Validation of the first annual increment deposition in the otoliths of European anchovy in the Bay of Biscay based on otolith microstructure analysis
Naroa Aldanondo A B , Unai Cotano A , Paula Álvarez A and Andrés Uriarte AA AZTI, Marine Research Unit, Herrera Kaia Portualdea z/g, E-20110, Pasaia, Basque Country, Spain.
B Corresponding author. Email: naldanondo@azti.es
Marine and Freshwater Research 67(7) 943-950 https://doi.org/10.1071/MF15083
Submitted: 28 February 2015 Accepted: 14 September 2015 Published: 6 November 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
In order to validate the first annual increment deposition in European anchovy otoliths, early juveniles were captured in October 2012 in the southern Bay of Biscay. These individuals were maintained under a continuous feeding regimen in a sea cage over a period of 6 months. From October 2012 to January 2013, lengths increased slightly or remained stable at ~9.8 cm. After this period, standard length increased significantly up to a mean value of 12.0 cm in April 2013. Likewise, the age of anchovies was estimated based on otolith microstructure analysis. The estimated age varied from 96 days (for individuals sampled in October 2012) to 293 days (for anchovies sampled in April 2013). A daily increment deposition rate was confirmed in otoliths of individuals maintained in the sea cage during the winter. The general otolith daily growth pattern showed that increment widths increased rapidly and were broadest between 51 and 56 days, with a mean of 19.1 µm. Thereafter, the widths decreased steadily to 1.5 µm and remained almost constant until the end of the experiment. The present study also revealed that the first translucent band formation started in autumn and was completed by spring.
Additional keywords: age, daily increment deposition, Engraulis encrasicolus.
Introduction
Otoliths contain information about the age and growth history of marine fish, at both a daily and annual level. On the annual level, otolith macrostructure analysis enables determination of fish age, which is fundamental to an understanding of population dynamics. It is largely assumed that the annual growth increment, known as an annulus, is laid down at regular intervals following a seasonal cycle (Beckman and Wilson 1995; Pilling et al. 2007; Hüssy et al. 2010). The annulus is composed of an opaque and translucent band, which differ optically under transmitted light (Neat et al. 2008). In temperate species, the general interpretation is that formation of the opaque band is related to a fast growth period during spring and summer, whereas the translucent band or ‘winter ring’ is generally associated with a period of slow growth rate in autumn and winter (Pannella 1974; Victor and Brothers 1982).
The alternation of opaque and translucent bands in otoliths has been used to estimate fish age (Campana 2001). In the case of the European anchovy in the Bay of Biscay, age determination has traditionally been conducted by interpretation of these structures (ICES 2009) based on an unpublished validation study (A. Uriarte, pers. comm.). In this area, the anchovy has a continuous spawning season between March and August (Motos 1996; Bellier et al. 2007) and larvae develop during spring and summer (Cotano et al. 2008). Juveniles are regularly observed in autumn (Aldanondo et al. 2010; Boyra et al. 2013) and, after overcoming the winter period, they are finally recruited to the adult population the following spring. Taking the timing of recruitment into account, in spring 1-year-old individual otolith shows a complete opaque and translucent band and the start of the second opaque band (ICES 2009).
Nevertheless, the factors regulating the formation of the opaque and translucent bands are unclear and substantial variations in the timing of translucent band formation at individual, population and species levels have been reported (Beckman and Wilson 1995). Therefore, to obtain reliable age estimates, it is necessary to validate the periodicity and timing of annual increment deposition in otoliths (Campana 2001).
Daily increment analysis has been used to validate annual increment formation for many fish species, particularly for the first annulus (e.g. Brothers et al. 1976; Victor and Brothers 1982; Wright et al. 2002; Sequeira et al. 2009; Hüssy et al. 2010). For European anchovy, daily increment deposition has been validated for larvae (Aldanondo et al. 2008) and early juveniles (Cermeño et al. 2003). However, certain variability in daily increment deposition was observed for individuals maintained in captivity for long periods (over a period of 2 years), suggesting an underestimation of the age in adults (Cermeño et al. 2003).
Several authors have reported that laboratory conditions often affect the growth of larval, juvenile and adult fish, and therefore the formation of increments in the otoliths (e.g. Geffen 1982; Folkvord et al. 2000; Feet et al. 2002; Aldanondo et al. 2008; Namiki et al. 2010). For validation studies, Geffen (1992) stated that the fish should be reared under conditions that contribute to good growth and natural behaviour, thus avoiding as much as possible the effect of captivity. Large outdoor enclosures, where photoperiod and temperature cycles reflect natural conditions, seem to ensure good fish growth and development, and are considered a suitable solution to overcome possible rearing-related artefacts (Geffen 1992; Folkvord et al. 1996, 1997; Feet et al. 2002). This approach enables an enclosed and well-defined population to be followed over time under seminatural conditions.
The main objectives of the present study were to: (1) assess temporal variations in juvenile length and weight during the winter under a continuous feeding regimen; (2) validate daily increment deposition in otoliths of the anchovy during the first slow growth period in the Bay of Biscay; and (3) validate the first annual increment deposition in otoliths of the anchovy, as well as to determine the timing of the formation of the first translucent band.
Material and methods
Rearing experiment
Early anchovy juveniles were captured by purse seine in October 2012 in the southern Bay of Biscay. These individuals, weighing ~1000 kg in total weight, were transferred to a cylindrical sea culture cage (16-m diameter, 7-m net depth, 4-mm mesh size) located in Mutriku (Gipuzkoa, Spain; 43°18′N, 02°22′W). Anchovies were fed by hand once daily with a commercial diet (Microbaq; Dibaq Acuicultura, Segovia, Spain) at 4% body weight (Tudela and Palomera 1995) and maintained in captivity until the formation of the first translucent band in otoliths was complete (April 2013). Juveniles were sampled at approximate 27-day intervals, weighed (W; to the nearest 0.1 g) and measured for standard length (SL; to the nearest 1 mm; Table 1).

|
Otolith analysis
In the laboratory, both sagittal otoliths were removed from each individual. To establish the time of first translucent band deposition, otolith pairs were placed in black containers with the sulcus facing down and examined in water under a dissecting microscope at 10× magnification with reflected light. Translucent band formation was determined according to criteria established by the ICES (2009).
For otolith microstructure analysis, five individuals for each sampling were processed to obtain a representative sample of the whole experiment. For each sample, the right otolith was processed following the methods described by Aldanondo et al. (2010). Otoliths were analysed under a light microscope coupled to an image analyser (TNPC Software, version 3.2; Visilog, Ifremer, France). The central part of otoliths was evaluated at a magnification of 1000× in immersion oil, whereas the outer opaque band was analysed at a magnification of 100×. For translucent band analysis, samples were analysed at a magnification of 630×. Composite image files were constructed to enable the reader to scroll across the complete otolith image during analysis.
All increments were counted starting at hatch increment (Aldanondo et al. 2008) and the distance between increments was measured along the same axis from the core to the edge of the otolith on the post-rostrum side. In addition, the distance from the core to the beginning and the end of the first translucent band was measured along the same axis. For daily increment interpretation, the group band reading method suggested by Cermeño et al. (2008) was used. Each otolith was read independently by two readers (N. Aldanondo and U. Cotano) and any otolith with a difference between readers >5% was rejected. Of 42 otoliths read, 40 were used in the analysis. Because daily increment deposition has been validated for European anchovy larvae (Aldanondo et al. 2008) and juveniles (Cermeño et al. 2003), the increment number was considered a proxy for age in days.
Condition factor
The length–weight relationship for anchovies in the experiment was established using the simple allometric equation
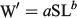
where W′ is predicted length-specific mean weight for the sample (g), a is a constant of proportionality, and b is the allometric factor. Because Fulton’s condition factor was significantly correlated with body length, relative condition factor (Kn; Le Cren 1951) was used to compare body weight as follows:

Results
Length and weight temporal variation
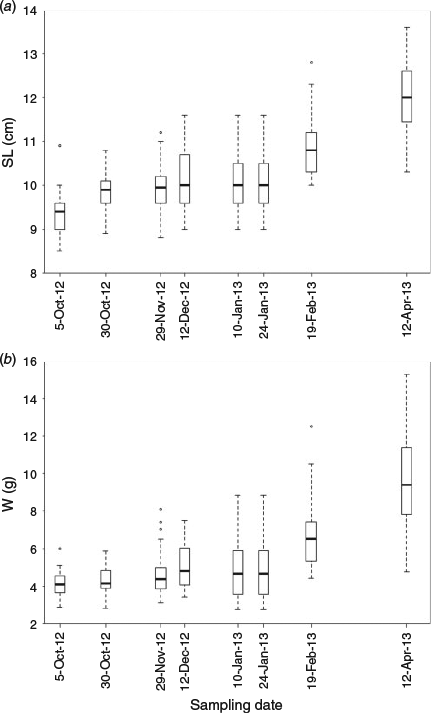
There was a significant increase in length during the initial 3 weeks of the experiment (P < 0.001, Kruskal–Wallis test; Fig. 1a). From the end of October 2012 until January 2013, anchovy length increased slightly or remained stable at around a mean ± s.d. value of 9.8 ± 0.6 cm. After this period, SL increased significantly up to a mean value of 12.0 ± 0.8 cm in April 2013 (P < 0.001, Kruskal–Wallis test).
|
Juvenile weight distribution showed a similar trend to that observed for length (Fig. 1b). In this case, individual weight was less variable from the beginning of the experiment until January 2013 (mean ± s.d. weight = 4.6 ± 1.2 g). Thereafter, weight increased significantly up to a mean value of 10.7 g at the termination of the experiment (P < 0.001, Kruskal–Wallis test).
The relationship between length and weight was significant, and was given by the equation:
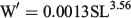
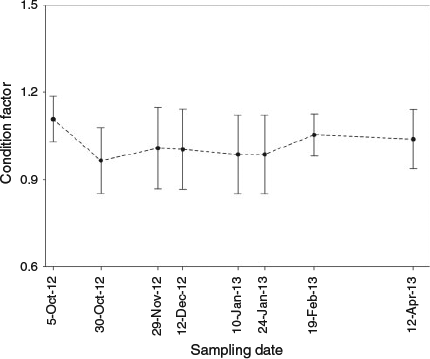
where n = 261; R2 = 0.89; and P < 0.001. There were significant differences in the condition of anchovies among samplings (P < 0.05, Kruskal–Wallis test; Fig. 2). Individuals sampled in February and April 2013 had the highest mean values (1.04 ± 0.09), indicating that these anchovies were better conditioned than those from the previous samplings. No significant differences were found among individuals sampled between the end of October 2012 and January 2013 (0.98 ± 0.13; P > 0.05, Kruskal–Wallis test).
|
Age estimation and increment deposition rate
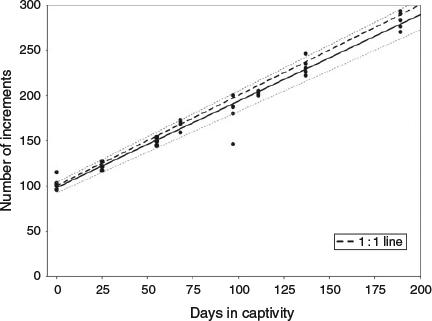
The age (in days) of anchovy juveniles was estimated based on otolith microstructure analysis. The estimated age varied from 96 days for individuals sampled in October 2012 to 293 days for anchovies sampled in April 2013 (Fig. 3). Otolith-derived hatch date distributions indicated that these anchovies originated from late spring and early summer (between 12 June and 16 July), with a mean hatch date of 1 July (±10.2 days).
|
The relationship between the number of increments (I) and days in captivity (D) was significant, and was given by the following equation
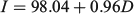
where n = 40; R2 = 0.97; and P < 0.001 (Fig. 3). The estimated number of increments was compared with the number of days in captivity using linear regression. To this end, and based on estimated mean age, it was assumed that juveniles were, on average, 100 days old at the beginning of the experiment. The regression analysis showed that the slope approached 1 (95% confidence interval 0.89–1.01) and therefore the number of increments was in accordance with a daily increment deposition rate in otoliths of anchovies maintained in the sea cage.
Somatic and otolith growth
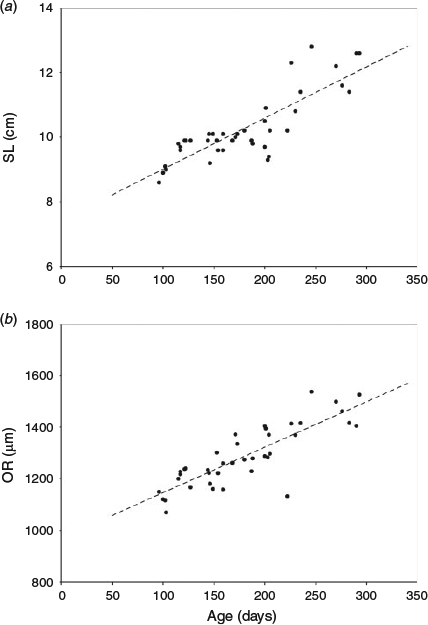
The length at age data were described by linear regression analysis (Fig. 4a). The estimated mean somatic growth rate for anchovies during the experiment was 0.16 mm day–1. The otolith growth pattern was similar to that observed for somatic growth. A linear regression line was fit for otolith radius as a function of age and the estimated mean otolith growth rate was 1.8 µm day–1 (Fig. 4b).
|
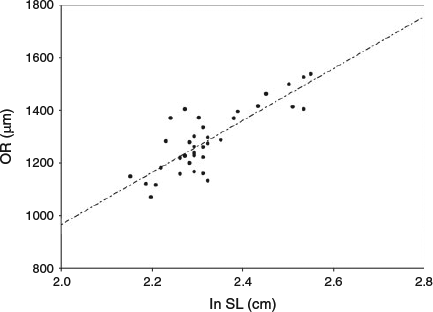
A linear relationship was found between otolith radius and length (ln transformed), indicating that otolith radius was a suitable proxy for anchovy size in the present study (otolith radius = 986.6 ln(SL) – 1006.8; n = 40, R2 = 0.69, P < 0.001; Fig. 5).
|
The general otolith daily growth pattern showed that increment widths increased rapidly and were broadest between 51 and 56 days, with a mean (± s.d.) of 19.1 ± 3.7 µm. Thereafter, the widths decreased steadily to ~1.5 µm on Day 173 and remained almost constant at approximately this value until the end of the experiment (Fig. 6).
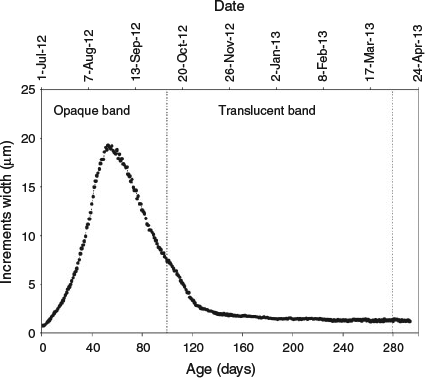
|
Deposition of the first translucent band
Deposition of the first translucent band was observed in otoliths of anchovies maintained in the sea cage. At the end of the experiment, anchovy otoliths showed a complete opaque and translucent band and the start of the second opaque band. Estimated mean hatch date in relation to otolith daily growth pattern indicated that the formation of the first translucent band started in October 2012 and was complete by April 2013 (Fig. 6). This translucent band was characterised by a decline in increment widths, which were significantly narrower than those in the adjacent opaque band.
Discussion
In the present study, juveniles were successfully maintained in a sea cage under continuous feeding conditions to assess anchovy length and weight temporal variation in the Bay of Biscay. Both length and weight remained almost constant during the autumn and early winter; thereafter they increased significantly until early spring (Fig. 1). The relative condition factor followed a similar trend to that observed for anchovy length and weight (Fig. 2). Unfortunately, no references to temporal variations in length and weight during the winter were found in the literature against which the results of the present study could be compared.
The mean length of individuals maintained in the sea cage until April 2013 falls within the range of 10.5–18.0 cm in length reported for 1-year-old anchovy in the Bay of Biscay (Junquera and Perez-Gándaras 1993). However, these anchovies were noticeably smaller than those caught in the field during the same period (14.9 ± 0.8 cm; I. Martín and I. Rico, unpubl. data). Similarly, the condition of the individuals maintained in the sea cage was lower than that of anchovies caught in the field (1.15 ± 0.08; I. Martín and I. Rico, unpubl. data). These differences could be attributable to the small size range of anchovies analysed in the present study, because only a homogeneous cohort of a juvenile population was sampled in autumn. The mean length of juveniles captured in autumn 2012 was 9.3 ± 0.4 cm, whereas the juvenile population size at that time ranged from 2.7 to 13.7 cm (ICES 2012).
Furthermore, anchovies in the sea cage were not subjected to predation mortality, which could affect individual size distribution in the cage. Several studies have reported a size-dependent vulnerability to predation (i.e. smaller individuals are more susceptible to predators and experience a higher mortality rate than larger individuals; Miller et al. 1988; Rice et al. 1993; Sogard 1997). Therefore, the absence of predators could also explain the observed differences in length, because the smallest individuals were not removed from the sea cage. In addition, overwinter mortality associated with either starvation or adverse environmental conditions could be an additional size-selective process in the field (Henderson et al. 1988; Johnson and Evans 1991; Cargnelli and Gross 1996; Schindler 1999).
Validation of daily increment deposition rate
Daily increment deposition rate has been validated in otoliths of anchovies maintained under a continuous feeding regimen during the winter. For this species, Cermeño et al. (2003) previously validated the daily periodicity of increment formation in otoliths of juveniles and adults in laboratory rearing experiments using oxytetracycline hydrochloride as a marker. However, they observed a loss of daily increment deposition in otoliths and suggested that this could be associated with a slow growth rate in the aquarium. The lack of apparent daily increment deposition because of a slow growth rate has also been reported by other authors (Folkvord et al. 2000; Fox et al. 2003; Aldanondo et al. 2008). However, in the present study, the daily periodicity of increment formation was confirmed for winter, when the growth rate decreases markedly (0.16 mm day–1). The observed differences in increment deposition rate between these studies could be associated with the rearing conditions, because in the present study anchovies were maintained under seminatural conditions, in contrast with the other study, where individuals were maintained under aquarium conditions. Therefore, it is expected that the cage conditions contribute to noticeable growth compared with the laboratory conditions. Conversely, as mentioned previously, anchovies in the sea cage at the end of the experiment were smaller than those captured in the field. Therefore, a higher growth rate is expected in the field, and consequently higher age accuracy, as suggested by others (Folkvord et al. 2000; Feet et al. 2002; Ivarjord et al. 2008).
For Japanese anchovy (Engraulis japonicus S.), Namiki et al. (2010) also reported non-daily increment deposition in otoliths of immature anchovies in winter. The authors argued that low water temperature conditions (below 13–14°C) reduced otolith growth rate, making interpretation of daily increments more difficult. In the present study, anchovies experienced similar temperatures during the winter to those reported for Japanese anchovy (Fig. 7); however, daily periodicity of increment deposition in the present study was not impaired at low temperatures, even though otolith growth noticeably decreased.
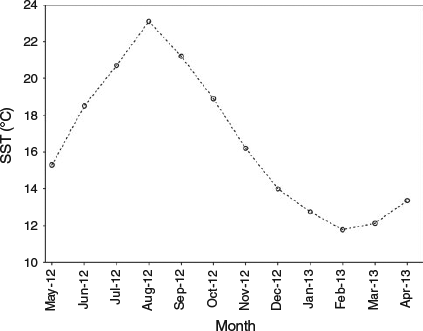
|
The general otolith growth pattern was characterised by a period of continuous increase in increment widths during the first 56 days, which corresponds to the larval and early juvenile stages. After this period, increment widths decreased until the age of 139 days; thereafter, they remained relatively stable until the termination of the experiment (Fig. 6). To our knowledge, the present study is the first to report otolith growth during the late juvenile stage. Conversely, several authors have studied the otolith growth of this species in the Bay of Biscay. A study by Cermeño et al. (2008) found a maximum average increment width of 18 µm day–1 between 40 and 60 days, similar to that measured in the present study. Similarly, Aldanondo et al. (2011) described otolith growth of anchovy during the first growth season with a Gompertz model and observed a similar pattern to that observed for juveniles in the sea cage. A similar growth pattern has been reported previously in other pelagic fish species (Cotano and Álvarez 2003; Xie and Watanabe 2005; Baumann et al. 2009).
The trend observed suggests that growth rates are primarily related to endogenous factors, although they are also modulated by environmental variables, such as temperature or food availability (Campana and Neilson 1985; Alemany et al. 2006; Baumann et al. 2006). In this case, the otolith growth pattern seems to reflect the seasonal temperature cycle in the Bay of Biscay (Figs 6, 7). The increase in growth rates was coincident with high water temperatures in summer, whereas the subsequent decline in growth rates occurred during the autumn, the time when sea surface temperature started to decline.
Growth discontinuities or underestimation of narrow increments because of resolution problems with the light microscope have been reported in many studies (e.g. Feet et al. 2002; Paul and Horn 2009; Hüssy et al. 2010; Namiki et al. 2010). However, in the present study, daily increments were well defined along the otolith, although they were narrow and with low contrast during the late juvenile stage. Unclear increments or non-defined daily deposition patterns were not observed. Furthermore, the average increment width during the slow growth period was 1.5 ± 0.2 µm, which is well above the resolution limit of the light microscope (0.3 µm according to Fox et al. 2003). Therefore, significant age underestimation is not expected.
In order to validate daily increment deposition, it was assumed that juveniles were, on average, 100 days old at the beginning of the experiment, because their actual age was not known. This assumption was based on estimated average age at that moment. It should be noted that individuals caught in the field in October 2012 belonged to a homogeneous cohort and originated from 1 July (±10.2 days). Therefore, the expected deviation is low and it could be assumed that there is negligible ageing error.
Validation of the first annual increment deposition
Based on otolith microstructure analysis, the first annual increment deposition has also been validated in anchovy otoliths in the Bay of Biscay. It is generally recognised that the translucent band is usually formed during winter–spring in the otoliths of temperate species (Beckman and Wilson 1995; Hüssy et al. 2010). The findings for the European anchovy are in accordance with this assumption, because the formation of the first translucent band starts in October and is complete by April. A similar deposition pattern has been observed in other species (Woodroffe et al. 2003; La Mesa 2007). The formation of the first annulus was also validated for South African anchovy using daily increment counts, but in this case the deposition of the first translucent band occurs in the austral summer (Waldron 1994).
Numerous studies have reported large variations in seasonality of otolith band formation between species (Beckman and Wilson 1995), as well as between populations within the same species (Williams et al. 2005; Høie et al. 2009). In order to explain these differences, several factors have been postulated, for example somatic growth (Wright et al. 2002), reproduction (Morales-Nin et al. 1998), photoperiod (Wright et al. 1992) and environmental temperature (Beckman and Wilson 1995; Pilling et al. 2007; Neat et al. 2008).
In this sense, the formation of opaque and translucent bands is expected to be associated with seasonality of somatic growth or general environmental conditions (Wright et al. 2002). In the case of the European anchovy, the daily growth pattern of the otolith reveals that the deposition of the opaque band is related to a fast growth period, whereas formation of the translucent band is linked to a slow growth period (Fig. 6). This pattern is consistent with previous descriptions of the translucent band in this species (ICES 2009) and in several other species (Brothers et al. 1976; Pannella 1980; Victor and Brothers 1982; Beckman and Wilson 1995; Wright et al. 2002). However, in the case of the South African anchovy, whether the formation of the translucent band is associated with a fast or slow growth period is unknown because it was not reported by Waldron (1994).
Seasonal changes in water temperature have also been suggested to promote translucent band formation in otoliths (Pannella 1980; Schramm 1989; Beckman and Wilson 1995). Nevertheless, the relationship between translucent band formation and temperature remains uncertain (Høie and Folkvord 2006; Pilling et al. 2007; Neat et al. 2008; Hüssy et al. 2010). In the case of the European anchovy, the formation of the opaque and translucent bands resembles the seasonal temperature variation in the Bay of Biscay (Fig. 7). Deposition of the opaque band coincides with high seasonal temperatures in summer and early autumn, whereas formation of the translucent band coincides with low temperatures in winter and early spring (Figs 6, 7).
Finally, the possibility that annulus formation could be under endogenous physiological control should be taken into account. Some studies have addressed this issue and reported that many fish form an annulus in the absence of variable water temperature (Schramm 1989; Johnson and Belk 2004; Neat et al. 2008).
In conclusion, the results of the present study indicate that daily increment deposition in otoliths of anchovies is not impaired during their first growth season. Therefore, the age and growth of juveniles during the winter can be estimated based on analysis of these structures. This information will enable overwinter mortality estimates to be made in order to determine the factors that control anchovy recruitment strength in the Bay of Biscay. In addition, the results of the present study confirm that the first annulus is composed of an opaque band, which is deposited during the spring and summer, and a translucent band, which is formed during autumn and winter. Consequently, the study validates age determination based on annulus interpretation and shows that this method is a reliable tool for age determination in the anchovy.
Acknowledgements
The authors thank Iñaki Rico for assistance with laboratory work and Victor Valencia for providing temperature data. This work was supported by the research project Jaula (Department of Agriculture, Fisheries and Food of the Basque Country Government). This paper is contribution number 732 from AZTI (Marine Research Division).
References
Aldanondo, N., Cotano, U., Etxebeste, E., Irigoien, X., Álvarez, P., Martínez de Murguía, A., and Herrero, D. L. (2008). Validation of daily increments deposition in the otoliths of European anchovy larvae (Engraulis encrasicolus L.) reared under different temperature conditions. Fisheries Research 93, 257–264.| Validation of daily increments deposition in the otoliths of European anchovy larvae (Engraulis encrasicolus L.) reared under different temperature conditions.Crossref | GoogleScholarGoogle Scholar |
Aldanondo, N., Cotano, U., Tiepolo, M., Boyra, G., and Irigoien, X. (2010). Growth and movement patterns of early juvenile European anchovy (Engraulis encrasicolus L.) in the Bay of Biscay based on otolith microstructure and chemistry. Fisheries Oceanography 19, 196–208.
| Growth and movement patterns of early juvenile European anchovy (Engraulis encrasicolus L.) in the Bay of Biscay based on otolith microstructure and chemistry.Crossref | GoogleScholarGoogle Scholar |
Aldanondo, N., Cotano, U., and Etxebeste, E. (2011). Growth of young-of-the-year anchovy (Engraulis encrasicolus L.) in the Bay of Biscay. Scientia Marina 75, 227–235.
Alemany, F., Álvarez, I., García, A., Cortés, D., Ramírez, T., Quintanilla, J., Álvarez, F., and Rodríguez, J. M. (2006). Postflexion larvae and juvenile daily growth patterns of the Alborán Sea sardine (Sardina pilchardus Walb.): influence of wind. Scientia Marina 70, 93–104.
Baumann, H., Gröhsler, T., Kornilovs, G., Makarchouk, A., Feldmann, V., and Temming, A. (2006). Temperature-induced regional and temporal growth differences in Baltic young-of-the-year sprat Sprattus sprattus. Marine Ecology Progress Series 317, 225–236.
| Temperature-induced regional and temporal growth differences in Baltic young-of-the-year sprat Sprattus sprattus.Crossref | GoogleScholarGoogle Scholar |
Baumann, H., Malzahn, A. M., Voss, R., and Temming, A. (2009). The German Bight (North Sea) is a nursery area for both locally and externally produced sprat juveniles. Journal of Sea Research 61, 234–243.
| The German Bight (North Sea) is a nursery area for both locally and externally produced sprat juveniles.Crossref | GoogleScholarGoogle Scholar |
Beckman, D., and Wilson, C. A. (1995). Seasonal timing of opaque zone formation in fish otoliths. In ‘Recent Developments in Fish Otolith Research’. (Eds D. H. Secor, J. M. Dean and S. E. Campana.) pp. 27–44. (University of South Carolina Press: Columbia.)
Bellier, E., Planque, B., and Petitgas, P. (2007). Historical fluctuations in spawning location of anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) in the Bay of Biscay during 1967–73 and 2000–2004. Fisheries Oceanography 16, 1–15.
| Historical fluctuations in spawning location of anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) in the Bay of Biscay during 1967–73 and 2000–2004.Crossref | GoogleScholarGoogle Scholar |
Boyra, G., Martínez, U., Cotano, U., Santos, M., Irigoien, X., and Uriarte, A. (2013). Acoustic surveys for juvenile anchovy in the Bay of Biscay: abundance estimate as an indicator of the next year’s recruitment and spatial distribution patterns. ICES Journal of Marine Science 70, 1354–1368.
| Acoustic surveys for juvenile anchovy in the Bay of Biscay: abundance estimate as an indicator of the next year’s recruitment and spatial distribution patterns.Crossref | GoogleScholarGoogle Scholar |
Brothers, E. B., Mathews, C. P., and Lasker, R. (1976). Daily growth increments in otoliths from larval and adult fishes. Fishery Bulletin 74, 1–8.
Campana, S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59, 197–242.
| Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., and Neilson, J. D. (1985). Microstructure of fish otoliths. Canadian Journal of Fisheries and Aquatic Sciences 42, 1014–1032.
| Microstructure of fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Cargnelli, L. M., and Gross, M. R. (1996). The temporal dimension in fish recruitment: birth date, body size, and size-dependent survival in a sunfish (bluegill: Lepomis macrochirus). Canadian Journal of Fisheries and Aquatic Sciences 53, 360–367.
| The temporal dimension in fish recruitment: birth date, body size, and size-dependent survival in a sunfish (bluegill: Lepomis macrochirus).Crossref | GoogleScholarGoogle Scholar |
Cermeño, P., Uriarte, A., De Munguía, A. M., and Morales-Nin, B. (2003). Validation of daily increment formation in otoliths of juvenile and adult European anchovy. Journal of Fish Biology 62, 679–691.
| Validation of daily increment formation in otoliths of juvenile and adult European anchovy.Crossref | GoogleScholarGoogle Scholar |
Cermeño, P., Uriarte, A., Morales-Nin, B., Cotano, U., and Álvarez, P. (2008). Setting up interpretation criteria for ageing juvenile european anchovy otoliths. Scientia Marina 72, 733–742.
Cotano, U., and Álvarez, P. (2003). Growth of young-of-the-year mackerel in the Bay of Biscay. Journal of Fish Biology 62, 1010–1020.
| Growth of young-of-the-year mackerel in the Bay of Biscay.Crossref | GoogleScholarGoogle Scholar |
Cotano, U., Irigoien, X., Etxebeste, E., Álvarez, P., Zarauz, L., Mader, J., and Ferrer, L. (2008). Distribution, growth and survival of anchovy larvae (Engraulis encrasicolus L.) in relation to hydrodynamic and trophic environment in the Bay of Biscay. Journal of Plankton Research 30, 467–481.
| Distribution, growth and survival of anchovy larvae (Engraulis encrasicolus L.) in relation to hydrodynamic and trophic environment in the Bay of Biscay.Crossref | GoogleScholarGoogle Scholar |
Feet, P., Ugland, K. I., and Moksness, E. (2002). Accuracy of age estimates in spring spawning herring (Clupea harengus L.) reared under different prey densities. Fisheries Research 56, 59–67.
| Accuracy of age estimates in spring spawning herring (Clupea harengus L.) reared under different prey densities.Crossref | GoogleScholarGoogle Scholar |
Folkvord, A., Ystanes, L., Johannessen, A., and Moksness, E. (1996). RNA : DNA ratios and growth of herring (Clupea harengus) larvae reared in mesocosms. Marine Biology 126, 591–602.
| RNA : DNA ratios and growth of herring (Clupea harengus) larvae reared in mesocosms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XntFOrurw%3D&md5=7eee7cc5b1e6c86e8ca7ecad5dafc00fCAS |
Folkvord, A., Rukan, K., Johannessen, A., and Moksness, E. (1997). Early life history of herring larvae in contrasting feeding environments determined by otolith microstructure analysis. Journal of Fish Biology 51, 250–263.
| Early life history of herring larvae in contrasting feeding environments determined by otolith microstructure analysis.Crossref | GoogleScholarGoogle Scholar |
Folkvord, A., Blom, G., Johannessen, A., and Moksness, E. (2000). Growth-dependent age estimation in herring (Clupea harengus L.) larvae. Fisheries Research 46, 91–103.
| Growth-dependent age estimation in herring (Clupea harengus L.) larvae.Crossref | GoogleScholarGoogle Scholar |
Fox, C. J., Folkvord, A., and Geffen, A. J. (2003). Otolith micro-increment formation in herring Clupea harengus larvae in relation to growth rate. Marine Ecology Progress Series 264, 83–94.
| Otolith micro-increment formation in herring Clupea harengus larvae in relation to growth rate.Crossref | GoogleScholarGoogle Scholar |
Geffen, A. J. (1982). Otolith ring deposition in relation to growth rate in herring (Clupea harengus) and turbot (Scophthalmus maximus) larvae. Marine Biology 71, 317–326.
| Otolith ring deposition in relation to growth rate in herring (Clupea harengus) and turbot (Scophthalmus maximus) larvae.Crossref | GoogleScholarGoogle Scholar |
Geffen, A. J. (1992). Validation of otolith increment deposition rate. In ‘Otolith Microstructure Examination and Analysis’. (Eds D. K. Stevenson and S. E. Campana.) Canadian Special Publication of Fisheries and Aquatic Sciences 117, pp. 101–113, Ottawa, ON, Canada.
Henderson, P. A., Holmes, R. H. A., and Bamber, R. N. (1988). Size-selective overwintering mortality in the sand smelt, Atherina boyeri Risso, and its role in population regulation. Journal of Fish Biology 33, 221–233.
| Size-selective overwintering mortality in the sand smelt, Atherina boyeri Risso, and its role in population regulation.Crossref | GoogleScholarGoogle Scholar |
Høie, H., and Folkvord, A. (2006). Estimating the timing of growth rings in Atlantic cod otoliths using stable oxygen isotopes. Journal of Fish Biology 68, 826–837.
| Estimating the timing of growth rings in Atlantic cod otoliths using stable oxygen isotopes.Crossref | GoogleScholarGoogle Scholar |
Høie, H., Millner, R. S., McCully, S., Nedreaas, K. H., Pilling, G. M., and Skada, J. (2009). Latitudinal differences in the timing of otolith growth: a comparison between the Barents Sea and southern North Sea. Fisheries Research 96, 319–322.
| Latitudinal differences in the timing of otolith growth: a comparison between the Barents Sea and southern North Sea.Crossref | GoogleScholarGoogle Scholar |
Hüssy, K., Hinrichsen, H.-H., Fey, D. P., and Velasco, A. (2010). The use of otolith microstructure to estimate age in adult Atlantic cod Gadus morhua. Journal of Fish Biology 76, 1640–1654.
| The use of otolith microstructure to estimate age in adult Atlantic cod Gadus morhua.Crossref | GoogleScholarGoogle Scholar | 20557621PubMed |
ICES (2009). Report of the Workshop on Age reading of European anchovy. ICES C.M. 2009/ACOM:43. International Council for the Exploration of the Sea, Copenhagen.
ICES (2012). Report of the Working Group on Acoustic and Egg Surveys for Sardine and Anchovy in ICES Areas VIII and IX.0 ICES C.M. 2012/SSGESST:16. International Council for the Exploration of the Sea, Copenhagen.
Ivarjord, T., Pedersen, T., and Moksness, E. (2008). Effects of growth rates on the otolith increments deposition rate in capelin larvae (Mallotus villosus). Journal of Experimental Marine Biology and Ecology 358, 170–177.
| Effects of growth rates on the otolith increments deposition rate in capelin larvae (Mallotus villosus).Crossref | GoogleScholarGoogle Scholar |
Johnson, J. B., and Belk, M. C. (2004). Temperate Utah chub form valid otolith annuli in the absence of fluctuating water temperature. Journal of Fish Biology 65, 293–298.
| Temperate Utah chub form valid otolith annuli in the absence of fluctuating water temperature.Crossref | GoogleScholarGoogle Scholar |
Johnson, T. B., and Evans, D. O. (1991). Behaviour, energetics, and associated mortality of young-of-the-year white perch (Morone americana) and yellow perch (Perca flavescens) under simulated winter conditions. Canadian Journal of Fisheries and Aquatic Sciences 48, 672–680.
| Behaviour, energetics, and associated mortality of young-of-the-year white perch (Morone americana) and yellow perch (Perca flavescens) under simulated winter conditions.Crossref | GoogleScholarGoogle Scholar |
Junquera, S., and Perez-Gándaras, G. (1993). Population diversity in Bay of Biscay anchovy (Engraulis encrasicolus L. 1758) as revealed by multivariate analysis of morphometric and meristic characters. ICES Journal of Marine Science 50, 383–391.
| Population diversity in Bay of Biscay anchovy (Engraulis encrasicolus L. 1758) as revealed by multivariate analysis of morphometric and meristic characters.Crossref | GoogleScholarGoogle Scholar |
La Mesa, M. (2007). The utility of otolith microstructure in determining the timing and position of the first annulus in juvenile Antarctic toothfish (Dissostichus mawsoni) from the South Shetland Islands. Polar Biology 30, 1219–1226.
| The utility of otolith microstructure in determining the timing and position of the first annulus in juvenile Antarctic toothfish (Dissostichus mawsoni) from the South Shetland Islands.Crossref | GoogleScholarGoogle Scholar |
Le Cren, E. D. (1951). The length–weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). Journal of Animal Ecology 20, 201–219.
| The length–weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis).Crossref | GoogleScholarGoogle Scholar |
Miller, T. J., Crowder, L. B., Rice, J. A., and Marschall, E. A. (1988). Larval size and recruitment mechanisms in fishes: toward a conceptual framework. Canadian Journal of Fisheries and Aquatic Sciences 45, 1657–1670.
| Larval size and recruitment mechanisms in fishes: toward a conceptual framework.Crossref | GoogleScholarGoogle Scholar |
Morales-Nin, B., Torres, G. J., Lombarte, A., and Recasens, L. (1998). Otolith growth and age estimation in the European hake. Journal of Fish Biology 53, 1155–1168.
| Otolith growth and age estimation in the European hake.Crossref | GoogleScholarGoogle Scholar |
Motos, L. (1996). Reproductive biology and fecundity of the Bay of Biscay anchovy population (Engraulis encrasicolus L.). Scientia Marina 60, 195–207.
Namiki, S., Tanaka, H., Katayama, S., Funaki, O., Aoki, I., and Oozeki, Y. (2010). Validation of daily increment formation in otoliths of immature and adult Japanese anchovy Engraulis japonicus. Fisheries Science 76, 951–959.
| Validation of daily increment formation in otoliths of immature and adult Japanese anchovy Engraulis japonicus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlygsr7N&md5=ce107ecbf28c4b8e80154c4a1b176f2dCAS |
Neat, F. C., Wright, P. J., and Fryer, R. J. (2008). Temperature effects on otolith pattern formation in Atlantic cod Gadus morhua. Journal of Fish Biology 73, 2527–2541.
| Temperature effects on otolith pattern formation in Atlantic cod Gadus morhua.Crossref | GoogleScholarGoogle Scholar |
Pannella, G. (1974). Otolith growth patterns: an aid in age determination in temperate and tropical fishes. In ‘Ageing of Fish’. (Ed. T. B. Bagenal.) pp. 28–39. (The Gresham Press: Old Woking, UK.)
Pannella, G. (1980). Growth patterns in fish sagittae. In ‘Skeletal Growth of Aquatic Organisms’. (Eds D. C. Rhoads and R. A. Lutz.) pp. 519–560. (Plenum Press: New York.)
Paul, L. J., and Horn, P. L. (2009). Age and growth of sea perch (Helicolenus percoides) from two adjacent areas off the east coast of South Island, New Zealand. Fisheries Research 95, 169–180.
| Age and growth of sea perch (Helicolenus percoides) from two adjacent areas off the east coast of South Island, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Pilling, G. M., Millner, R. S., Easey, M. W., Maxwell, D. L., and Tidd, A. N. (2007). Phenology and North Sea cod Gadus morhua L.: has climate change affected otolith annulus formation and growth? Journal of Fish Biology 70, 584–599.
| Phenology and North Sea cod Gadus morhua L.: has climate change affected otolith annulus formation and growth?Crossref | GoogleScholarGoogle Scholar |
Rice, J. A., Miller, T. J., Rose, K. A., Crowder, L. B., Marschall, E. A., Trebitz, A. S., and DeAngelis, D. L. (1993). Growth rate variation and larval survival: inferences from an individual-based size-dependet predtion model. Canadian Journal of Fisheries and Aquatic Sciences 50, 133–142.
| Growth rate variation and larval survival: inferences from an individual-based size-dependet predtion model.Crossref | GoogleScholarGoogle Scholar |
Schindler, D. E. (1999). Migration strategies of young fishes under temporal constraints: the effect of size-dependent overwinter mortality. Canadian Journal of Fisheries and Aquatic Sciences 56, 61–70.
| Migration strategies of young fishes under temporal constraints: the effect of size-dependent overwinter mortality.Crossref | GoogleScholarGoogle Scholar |
Schramm, H. L. (1989). Formation of annuli in otoliths of bluegills. Transactions of the American Fisheries Society 118, 546–555.
| Formation of annuli in otoliths of bluegills.Crossref | GoogleScholarGoogle Scholar |
Sequeira, V., Neves, A., Vieira, A. R., Figueiredo, I., and Gordo, L. S. (2009). Age and growth of bluemouth, Helicolenus dactylopterus, from the Portuguese continental slope. ICES Journal of Marine Science 66, 524–531.
| Age and growth of bluemouth, Helicolenus dactylopterus, from the Portuguese continental slope.Crossref | GoogleScholarGoogle Scholar |
Sogard, S. M. (1997). Size-selective mortality in the juvenile stage of teleost fishes: a review. Bulletin of Marine Science 60, 1129–1157.
Tudela, S., and Palomera, I. (1995). Diel feeding intensity and daily ration in the anchovy Engraulis encrasicolus in the northwest Mediterranean Sea during the spawning period. Marine Ecology Progress Series 129, 55–61.
| Diel feeding intensity and daily ration in the anchovy Engraulis encrasicolus in the northwest Mediterranean Sea during the spawning period.Crossref | GoogleScholarGoogle Scholar |
Victor, B. C., and Brothers, E. B. (1982). Age and growth of the fallfish Semotilus corporalis with daily otolith increments as a method of annulus verification. Canadian Journal of Zoology 60, 2543–2550.
| Age and growth of the fallfish Semotilus corporalis with daily otolith increments as a method of annulus verification.Crossref | GoogleScholarGoogle Scholar |
Waldron, M. (1994). Validation of annuli of the South African anchovy, Engraulis capensis, using daily otolith growth increments. ICES Journal of Marine Science 51, 233–234.
| Validation of annuli of the South African anchovy, Engraulis capensis, using daily otolith growth increments.Crossref | GoogleScholarGoogle Scholar |
Williams, A. J., Davies, C. R., and Mapstone, B. D. (2005). Variations in the periodicity and timing of increment formation in red throat emperor (Lethrinus miniatus) otoliths. Marine and Freshwater Research 56, 529–538.
| Variations in the periodicity and timing of increment formation in red throat emperor (Lethrinus miniatus) otoliths.Crossref | GoogleScholarGoogle Scholar |
Woodroffe, D. A., Wright, P. J., and Gordon, J. D. M. (2003). Verification of annual increment formation in the white anglerfish, Lophius piscatorius using the illicia and sagitta otoliths. Fisheries Research 60, 345–356.
| Verification of annual increment formation in the white anglerfish, Lophius piscatorius using the illicia and sagitta otoliths.Crossref | GoogleScholarGoogle Scholar |
Wright, P. J., Talbot, C., and Thorpe, J. E. (1992). Otolith calcification in Atlantic salmon parr, Salmo salar L., and its relation to photoperiod and calcium metabolism. Journal of Fish Biology 40, 779–790.
| Otolith calcification in Atlantic salmon parr, Salmo salar L., and its relation to photoperiod and calcium metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XksVGjtr8%3D&md5=c7eb89b6cbb893e52605d1951069daf2CAS |
Wright, P. J., Woodroffe, D. A., Gibb, F. M., and Gordo, J. D. M. (2002). Verification of first annulus formation in the illiaca and otoliths of white anglerfish, Lophius piscatorius using otolith microstructure. ICES Journal of Marine Science 59, 587–593.
| Verification of first annulus formation in the illiaca and otoliths of white anglerfish, Lophius piscatorius using otolith microstructure.Crossref | GoogleScholarGoogle Scholar |
Xie, S., and Watanabe, Y. (2005). Hatch date-dependent differences in early growth and development recorded in the otolith microstructure of Trachurus japonicus. Journal of Fish Biology 66, 1720–1734.
| Hatch date-dependent differences in early growth and development recorded in the otolith microstructure of Trachurus japonicus.Crossref | GoogleScholarGoogle Scholar |


