect of temperature and salinity on otolith element incorporation in juvenile gray snapper Lutjanus griseus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptlSltw%3D%3D&md5=a6b9afb7686f12bc04ff0ac732569c11CAS |
McCormick, M. I., and Meekan, M. G. (2010). The importance of attitude: the influence of behaviour on survival at an ontogenetic boundary. Marine Ecology Progress Series 407, 173–185.
| The importance of attitude: the influence of behaviour on survival at an ontogenetic boundary.Crossref | GoogleScholarGoogle Scholar |
McMahon, K. W., Fogel, M. L., Johnson, B. J., Houghton, L. A., and Thorrold, S. R. (2011). A new method to reconstruct fish diet and movement patterns from δ13C values in otolith amino acids. Canadian Journal of Fisheries and Aquatic Sciences 68, 1330–1340.
| A new method to reconstruct fish diet and movement patterns from δ13C values in otolith amino acids.Crossref | GoogleScholarGoogle Scholar |
McMahon, K. W., Hamady, L. L., and Thorrold, S. R. (2013). Ocean ecogeochemistry: a review. Oceanography and Marine Biology – an Annual Review 51, 327–373.
Meekan, M. G., and Fortier, L. (1996). Selection for fast growth during the larval life of Atlantic cod Gadus morhua on the Scotian Shelf. Marine Ecology Progress Series 137, 25–37.
| Selection for fast growth during the larval life of Atlantic cod Gadus morhua on the Scotian Shelf.Crossref | GoogleScholarGoogle Scholar |
Metcalfe, N. B. (1986). Intraspecific variation in competitive ability and food-intake in salmonids: consequences for energy budgets and growth-rates. Journal of Fish Biology 28, 525–531.
| Intraspecific variation in competitive ability and food-intake in salmonids: consequences for energy budgets and growth-rates.Crossref | GoogleScholarGoogle Scholar |
Metcalfe, N. B. (1998). The interaction between behavior and physiology in determining life history patterns in Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 55, 93–103.
| The interaction between behavior and physiology in determining life history patterns in Atlantic salmon (Salmo salar).Crossref | GoogleScholarGoogle Scholar |
Metcalfe, N. B., Wright, P. J., and Thorpe, J. E. (1992). Relationships between social-status, otolith size at 1st feeding and subsequent growth in Atlantic salmon (Salmo salar). Journal of Animal Ecology 61, 585–589.
| Relationships between social-status, otolith size at 1st feeding and subsequent growth in Atlantic salmon (Salmo salar).Crossref | GoogleScholarGoogle Scholar |
Millidine, K. J., Armstrong, J. D., and Metcalfe, N. B. (2009). Juvenile salmon with high standard metabolic rates have higher energy costs but can process meals faster. Proceedings of the Royal Society – B. Biological Sciences 276, 2103–2108.
| Juvenile salmon with high standard metabolic rates have higher energy costs but can process meals faster.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M3ps1yqsw%3D%3D&md5=b75dde8c40df50bae4e3b0d5a731affcCAS | 19324750PubMed |
Millner, R. S., Pilling, G. M., McCully, S. R., and Hoie, H. (2011). Changes in the timing of otolith zone formation in North Sea cod from otolith records: an early indicator of climate-induced temperature stress? Marine Biology 158, 21–30.
| Changes in the timing of otolith zone formation in North Sea cod from otolith records: an early indicator of climate-induced temperature stress?Crossref | GoogleScholarGoogle Scholar |
Morales-Nin, B. (2000). Review of the growth regulation processes of otolith daily increment formation. Fisheries Research 46, 53–67.
| Review of the growth regulation processes of otolith daily increment formation.Crossref | GoogleScholarGoogle Scholar |
Morales-Nin, B., and Panfili, J. (2005). Seasonality in the deep sea and tropics revisited: what can otoliths tell us? Marine and Freshwater Research 56, 585–598.
| Seasonality in the deep sea and tropics revisited: what can otoliths tell us?Crossref | GoogleScholarGoogle Scholar |
Mosegaard, H. (1990). What is reflected by otolith size at emergence: a reevaluation of the results. Canadian Journal of Fisheries and Aquatic Sciences 47, 225–228.
Mosegaard, H., Svedang, H., and Taberman, K. (1988). Uncoupling of somatic and otolith growth rates in Arctic char (Salvelinus alpinus) as an effect of differences in temperature response. Canadian Journal of Fisheries and Aquatic Sciences 45, 1514–1524.
| Uncoupling of somatic and otolith growth rates in Arctic char (Salvelinus alpinus) as an effect of differences in temperature response.Crossref | GoogleScholarGoogle Scholar |
Mugiya, Y. (1984). Diurnal rhythm in otolith formation in the rainbow trout, Salmo gairdneri: seasonal reversal of the rhythm in relation to plasma calcium concentrations. Comparative Biochemistry and Physiology – A. Physiology 78, 289–293.
| Diurnal rhythm in otolith formation in the rainbow trout, Salmo gairdneri: seasonal reversal of the rhythm in relation to plasma calcium concentrations.Crossref | GoogleScholarGoogle Scholar |
Narum, S. R., Buerkle, C. A., Davey, J. W., Miller, M. R., and Hohenlohe, P. A. (2013). Genotyping-by-sequencing in ecological and conservation genomics. Molecular Ecology 22, 2841–2847.
| Genotyping-by-sequencing in ecological and conservation genomics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXos12nurc%3D&md5=c1482f6fdf5f70d071ce234b8c9f3619CAS | 23711105PubMed |
Neat, F. C., Wright, P. J., and Fryer, R. J. (2008). Temperature effects on otolith pattern formation in Atlantic cod Gadus morhua. Journal of Fish Biology 73, 2527–2541.
| Temperature effects on otolith pattern formation in Atlantic cod Gadus morhua.Crossref | GoogleScholarGoogle Scholar |
Newman, S. J., and Dunk, I. J. (2003). Age validation, growth, mortality, and additional population parameters of the goldband snapper (Pristipomoides multidens) off the Kimberley coast of northwestern Australia. Fishery Bulletin 101, 116–128.
Nielsen, E. E., Hemmer-Hansen, J., Larsen, P. F., and Bekkevold, D. (2009). Population genomics of marine fishes: identifying adaptive variation in space and time. Molecular Ecology 18, 3128–3150.
| Population genomics of marine fishes: identifying adaptive variation in space and time.Crossref | GoogleScholarGoogle Scholar | 19627488PubMed |
Panfili, J., Darnaude, A. M., Vigliola, L., Jacquart, A., Labonne, M., and Gilles, S. (2015). Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths. Journal of Experimental Marine Biology and Ecology 467, 65–70.
| Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXks1agu7o%3D&md5=09079a3defc6586ac9e7a3aeab8dc029CAS |
Pecquerie, L., Fablet, R., de Pontual, H., Bonhommeau, S., Alunno-Bruscia, M., Petitgas, P., and Kooijman, S. A. L. M. (2012). Reconstructing individual food and growth histories from biogenic carbonates. Marine Ecology Progress Series 447, 151–164.
| Reconstructing individual food and growth histories from biogenic carbonates.Crossref | GoogleScholarGoogle Scholar |
Petersen, M. F., and Steffensen, J. F. (2003). Preferred temperature of juvenile Atlantic cod Gadus morhua with different haemoglobin genotypes at normoxia and moderate hypoxia. The Journal of Experimental Biology 206, 359–364.
| Preferred temperature of juvenile Atlantic cod Gadus morhua with different haemoglobin genotypes at normoxia and moderate hypoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsFOhsrw%3D&md5=dc06dfc4f2f2b71d3980e822959a1fa4CAS | 12477905PubMed |
Pilling, G. M., Millner, R. S., Easey, M. W., Maxwell, D. L., and Tidd, A. N. (2007). Phenology and North Sea cod Gadus morhua L.: has climate change affected otolith annulus formation and growth? Journal of Fish Biology 70, 584–599.
| Phenology and North Sea cod Gadus morhua L.: has climate change affected otolith annulus formation and growth?Crossref | GoogleScholarGoogle Scholar |
Radtke, R. L., and Shafer, D. J. (1992). Environmental sensitivity of fish otolith microchemistry. Australian Journal of Marine and Freshwater Research 43, 935–951.
| Environmental sensitivity of fish otolith microchemistry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXltFelu7w%3D&md5=f6da67e322b2a18f2656c4826ec1342fCAS |
Radtke, R. L., Williams, D. F., and Hurley, P. C. F. (1987). The stable isotopic composition of bluefin tuna (Thunnus thynnus) otoliths: evidence for physiological regulation. Comparative Biochemistry and Physiology – A. Comparative Physiology 87, 797–801.
| The stable isotopic composition of bluefin tuna (Thunnus thynnus) otoliths: evidence for physiological regulation.Crossref | GoogleScholarGoogle Scholar |
Reznick, D., Lindbeck, E., and Bryga, H. (1989). Slower growth results in larger otoliths: an experimental test with guppies (Poecilia reticulata). Canadian Journal of Fisheries and Aquatic Sciences 46, 108–112.
| Slower growth results in larger otoliths: an experimental test with guppies (Poecilia reticulata).Crossref | GoogleScholarGoogle Scholar |
Righton, D. A., Andersen, K. H., Neat, F., Thorsteinsson, V., Steingrund, P., Svedang, H., Michalsen, K., Hinrichsen, H. H., Bendall, V., Neuenfeldt, S., Wright, P., Jonsson, P., Huse, G., van der Kooij, J., Mosegaard, H., Hussy, K., and Metcalfe, J. (2010). Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima. Marine Ecology Progress Series 420, 1–13.
| Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima.Crossref | GoogleScholarGoogle Scholar |
Romanek, C. S., Grossman, E. L., and Morse, J. W. (1992). Carbon isotopic fractionation in synthetic aragonite and calcite: effects of temperature and precipitation rate. Geochimica et Cosmochimica Acta 56, 419–430.
| Carbon isotopic fractionation in synthetic aragonite and calcite: effects of temperature and precipitation rate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xhtlarurg%3D&md5=29c2988b12f9015ef8af2ca360fec550CAS |
Ross, S. D., Behrens, J. W., Brander, K., Methling, C., and Mork, J. (2013). Haemoglobin genotypes in cod (Gadus morhua L): their geographic distribution and physiological significance. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 166, 158–168.
| Haemoglobin genotypes in cod (Gadus morhua L): their geographic distribution and physiological significance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVKns7nK&md5=c091e4f8136264e88b04afc2e33b7810CAS |
Secor, D. H., and Rooker, J. R. (2000). Is otolith strontium a useful scalar of life cycles in estuarine fishes? Fisheries Research 46, 359–371.
| Is otolith strontium a useful scalar of life cycles in estuarine fishes?Crossref | GoogleScholarGoogle Scholar |
Stanley, R. R. E., Bradbury, I. R., DiBacco, C., Snelgrove, P. V. R., Thorrold, S. R., and Killen, S. S. (2015). Environmentally mediated trends in otolith composition of juvenile Atlantic cod (Gadus morhua). ICES Journal of Marine Science 72, 2350–2363.
| Environmentally mediated trends in otolith composition of juvenile Atlantic cod (Gadus morhua).Crossref | GoogleScholarGoogle Scholar |
Stewart, B. D., Fenton, G. E., Smith, D. C., and Short, S. A. (1995). Validation of otolith increment age estimates for a deep water fish species, the warty oreo Allocyttus verrucosus, by radiometric analysis. Marine Biology 123, 29–38.
| Validation of otolith increment age estimates for a deep water fish species, the warty oreo Allocyttus verrucosus, by radiometric analysis.Crossref | GoogleScholarGoogle Scholar |
Sturrock, A. M., Trueman, C. N., Darnaude, A. M., and Hunter, E. (2012). Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? Journal of Fish Biology 81, 766–795.
| Can otolith elemental chemistry retrospectively track migrations in fully marine fishes?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtl2ltLvN&md5=9546ccc49c9558b3fe7de3bf4a0f68efCAS | 22803735PubMed |
Sturrock, A. M., Trueman, C. N., Milton, J. A., Waring, C. P., Cooper, M. J., and Hunter, E. (2014). Physiological influences can outweigh environmental signals in otolith microchemistry research. Marine Ecology Progress Series 500, 245–264.
| Physiological influences can outweigh environmental signals in otolith microchemistry research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpslylsL4%3D&md5=529800d6fb7688e2564d96449d3611a5CAS |
Sturrock, A.M., Hunter, E., Milton, J.A., EIMF Johnson, R.C., Waring, C.P., and Trueman, C.N. (2015). Quantifying physiological influences on otolith microchemistry. Methods in Ecology and Evolution 6, 806–816.
| Quantifying physiological influences on otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Titus, R. G., and Mosegaard, H. (1991). Selection for growth potential among migratory brown trout (Salmo trutta) fry competing for territories: evidence from otoliths. Canadian Journal of Fisheries and Aquatic Sciences 48, 19–27.
| Selection for growth potential among migratory brown trout (Salmo trutta) fry competing for territories: evidence from otoliths.Crossref | GoogleScholarGoogle Scholar |
Van Leeuwen, T. E., Rosenfeld, J. S., and Richards, J. G. (2012). Effects of food ration on SMR: influence of food consumption on individual variation in metabolic rate in juvenile coho salmon (Onchorhynchus kisutch). Journal of Animal Ecology 81, 395–402.
| Effects of food ration on SMR: influence of food consumption on individual variation in metabolic rate in juvenile coho salmon (Onchorhynchus kisutch).Crossref | GoogleScholarGoogle Scholar | 22066987PubMed |
Vignon, M., and Morat, F. (2010). Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish. Marine Ecology Progress Series 411, 231–241.
| Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish.Crossref | GoogleScholarGoogle Scholar |
Walther, B. D., Kingsford, M. J., O’Callaghan, M. D., and McCulloch, M. T. (2010). Interactive effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish. Environmental Biology of Fishes 89, 441–451.
| Interactive effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish.Crossref | GoogleScholarGoogle Scholar |
Wright, P. J. (1991). The influence of metabolic rate on otolith increment width in Atlantic salmon parr, Salmo salar L. Journal of Fish Biology 38, 929–933.
| The influence of metabolic rate on otolith increment width in Atlantic salmon parr, Salmo salar L.Crossref | GoogleScholarGoogle Scholar |
Wright, P. J., Rowe, D., and Thorpe, J. E. (1991). Daily growth increments in the otoliths of Atlantic salmon parr, Salmo salar L, and the influence of environmental factors on their periodicity. Journal of Fish Biology 39, 103–113.
| Daily growth increments in the otoliths of Atlantic salmon parr, Salmo salar L, and the influence of environmental factors on their periodicity.Crossref | GoogleScholarGoogle Scholar |
Wright, P. J., Fallon-Cousins, P., and Armstrong, J. D. (2001). The relationship between otolith accretion and resting metabolic rate in juvenile Atlantic salmon during a change in temperature. Journal of Fish Biology 59, 657–666.
| The relationship between otolith accretion and resting metabolic rate in juvenile Atlantic salmon during a change in temperature.Crossref | GoogleScholarGoogle Scholar |
Wright, P. J., Woodroffe, D. A., Gibb, F. M., and Gordon, J. D. M. (2002). Verification of first annulus formation in the illicia and otoliths of white anglerfish, Lophius piscatorius using otolith microstructure. ICES Journal of Marine Science 59, 587–593.
| Verification of first annulus formation in the illicia and otoliths of white anglerfish, Lophius piscatorius using otolith microstructure.Crossref | GoogleScholarGoogle Scholar |
Yamamoto, T., Ueda, H., and Higashi, S. (1998). Correlation among dominance status, metabolic rate and otolith size in masu salmon. Journal of Fish Biology 52, 281–290.
| Correlation among dominance status, metabolic rate and otolith size in masu salmon.Crossref | GoogleScholarGoogle Scholar |


