Phosphorus dynamics in sediments of a eutrophic lake derived from 31P nuclear magnetic resonance spectroscopy
Deniz Özkundakci A D , David P. Hamilton A , Richard McDowell B and Stefan Hill CA Environmental Research Institute, University of Waikato, Private Bag 3105, Hamilton 3240, New Zealand.
B AgResearch, Invermay Agricultural Centre, Private Bag 50034, Mosgiel, New Zealand.
C Scion Research, Te Papa Tipu Innovation Park, 49 Sala Street, Private Bag 3020, Rotorua, New Zealand.
D Corresponding author. Email: d.ozkundakci@gmail.com
Marine and Freshwater Research 65(1) 70-80 https://doi.org/10.1071/MF13033
Submitted: 4 February 2013 Accepted: 19 June 2013 Published: 18 September 2013
Abstract
The determination of organic phosphorus (P) compounds in lake sediments can provide information on the potential for internal P loading. Settling seston and vertical sediment core samples from highly eutrophic Lake Okaro, New Zealand, were collected during a mixed winter and stratified summer period, representing, respectively, when the water column was well oxygenated and when the bottom waters were anoxic. Samples were analysed with 31P nuclear magnetic resonance (31P NMR) spectroscopy, which showed that both bottom sediments and settling seston contained orthophosphate, orthophosphate monoesters and diesters, pyrophosphates, polyphosphates and phosphonates. Phosphorus concentration in settling seston increased ~2.5-fold in winter as a result of seasonally induced changes in phytoplankton biomass, with a marked increase in the concentration of orthophosphate. Several potentially bioavailable P compounds in the bottom sediments were identified that were likely to contribute to recycling of P from the sediment to the water column. An ‘apparent half-life’ was used to quantify the time scales on which these compounds were recycled to the overlying water column. Orthophosphate monoesters that include inositol phosphates were the most persistent P compound. On the basis of half-lives, high internal P loadings may persist for more than 20 years, potentially hindering restoration efforts in Lake Okaro.
Additional keywords: internal loading, Lake Okaro, sedimentation, temporal variability.
Introduction
Phosphorus (P) has long been recognised as a key nutrient in many aquatic ecosystems because of its potential to limit primary productivity (Vollenweider 1976; Carpenter 2008). In addition to contributions from surface and ground water and, to a lesser extent, atmospheric deposition (Reed-Andersen et al. 2000), bottom sediments are a key source of P, especially in eutrophic lakes (Søndergaard et al. 2003). Whereas the interactions among physical, chemical and biological processes influencing sediment P concentrations have been intensively investigated (Smolders et al. 2006), few studies have investigated the compounds and the reactivity of organic P compounds in lake sediments (e.g. Hupfer et al. 2004; Ahlgren et al. 2005; Shinohara et al. 2012).
Since the first use of 31P nuclear magnetic resonance (NMR) to investigate organic P extracted from New Zealand grassland soils in 1980 (Newman and Tate 1980), this method has become a valuable tool to determine either specific compounds (e.g. orthophosphate) or differentiate organic P-compound classes (e.g. orthophosphate monoesters or diesters) in soils and sediments worldwide (Cade-Menun 2005). Although several studies have discussed organic P groups in agricultural soils (e.g. McDowell et al. 2005), fewer studies have examined speciation of organic P in marine sediments and fewer still in freshwater lake sediments (Cade-Menun 2005). Lake sediment studies have identified several classes of organic P compounds similar to those found in terrestrial soils, including orthophosphate monoesters, orthophosphate diesters and polyphosphates. The relative proportion of these compounds varies, however, suggesting variations among sediment types and prevailing environmental conditions (Hupfer et al. 1995; Ahlgren et al. 2006).
Differences among the proportions of organic P-compound classes in lake sediments have been related to the oxidation–reduction status of the sediments. Carman et al. (2000) described the presence of pyro- and polyphosphates in oxic surface sediments, noting that these compounds were absent in anoxic conditions. Similarly, McDowell (2009) found more bioavailable P-compound classes, particularly orthophosphate diesters, in dry (oxic) sediment than in wet (anoxic) stream sediments of agricultural catchments. Changes in redox chemistry appear to play an important role in the diagenesis of phosphate diesters (Ingall and Jahnke 1994). Very little work has used the added insight available by 31P NMR to further understanding of P compounds and compound classes for both settling seston and bottom sediments of lakes (Shinohara et al. 2012).
In New Zealand, water quality of many of the Te Arawa lakes of the Rotorua region has declined significantly with development of surrounding catchments for pastoral agriculture (Hamilton 2005; Paul et al. 2012). Several of the lakes have high internal nutrient loadings as a result of organic enrichment of the bottom sediments and remobilisation of bottom-sediment nutrients with eutrophication-driven depletion of dissolved oxygen in bottom waters (White et al. 1978; Burger et al. 2007; Trolle et al. 2008). Lake Okaro, the focus site of the present study, is the most eutrophic of the Te Arawa lakes. It is a monomictic lake with a hypolimnion that becomes anoxic approximately 1 month after the onset of seasonal stratification, which lasts for approximately 9 months (Özkundakci et al. 2010). The concentration of P compounds in settling material in lakes can vary substantially with season, and therefore may have an important role in changing the composition of bottom sediments (Pettersson 2001). For the present study, we chose periods of seasonal stratification, when bottom waters were anoxic, as well as periods of mixing, when the entire water column was well oxygenated, to test for the effects of seasonal variations caused by mixing patterns and redox status on speciation of sediment P.
We hypothesised that sediment organic P is an important source of bioavailable P in Lake Okaro. Because seasonal changes in the water column of Lake Okaro (oxic and anoxic hypolimnion) could lead to changes in rates of P sedimentation and mobilisation, we further hypothesised that the supply and bioavailability of the organic P would change with season. To test these hypotheses, we used 31P NMR to determine the P compounds and compound classes in settling seston and at different depths and in different seasons in the bottom sediments of Lake Okaro.
Materials and methods
Study site
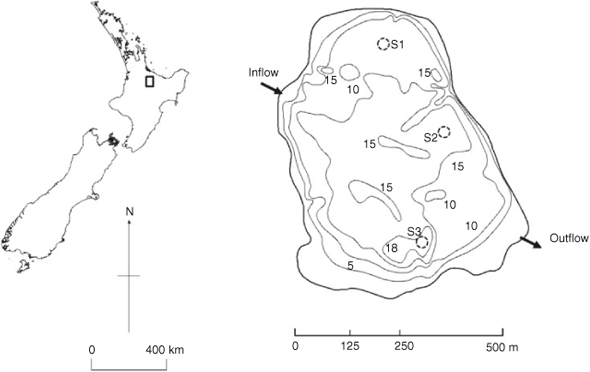
Lake Okaro is a small lake (0.32 km2) with a maximum depth of 18 m (Fig. 1). The catchment land-use area (3.89 km2) is now mostly in dairy farming. The lake has been eutrophic since the early 1960s (Jolly 1977), with regular cyanobacterial blooms and an anoxic hypolimnion during summer stratification (Forsyth et al. 1988; Paul et al. 2008). Two unnamed streams enter the lake from the north-west. In 2006, these streams were diverted through a 2.3-ha constructed wetland as part of a lake restoration strategy (Tanner et al. 2007). Further, on 16 and 17 December 2003, 13 m3 of alum, equivalent to a dose rate of 0.6 g aluminium m–3 in the epilimnion, was applied to the lake to attempt to control internal P loads (Paul et al. 2008). In September 2007, 110 t of aluminium-modified zeolite (Z2G1, Blue Pacific Minerals, Tokoroa, New Zealand) was applied to Lake Okaro to act as a sediment capping agent to further decrease internal loading of P (Özkundakci et al. 2010).
|
Sedimentation rates
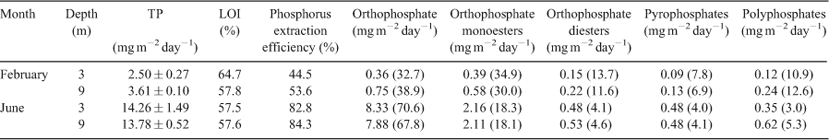
Sedimentation rates of seston in Lake Okaro were measured with cylindrical sediment traps (PVC, 0.065 m in diameter, 0.65 m in height). Traps were placed at 3- and 9-m depths, deployed at three sites where bottom depths were 11 m (Site S1), 14 m (Site S2) and 18 m (Site S3) (Fig. 1). Traps were deployed when the water column was thermally stratified between 26 January 2007 and 28 February 2007 and fully mixed between 23 May and 22 June 2007. No inhibitors or preservatives were added a priori to the traps; so, P compounds analysed in the study potentially include the effects from degradation over the deployment period. At each trapping depth, four replicate traps were supported by a wooden frame and attached to a central rope suspended vertically in the water column between a bottom anchor and a subsurface buoy (1-m depth). Replicate traps were separated by a distance of at least three times the trap diameter (0.065 m) to minimise effects of trap interactions on water movement and sedimentation. Once retrieved, traps were placed in a chilled container for 30 min before the supernatant was siphoned off and the remaining ~500 mL of fluid was capped and placed on ice for return to the laboratory. This material was stirred immediately on return to the laboratory and subsamples were taken for analysis of total P (TP) and total particulate matter (TPM). Samples for the analysis of TP were first digested using persulphate (Ebina et al. 1983), followed by analysis as molybdate-reactive P on a Lachat QuickChem Flow Injection Analyzer (FIA+ 8000 Series, Zellweger Analytics, Univeristy of Waikato, New Zealand). Subsamples were filtered through pre-weighed Whatman GF/C filters (GE Healthcare, Australia), which were then dried at 105°C for 24 h to determine TPM, and then combusted at 550°C for 4 h to determine loss on ignition (LOI). Subsamples from trapped material were taken from each depth at Site S2 for 31P NMR analysis, on each sampling occasion. Because of the expense of conducting 31P NMR, P speciation of settling seston was conducted only at Site S2.
Sediment sampling
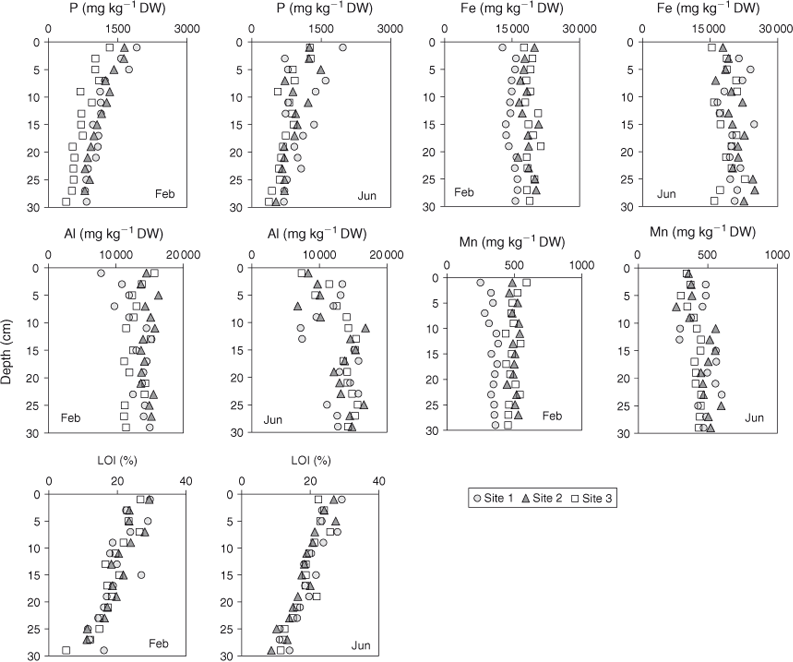
An undisturbed sediment core was collected with a gravity corer from each of the three sampling sites (S1, S2 and S3; Fig. 1) on 28 February 2007 (stratified water column) and 22 June 2007 (mixed water column). In the field, sediment cores were sliced into 2-cm intervals down to 30-cm depth, and stored on ice in 50-mL plastic tubes until return to the laboratory where each tube was centrifuged for 40 min at 66.6 Hz to separate pore waters from solid material. Sediment dry weight (DW) was measured by drying sediment samples at 105°C over ~24 h or until constant weight was obtained. Organic content was measured as LOI, by combusting the dried sample for 4 h at 550°C. Analysis of TP, iron (Fe), manganese (Mn) and aluminium (Al) was carried out on aqua regia digests of dried sediment samples that had been homogenised by grinding beforehand, following the procedure of Trolle et al. (2008). Sediment core samples from Site S2 were sliced horizontally at 2-cm intervals down to 10 cm for 31P NMR analysis.
31P NMR analysis
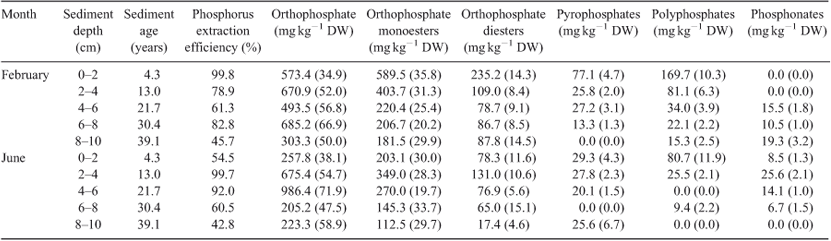
A subsample from bottom sediments and seston of ~10-g wet weight was shaken with 30 mL of 0.25 M NaOH + 0.05 M Na2-EDTA (1 : 15 dry sediment : extractant ratio) for 16 h (the subsample of sediment-trap material consisted of pooled samples from four replicate sediment traps; Cade-Menun 1996). Samples were then centrifuged (15 000 rpm) before filtering supernatant through Whatman GF/C filters. Subsamples of the extract were analysed by inductively coupled plasma–mass spectrometry (ICP–MS) for TP. Each extract was pre-concentrated (~10-fold) by rotary evaporation (Büchi Rotavopor R110, BUCHI Labortechnik AG, Flawil, Switzerland) at 30°C and then frozen. This process has been shown to yield a wider range of P compounds and compound classes than the resuspension and analysis of freeze-dried samples (McDowell 2003; Hupfer et al. 2004). Prior to analysis, samples (3 mL) were centrifuged at 66.6 Hz, transferred to a NMR (5-mm-diameter) tube and 1 mL of deuterium oxide (D2O) was added to the samples to obtain a stable signal lock. The 31P NMR spectra were obtained on solutions at a frequency of 161.97 MHz on a Bruker Avance DPX 400 spectrometer fitted with a 5-mm dual inverse board-band (H-X) probe (Bruker, Billerica, MA, USA). An identical number of scans (10 000) was used for each sample by using a pulse angle of 90° with a pulse delay of 5 s and an acquisition time of 0.34 s. The delay between pulses was sufficient to exceed five times the spin-lattice relaxation time (T1), as examined by an inversion recovery sequence, thereby generating quantitative spectra (McDowell et al. 2006). Chemical shifts were recorded relative to an external phosphoric acid standard (δ = 0 ppm). Spectra were processed with a 10-Hz exponential line broadening by using Mestre-C software (V. 2.3a, Santiago de Compostela, Spain). Peak areas were calculated by integration on processed spectra and, subsequently, grouped into distinct P compounds (orthophosphate, pyrophosphate and polyphosphate) or compound classes (orthophosphate monoesters and diesters and phosphonates) by using peak assignments derived from the literature (Cade-Menun 2005; Hupfer et al. 2004; Ahlgren et al. 2006). Peak assignments (chemical shifts) to identify P compounds and compound classes were 19.58 ppm for phosphonates, 6.68–5.20 ppm for orthophosphate, 5.21–3.37 ppm for orthophosphate monoesters, 3.34 to –3.43 ppm for orthophosphate diesters, –3.87 to –4.97 ppm for pyrophosphates, and –18.85 to –21.67 ppm for polyphosphates. The concentration of each P compound group was calculated via the percentage of the total area under each peak occupied in the spectra and the TP in the original extract.
Data analysis
Sedimentation rates of TP and TPM from traps were examined for spatial (i.e. among sites and between depths) and temporal (i.e. between sampling times) differences by using a one-way ANOVA with post hoc analysis using a Newman–Keuls test at a confidence interval of 95%. Before analysis, data were first tested for normality and log-transformed if necessary.
Statistical relationships of the elemental composition of lake bed sediments were tested among TP, Fe, Mn, Al and LOI by using Pearson correlation coefficients.
The decay of P compounds and compound classes was modelled as P(t) = P0 × e–kt, where P(t) is the amount of the P compound that has not yet decayed at time t, P0 is the initial amount of the P compound that will decay and k is the decay constant (year–1). Half-lives (τ) of P compounds in sediments were then calculated as T1/2 = ln(2)/k (Reitzel et al. 2007). Half-lives were determined from the slope of a significant (P < 0.05) regression fit to the exponential decay of P concentration with sediment profile depth, using averaged values of the corresponding 2-cm intervals between February and June 2007 to account for potential short-term variations in half-lives. Sediment age for each 2-cm layer was calculated according to the net sedimentation rate in Lake Okaro (Trolle et al. 2008). They derived sedimentation rates from a volcanic eruption of nearby Mount Tarawera in 1886, which dispersed volcanic ash over much of the Rotorua district, including Lake Okaro, and provided a well delineated tephra in the lake sediments. Therefore, the net sedimentation rate (2.3 mm year–1, Trolle et al. 2008) for the lake represents averages for the entire period 1886–2007, but is likely to have varied within this period, especially between pre- and post-1960, when the lake has undergone eutrophication (Forsyth et al. 1988). The upper 10 cm of the sediment (the focus of this part of the analysis), however, is assumed to represent a time period during which the lake was highly eutrophic, with no significant land-use change in the catchment, and may therefore reflect sediment deposition of relatively consistent sedimentation rates.
Results
Sedimentation rates
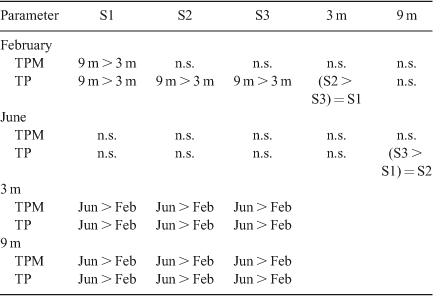
Mean sedimentation rates of TP across the three sites for the period from 26 January 2007 to 28 February 2007, when the water column was stratified, were 2.06 mg m–2 day–1 at 3-m depth and 3.52 mg m–2 day–1 at 9 m (Fig. 2). Rates were approximately five-fold greater from 23 May 2007 to 22 June 2007, when the water column was mixed, at 14.30 mg m–2 day–1 and 14.26 mg m–2 day–1 at 3- and 9-m depths, respectively. Rates of sedimentation of TPM and TP for individual stations at 3 and 9 m were, in several instances, significantly (P < 0.05) lower for the stratified period than for the mixed period (Table 1). Differences in sedimentation rates among sites and between depths occurred less often than between the two time periods of June and February (Table 1) corresponding to thermally mixed and stratified conditions, respectively. Sedimentation rates of both TP and TPM were significantly (P < 0.05) greater at 9-m depth than at 3 m during the stratified period but not during the mixed period. The sedimentation rate of TPM averaged across sites during stratification was 0.56 mg m–2 day–1 at 3 m and 0.80 mg m–2 day–1 at 9 m compared with 1.40 mg m–2 day–1 at 3 m and 1.42 mg m–2 day–1 at 9 m during mixing (Table 1). On average, LOI constituted 61% of TPM across all sites and depths during stratification and 56% of TPM during mixing (Fig. 2).
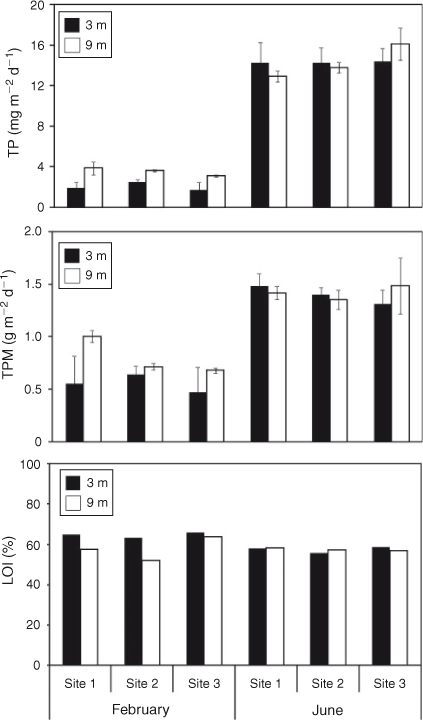
|
|
Vertical sediment profiles
Phosphorus concentrations in bottom-sediment slices at all three sites and both sampling occasions ranged from 1965 mg P kg–1 DW (0–2 cm, S1, mixed period) to 366 mg P kg–1 DW (28–30 cm, S3, mixed period; Fig. 3). Generally, P concentrations in the sediment decreased with depth. Across all samples, concentrations of Fe, Mn and Al in sediment slices ranged from 24 901 to 11 208 mg Fe kg–1 DW, from 600 to 244 mg Mn kg–1 DW, and from 16 833 to 6711 mg Al kg–1 DW, respectively. LOI generally decreased with depth and ranged from 29.5% (0–2 cm, S1, stratified period) to 5.1% (28–30 cm, S1, stratified period). The concentration of TP was significantly correlated with LOI on the basis of data for all slices (S1: r = 0.71; S2: r = 0.82; S3: r = 0.81; P < 0.001). However, sediment P was correlated with Fe only at S2 (r = 0.45; P ≤ 0.05) and showed no correlation with either Al or Mn (P > 0.05).
31P NMR analysis
Eight different P compounds or compound classes were identified in the bottom sediment extracts at S2; however, only seven were found in the sediment trap samples at S2 (Fig. 4). The eight P compounds included phosphonates, orthophosphate, orthophosphate monoesters, pyrophosphates, polyphosphates, and three different compounds in the orthophosphate diester region. The latter compounds were suggested by Ahlgren et al. (2006) and others to be DNA P (–0.83 ppm), microbial P lipids (from 2.66 to –0.15 ppm) and teichoic acid P (2.97 ppm).

|
The total extracted P using NaOH + EDTA was compared to the extracted TP using aqua regia and the difference is referred to as extraction efficiency. The average percentage of TP extracted from the bottom sediments was 75%, but extraction of TP appeared to become less efficient with depth (Table 2). Orthophosphate was the most abundant P compound in the bottom sediment extracts from S2, contributing up to 71.9% of the total extracted P, whereas orthophosphate monoesters contributed up to 37.2%, orthophosphate diesters up to 21.7%, polyphosphates up to 11.9%, pyrophosphates up to 6.7% and phosphonates only as much as 3.2%, on average, of the total P extracted. Orthophosphate concentrations generally increased with increasing depth, whereas all other identified P compounds or classes decreased with depth.
The signal to noise ratio of the 31P NMR signal was 133 in the mixed period (June 2007), which was lower than in the stratified period (February 2007), when it was 148. During both periods, the signal to noise ratio was mostly lower in the surface sediments, with less well developed peaks for microbial-P lipids and teichoic-acid P in particular. Although polyphosphates were present throughout the profile in the stratified period, this group was not observed in sediment layers of 4–6 and 8–10 cm in June 2007. Because of the low concentrations of phosphonates through the profiles, it is not possible to distinguish whether differences between sampling days were due to the limitations in analytical sensitivity or a distinct change with time.
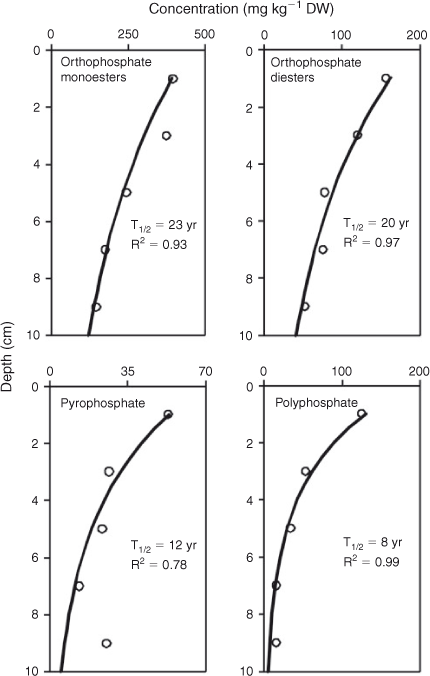
The model used to determine the decay of P compounds and compound classes resulted in a significant function for orthophosphate monoesters, orthophosphate diesters, pyrophosphates and polyphosphates (Fig. 5). These functions were used to calculate the half-lives for the different P compounds and compound classes, which ranged from 8 to 23 years, with the half-life being longest for orthophosphate monoesters and shortest for polyphosphates.
|
The mean extraction efficiency across all depths in the sediment trap material increased from 50% in February 2007 to 83% in June 2007 (Table 3). The mean percentage of orthophosphate in the sediment-trap extract was 35% across all depths in February and 69% in June, whereas other P compound classes decreased.
Discussion
Sedimentation and supply of P to sediments
Sedimentation rates of TPM in Lake Okaro were within the same range as those observed in other eutrophic lakes (Koski-Vähälä et al. 2000; Chalar and Tundisi 2001; Pettersson 2001). Rates were greatest during mixing when the P concentration of TPM increased ~2.5-fold, whereas the concentration of organic matter in the trapped material varied little between the mixed and stratified periods. On average, particulate matter in traps in Lake Okaro was enriched in P (7.1 mg TP g–1 TPM) compared with that in other eutrophic lakes (Pettersson 2001, 1.3–5.8 mg TP g–1 TPM). Seasonality of sedimentation rates can be strongly influenced by the discharge of the inflows to the lake (Fuentes et al. 2013). Precipitation in the Rotorua Region is highly seasonal, with a total amount of 167 and 47 mm, respectively, during the mixed (i.e. June) and stratified (February) sediment trap deployment period. The resulting increase in inflow discharge to Lake Okaro during winter may explain the increase in sedimentation rates of TPM. A disproportional increase in TP content of settling seston suggests high mobilisation rates of particulate P through erosion during high-flow events, as has been observed in other streams in the same region (Abell et al. 2013), and/or seasonality of in-lake phytoplankton biomass (Paul et al. 2008). The continuous supply of P-rich organic material to the surficial sediments and its resulting decomposition may enhance sediment release of P with the prolonged periods of anoxia in Lake Okaro, but will also be dependent on the bioavailability of P forms in seston (Søndergaard et al. 2003; Smolders et al. 2006).
Forms and bioavailability of phosphorus detected by 31P NMR in sediment
Central to our hypothesis that organic P compounds may regulate internal P loading in Lake Okaro is that the sediment contains and/or produces bioavailable organic P compounds. Most 31P NMR studies have found that organic P compounds such as orthophosphate diesters are more bioavailable than are orthophosphate monoesters (e.g. McDowell and Stewart 2005; Turner et al. 2005). To further examine the bioavailability, we also determined half-lives for each P compound and compound class detected by 31P NMR. Half-lives could not be established for orthophosphate because there was no significant trend in concentration with sediment depth, presumably because there is a balance of its consumption and production in the bottom sediments. The apparent half-lives calculated in the present study should be interpreted as indicative, representing the net effect of concurrent P decay and generation processes for one site in the lake.
The P compounds and compound classes detected in the sediments of Lake Okaro, with the exception of phosphonates, have also been detected in sediments of other eutrophic lakes (Reitzel et al. 2006). The relative proportions of each are mostly comparable to those in less eutrophic lakes (Ahlgren et al. 2005; Reitzel et al. 2007). However, care should be taken when making direct comparisons among different studies because of differences in the treatment of the sample, extractants, spectrometers, and whether or not delay times between pulses were sufficient to exceed T1 and generate quantitative spectra (Cade-Menun 2005; McDowell et al. 2006). Although the extractant used for preparation of 31P NMR sediment material in our study was similar to those used in other studies (Ahlgren et al. 2005; Turner et al. 2005; Reitzel et al. 2007), any extraction procedure for organic P is likely to induce some hydrolysis (Cade-Menun 2005). The combination of NaOH + EDTA appears to be the most universally accepted extraction technique and decreases degradation of polyphosphates as a result of the complexation of free metals with EDTA (Turner et al. 2003, 2005). However, pH stress of the alkaline extraction procedure can alter extracted compounds from their original form (McDowell and Stewart 2005). For example, altering the pH of lake water samples, or soil extracts to >13 has been shown to decrease the proportion of phosphonates, orthophosphate diesters and polyphosphates through hydrolysis or precipitation (McDowell and Stewart 2005). Hence, it is possible that some of these compound classes may be under-represented in our NMR spectra, whereas orthophosphate and orthophosphate monoesters may be over-represented.
Orthophosphate monoesters are usually the major organic P compound in soils and sediments (Turner et al. 2003) and were anticipated to be the most abundant in Lake Okaro. This group comprises several compounds with varying lability, of which the inositol phosphates (phytate) have often been found to be the most recalcitrant and are therefore usually dominant (DeGroot and Golterman 1993). However, Doolette et al. (2011) argued that the abundance of phytate in soil extracts can often be overestimated in the presence of broad humic P signals in the monoester region and that phytate is far less stable than was previously assumed. The expanded view of the orthophosphate monoester region in Fig. 4 shows four distinct resonances, which have previously been identified as phytate (Cade-Menun 2005; Turner et al. 2005); however, we were unable to quantify the abundance of phytate directly. It is likely that other P forms within monoesters were dominant in the present study, which is reflected in our half-life value of 23 years, being longer than for all other P compounds and compound classes. Given the presence and potential lability, phytates may provide a continuous low-level source of internal P loading in Lake Okaro during prolonged periods of anoxia of bottom waters in this lake (Özkundakci et al. 2010).
Orthophosphate diesters derived from soil organic P tend to be more labile and readily mineralised than are monoesters (Makarov et al. 2002). However, the apparent half-life of orthophosphate diesters found in our study was relatively long at 20 years, and similar to what has been found in other studies (Ahlgren et al. 2005). The presence of two orthophosphate diester compounds, phospholipids and techoic acids, may indicate low rates of microbial activity (Makarov et al. 2002), which may partially explain the relatively long half-life of the diesters. The greatest proportion of orthophosphate diesters was occupied by DNA-P, which arises mostly from bacterial DNA and to some extent decomposing phytoplankton and has been found to have a half-life of less than a decade (Ahlgren et al. 2006). Given the high decay rate of this major constituent of a comparably large P pool in the sediment, DNA-P may be an important contribution to internal P loading relative to monoesters at 23 years (Ahlgren et al. 2005).
Pyrophosphate, a short-chain version of polyphosphates, is bioavailable and can support the growth of heterotrophic bacteria from different environments, even in orthophosphate-rich environments (Liu et al. 1982; Sundareshwar et al. 2001). We found an apparent half-life for pyrophosphate of 12 years, which is similar to the 13-year half-life of pyrophosphate found in mesotrophic Lake Erken by Ahlgren et al. (2005), but much longer than the 3-year half-life found for sediments from the Baltic Sea (Ahlgren et al. 2006). There may be several possible reasons for the longer half-life in our study and in Ahlgren et al. (2005) than in deep-sea sediment and it could be related to low concentrations or activity of specific pyrophosphatase enzymes and high mineralisation rates in seasonally anoxic hypolimnia of lakes that could cause degradation of substantial fractions of labile P in the settling seston before it reaches the sediment surface.
Phosphonates (direct C–P bond) were detected in the bottom sediment of Lake Okaro. These compounds are thought to be a by-product of protozoan metabolism, which is common to almost all aquatic habitats with high primary productivity (Carman et al. 2000; Ahlgren et al. 2005). They also accumulate in a wide range of soils as a result of bacterial activity (Tate and Newman 1982; Hawkes et al. 1984). Phosphonates are considered to be very stable compounds that are not readily hydrolysed even after long exposure to acidic and alkaline conditions (Ahlgren et al. 2006). Hence, their role in the internal cycling of P within lakes is thought to be minimal.
The half-life of polyphosphates was only 8 years. Polyphosphates contribute significantly to P-release following diagenesis, at rates comparable to P-release from iron oxyhydroxides (Hupfer et al. 2004). The short half-life of polyphosphates found in our study suggests that they may play a significant role in internal P loading in Lake Okaro. These results are not consistent with those of Kenney et al. (2001) who considered that polyphosphates are not geochemically reactive.
Vertical sediment profiles
The interpretation of vertical sediment P concentration profiles in lakes is inherently difficult because of complex interactions of diagentic processes (Trolle et al. 2010). The correlation analysis between vertical profiles of TP and LOI, Fe, Al and Mn used in the present study may, however, provide some insight into the drivers affecting the vertical distribution of TP in Lake Okaro, albeit causation may not be inferred. Nonetheless, given a consistently strong relationship between TP and LOI in the sediments of Lake Okaro, it is reasonable to assume a causal relationship between these two variables. A lack of relationships between vertical sediment concentration profiles of TP and Fe, Al and Mn, conversely, does not imply that sediment P in Lake Okaro is not associated with these elements because they may be influenced by a disproportionate allochthonous supply and pH and redox conditions (Davison 1993).
Forms of phosphorus detected by 31P NMR in seston
The range of P compounds and compound classes in the settling seston in Lake Okaro was similar to that in the top layer of the sediment, except that no phosphonates were present in the settling seston, although there were differences in the relative concentrations and proportions of each compound class. Care must be taken, however, when directly comparing P compounds and compound classes in settling seston and surface sediments, because surface sediment P composition is influenced by both settled material and P that migrates upward from deeper sediment layers. Seasonally induced changes as a result of mixing and the resulting changes in oxygen status in bottom waters appear to affect not only P concentrations, but also the relative contributions of P compounds in the settling seston. These changes were largely evident as an enriched P concentration of the settling seston and increased recovery as orthophosphate, compared with the surface sediments of Lake Okaro (Table 3). There appeared to be no discernible difference in polyphosphate concentrations in the surface sediments of Lake Okaro during periods of stratification and mixing, corresponding to anoxic and oxic periods, respectively, in bottom waters. In contrast, Hupfer et al. (2004) found a general decrease of polyphosphate concentrations in surface sediments during mixing periods, which they attributed to changes in accumulation and hydrolysis (i.e. uptake and release, respectively) by the different communities of microorganisms prevailing under oxic and anoxic conditions. Although the present study did not prove that there was no such synthesis of polyphosphate by microorganisms, it is possible that either a suitable carbon substrate was not available to facilitate luxury uptake and storage of P as polyphosphate (Khoshmanesh et al. 2002) or that changes in the communities of sediment microorganisms as a result of mixing may be less important during the early diagenesis of polyphosphates in Lake Okaro.
It has been suggested that pyrophosphates and polyphosphates in settling seston originate from the bacterial community colonising and mineralising settling particles (Hupfer et al. 2004; Reitzel et al. 2007). Such organisms accumulate and store phosphate as cellular polyphosphates under oxic conditions and these polyphosphates are then hydrolysed during anoxia, and released as orthophosphates (Hupfer et al. 2004). The reactivity and the relatively high abundance of polyphosphates in the settling seston in Lake Okaro suggested that this compound is likely to significantly contribute to enrichment of P in the water column before P reaches the bottom sediments, particularly during summer stratification when the hypolimnion is anoxic for several months (Özkundakci et al. 2010).
In conclusion, the deposition of seston in Lake Okaro was ~five-fold higher in the mixing period (June) than in the stratified period (February). Concentration of P compounds and compound classes in the surface sediments of Lake Okaro showed a much lower seasonal variability and mostly reflected the deposition of P composition during stratification. The exponential decrease of organic P in the bottom sediments indicated that organic P is an important long-term source of internal P loading in Lake Okaro. The approach used in the present study, however, only partially identified the source of the organic P compounds in the sediment from the water column. Future work should include the analysis of catchment soils, which will contribute to a better understanding of the origin of different P compounds and compound classes in the lake sediment and, ultimately, provide for a targeted management approach for reducing external and/or internal P loadings.
Acknowledgements
The first author held a Ph.D. scholarship within the Lake Biodiversity Restoration program funded by the New Zealand Ministry of Business, Innovation & Employment (Contract UOWX 0505). We gratefully acknowledge SCION Research, Rotorua, for technical support for the 31P NMR analysis and Bay of Plenty Regional Council for additional funding.
References
Abell, J. M., Hamilton, D. H., and Rutherford, J. C. (2013). Quantifying temporal and spatial variations in sediment, nitrogen and phosphorus transport in stream inflows to a large eutrophic lake. Environmental Science Processes & Impacts 15, 1137–1152.| Quantifying temporal and spatial variations in sediment, nitrogen and phosphorus transport in stream inflows to a large eutrophic lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXosVCltr4%3D&md5=f47a0420bdcaef965449290c3196d529CAS |
Ahlgren, J., Tranvik, L., Gogoll, A., Waldbäck, M., Markides, K., and Rydin, E. (2005). Sediment depth attenuation of biogenic phosphorus compounds measured by 31P NMR. Environmental Science & Technology 39, 867–872.
| Sediment depth attenuation of biogenic phosphorus compounds measured by 31P NMR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFSltbbN&md5=de5b1f4441b0c3695c0bc674a70d3dc6CAS |
Ahlgren, J., Reitzel, K., Tranvik, L., Gogoll, A., and Rydin, E. (2006). Degradation of organic phosphorus compounds in anoxic Baltic Sea sediments: a 31P nuclear magnetic resonance study. Limnology and Oceanography 51, 2341–2348.
| Degradation of organic phosphorus compounds in anoxic Baltic Sea sediments: a 31P nuclear magnetic resonance study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVOju7fI&md5=e73b581cfab9365cc5735e3cdc065505CAS |
Burger, D. F., Hamilton, D. P., Pilditch, C. P., and Gibbs, M. M. (2007). Benthic nutrient fluxes in a eutrophic, polymictic lake. Hydrobiologia 584, 13–25.
| Benthic nutrient fluxes in a eutrophic, polymictic lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlsVeqtb8%3D&md5=594909d344854d6ba52d11c3991f9d73CAS |
Cade-Menun, B. J. (1996). A comparison of soil extraction procedures for P-31 NMR spectroscopy. Soil Science 161, 770–785.
| A comparison of soil extraction procedures for P-31 NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XntVOitLo%3D&md5=c4c380ed03dcd72e0dbd994c0e7b1ed4CAS |
Cade-Menun, B. J. (2005). Characterizing phosphorus in environmental and agricultural samples by 31P nuclear magnetic resonance spectroscopy. Talanta 66, 359–371.
| Characterizing phosphorus in environmental and agricultural samples by 31P nuclear magnetic resonance spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjvVClt7w%3D&md5=86000be150e25329ae2957c9cb79296bCAS | 18969999PubMed |
Carman, R., Edlund, G., and Damberg, C. (2000). Distribution of organic and inorganic phosphorus compounds in marine and lacustrine sediments: a 31P NMR study. Chemical Geology 163, 101–114.
| Distribution of organic and inorganic phosphorus compounds in marine and lacustrine sediments: a 31P NMR study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXntlaguw%3D%3D&md5=4720d29bc96861eb9ea52b689835dabbCAS |
Carpenter, S. R. (2008). Phosphorus control is critical to mitigating eutrophication. Proceedings of the National Academy of Sciences, USA 105, 11039–11040.
| Phosphorus control is critical to mitigating eutrophication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVSnsL%2FK&md5=6da7e5acb591291d3b54e7e0138f1e3eCAS |
Chalar, G., and Tundisi, J. G. (2001). Phosphorus fractions and fluxes in the water column and sediments of a tropical reservoir (Lobo-Broa – SP). International Review of Hydrobiology 86, 183–194.
| Phosphorus fractions and fluxes in the water column and sediments of a tropical reservoir (Lobo-Broa – SP).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtlyjsrg%3D&md5=374ddc32a709923e965ffcb0759a42d1CAS |
Davison, W. (1993). Iron and manganese in lakes. Earth-Science Reviews 34, 119–163.
| Iron and manganese in lakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXmsFKjsb0%3D&md5=d51a93a4249d0fccd61f8f0ef71d31bcCAS |
DeGroot, C. J., and Golterman, H. L. (1993). On the presence of organic phosphate in some Camargue sediments: evidence for the importance of phytate. Hydrobiologia 252, 117–126.
| On the presence of organic phosphate in some Camargue sediments: evidence for the importance of phytate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXktlersb8%3D&md5=8efa283afa80813918cc2cf8194f25c2CAS |
Doolette, A. L., Smernick, R. J., and Dougherty, W. J. (2011). Overestimation of the importance of phytate in NaOH–EDTA soil extracts as assessed by 31P NMR analyses. Organic Geochemistry 42, 955–964.
| Overestimation of the importance of phytate in NaOH–EDTA soil extracts as assessed by 31P NMR analyses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWjtbzL&md5=70a8007b537a6648d0c40f227a4f3405CAS |
Ebina, J., Tsutsui, T., and Shirai, T. (1983). Simultaneous determination of total nitrogen and total phosphorous in water using peroxodisulphate oxidation. Water Research 17, 1721–1726.
| Simultaneous determination of total nitrogen and total phosphorous in water using peroxodisulphate oxidation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXmtFygsrY%3D&md5=ec5af8c8687237adc06d1ffe281e6fe6CAS |
Forsyth, D. J., Dryden, S. J., James, M. R., and Vincent, W. F. (1988). Lake Okaro ecosystem 1. Background limnology. New Zealand Journal of Marine and Freshwater Research 49, 21–31.
Fuentes, N., Güde, H., Wessels, M., and Straile, D. (2013). Allochthonous contribution to seasonal and spatial variability of organic matter sedimentation in a deep oligotrophic lake (Lake Constance). Limnologica 43, 122–130.
| Allochthonous contribution to seasonal and spatial variability of organic matter sedimentation in a deep oligotrophic lake (Lake Constance).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXivVKhurg%3D&md5=5c9e15122e8c1b026df1ca79bd4508f6CAS |
Hamilton, D. P. (2005). Land use impacts on nutrient export in the Central Volcanic Plateau, North Island. New Zealand Journal of Forestry 49, 27–31.
Hawkes, G. E., Powlson, D. S., Randall, E. W., and Tate, K. R. (1984). A 31P nuclear magnetic resonance study of the phosphorus species in alkali extracts of soils from long-term field experiments. European Journal of Soil Science 35, 35–45.
| A 31P nuclear magnetic resonance study of the phosphorus species in alkali extracts of soils from long-term field experiments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXhvFSkuro%3D&md5=9658a6898aa76289f280122053532ac5CAS |
Hupfer, M., Gächter, R., and Rüegger, H. (1995). Polyphosphate in lake sediments: 31P NMR spectroscopy as a tool for its identification. Limnology and Oceanography 40, 610–617.
| Polyphosphate in lake sediments: 31P NMR spectroscopy as a tool for its identification.Crossref | GoogleScholarGoogle Scholar |
Hupfer, M., Rübe, B., and Schmeider, P. (2004). Origin and diagenesis of polyphosphate in lake sediments: a 31P NMR study. Limnology and Oceanography 49, 1–10.
| Origin and diagenesis of polyphosphate in lake sediments: a 31P NMR study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhsVeltrg%3D&md5=4d42afa04b1da2d89b3799399e078402CAS |
Ingall, E., and Jahnke, R. (1994). Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochimica et Cosmochimica Acta 58, 2571–2575.
| Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXks1CqsL0%3D&md5=98cadfe5fbdfdb0bd5e5e2df466d3aebCAS |
Jolly, V. H. (1977). The comparative limnology of some New Zealand lakes. New Zealand Journal of Marine and Freshwater Research 11, 307–340.
| The comparative limnology of some New Zealand lakes.Crossref | GoogleScholarGoogle Scholar |
Kenney, W. F., Schelske, C. L., and Chapman, A. D. (2001). Changes in polyphosphate sedimentation: a response to excessive phosphorus enrichment in a hypereutrophic lake. Canadian Journal of Fisheries and Aquatic Sciences 58, 879–887.
| Changes in polyphosphate sedimentation: a response to excessive phosphorus enrichment in a hypereutrophic lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXks1Khu7c%3D&md5=295aeb549b4b27c5704f0ca7b16606b9CAS |
Khoshmanesh, A., Hart, B. T., Duncan, A., and Beckett, R. (2002). Luxury uptake of phosphorus by sediment bacteria. Water Research 36, 774–778.
| Luxury uptake of phosphorus by sediment bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovFCntbo%3D&md5=03b8e3d7ff8cba80a3bfa1bde1b52c93CAS | 11827338PubMed |
Koski-Vähälä, J., Hartikainen, H., and Kairesalo, T. (2000). Resuspension in regulating sedimentation dynamics in Lake Vesijärvi. Archiv fuer Hydrobiologie 148, 357–381.
Liu, C. L., Hart, N., and Peck, H. D. (1982). Inorganic pyrophosphate: Energy source for sulfate-reducing bacteria of the genus Desulfotomaculum. Science 217, 363–364.
| Inorganic pyrophosphate: Energy source for sulfate-reducing bacteria of the genus Desulfotomaculum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xks12ju7s%3D&md5=dcffcaf178822286faeb2a4cb3afba0cCAS | 17791517PubMed |
Makarov, M. I., Haumaier, L., and Zech, W. (2002). The nature and origins of diester phosphates in soils: a 31P-NMR study. Biology and Fertility of Soils 35, 136–146.
| The nature and origins of diester phosphates in soils: a 31P-NMR study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisF2qtL0%3D&md5=63df02d50f45890e45a8e9b282adb792CAS |
McDowell, R. W. (2003). Identification of phosphorus species in extracts of soils with contrasting management histories. Communications in Soil Science and Plant Analysis 34, 1083–1095.
| Identification of phosphorus species in extracts of soils with contrasting management histories.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjs1aqurk%3D&md5=22cf2d7c779bc87ab3ea928e48f78821CAS |
McDowell, R. W. (2009). Effect of land use and moisture on phosphorus forms in upland stream beds in South Otago, New Zealand. Marine and Freshwater Research 60, 619–625.
| Effect of land use and moisture on phosphorus forms in upland stream beds in South Otago, New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptVChs74%3D&md5=a855a3c6b7b66ea6afb598c6c73bc66fCAS |
McDowell, R. W., and Stewart, I. (2005). Peak assignments for phosphorus-31 nuclear magnetic resonance spectroscopy in pH range 5–13 and their application in environmental samples. Chemistry and Ecology 21, 211–226.
| Peak assignments for phosphorus-31 nuclear magnetic resonance spectroscopy in pH range 5–13 and their application in environmental samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFSju7fJ&md5=fb3cd6d82360ec071ec77c0a453aa850CAS |
McDowell, R. W., Condron, L. M., Stewart, I., and Cave, V. (2005). Chemical nature and diversity of phosphorus in New Zealand pasture soils using 31P nuclear magnetic resonance spectroscopy and sequential fractionation. Nutrient Cycling in Agroecosystems 72, 241–254.
| Chemical nature and diversity of phosphorus in New Zealand pasture soils using 31P nuclear magnetic resonance spectroscopy and sequential fractionation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFyntrnI&md5=af5bb2e75ceac389bded558542702ce3CAS |
McDowell, R. W., Stewart, I., and Cade-Menun, B. J. (2006). An examination of spin-lattice relaxation times for analysis of soil and manure extracts by liquid state phosphorus-31 nuclear magnetic resonance spectroscopy. Journal of Environmental Quality 35, 293–302.
| An examination of spin-lattice relaxation times for analysis of soil and manure extracts by liquid state phosphorus-31 nuclear magnetic resonance spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFGitrc%3D&md5=ef79eb75e72fccfdfc76b564c9f7a016CAS | 16397105PubMed |
Newman, R. H., and Tate, K. R. (1980). Soil phosphorus characterisation by 31P nuclear magnetic resonance. Communications in Soil Science and Plant Analysis 11, 835–842.
| Soil phosphorus characterisation by 31P nuclear magnetic resonance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXlvVCrtbw%3D&md5=9241fe33ba697e6fab0ab7dd9f87d7fcCAS |
Özkundakci, D., Hamilton, D. P., and Scholes, P. (2010). Effect of intensive catchment and in-lake restoration procedures on phosphorus concentrations in a eutrophic lake. Ecological Engineering 36, 396–405.
| Effect of intensive catchment and in-lake restoration procedures on phosphorus concentrations in a eutrophic lake.Crossref | GoogleScholarGoogle Scholar |
Paul, W. J., Hamilton, D. P., and Gibbs, M. M. (2008). Low-dose alum application trialled as a management tool for internal nutrient loads in Lake Okaro, New Zealand. New Zealand Journal of Marine and Freshwater Research 42, 207–217.
| Low-dose alum application trialled as a management tool for internal nutrient loads in Lake Okaro, New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVeis7nJ&md5=51c45adba0df2ead3462d57ff5f0b7fbCAS |
Paul, W. J., Hamilton, D. P., Ostrovski, I., Miller, S. D., Zhang, A., and Muraoka, K. (2012). Catchment land use and trophic state impacts on phytoplankton composition: a case study from the Rotorua lakes’ district, New Zealand. Hydrobiologia 698, 133–146.
| Catchment land use and trophic state impacts on phytoplankton composition: a case study from the Rotorua lakes’ district, New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVagtbvN&md5=70c5ad7654d436e64465dcedfe59d214CAS |
Pettersson, K. (2001). Phosphorus characteristics of settling and suspended particles in Lake Erken. The Science of the Total Environment 266, 79–86.
| Phosphorus characteristics of settling and suspended particles in Lake Erken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtFSnu7k%3D&md5=943716ac5fb61b6b26b9e04c77139076CAS | 11258837PubMed |
Reed-Andersen, T., Carpenter, S. T., and Lathrop, R. C. (2000). Phosphorus flow in a watershed–lake ecosystem. Ecosystems 3, 561–573.
| Phosphorus flow in a watershed–lake ecosystem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnvF2rtg%3D%3D&md5=06fc888f01cfb0c0f3010071816ea71aCAS |
Reitzel, K., Ahlgren, J., Gogoll, A., and Rydin, E. (2006). Effects of aluminium treatment on phosphorus, carbon, and nitrogen distribution in lake sediment: a 31NMR study. Water Research 40, 647–654.
| Effects of aluminium treatment on phosphorus, carbon, and nitrogen distribution in lake sediment: a 31NMR study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsFCrsL0%3D&md5=65ad21fb5790718f32abe1d26bab921aCAS | 16427681PubMed |
Reitzel, K., Ahlgren, J., DeBrabandere, H., Walbebäck, M., Gogoll, A., Tranvik, L., and Rydin, E. (2007). Degradation rates of organic phosphorus in lake sediment. Biogeochemistry 82, 15–28.
| Degradation rates of organic phosphorus in lake sediment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXislWktrw%3D&md5=8d388505071823c79bc5a9bdf743ae70CAS |
Shinohara, R., Imai, A., Kawasaki, N., Komatsu, K., Kohzu, A., Miura, S., Sano, T., Satou, T., and Tomioka, N. (2012). Biogenic phosphorus compounds in sediment and suspended particles in a shallow eutrophic lake: a 31P-nuclear magnetic resonance (31P NMR) study. Environmental Science & Technology 46, 10572–10578.
| Biogenic phosphorus compounds in sediment and suspended particles in a shallow eutrophic lake: a 31P-nuclear magnetic resonance (31P NMR) study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlOjt73J&md5=1e2b7a57ef7adb9e1ea5b45c4aae8234CAS |
Smolders, A. J. P., Lamers, L. P. M., Lucasseu, E. C. H. E. T., van der Velde, G., and Roelops, J. G. M. (2006). Internal eutrophication: how it works and what to do about it – a review. Chemistry and Ecology 22, 93–111.
| Internal eutrophication: how it works and what to do about it – a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjt1Kltrk%3D&md5=2a3e5786060367fbc38b9dc56682d752CAS |
Søndergaard, M., Jensen, J. P., and Jeppesen, E. (2003). Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506–509, 135–145.
| Role of sediment and internal loading of phosphorus in shallow lakes.Crossref | GoogleScholarGoogle Scholar |
Sundareshwar, P. V., Morris, J. T., Pellechia, P. J., Cohen, H. J., Porter, D. E., and Jones, B. C. (2001). Occurrence and ecological implications of pyrophosphate in estuaries. Limnology and Oceanography 46, 1570–1577.
| Occurrence and ecological implications of pyrophosphate in estuaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnsVSkurk%3D&md5=06d402a0ef196891a63cb633c2030970CAS |
Tanner, C. C., Caldwell, K., Ray, D., and McIntosh, J. (2007). Constructing wetland to treat nutrient-rich inflow to Lake Okaro, Rotorua. In ‘Proceedings of Stormwater 2007: 5th South Pacific Stormwater Conference, 16–18 May, Auckland, New Zealand’, 17p.
Tate, K. R., and Newman, R. H. (1982). Phosphorus fractions in a climosequence of soils in New Zealand tussock grassland. Soil Biology & Biochemistry 14, 191–196.
| Phosphorus fractions in a climosequence of soils in New Zealand tussock grassland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XoslGksA%3D%3D&md5=159b92e92ea3612d5b1d55b8f9c9587dCAS |
Trolle, D., Hamilton, D. P., Hendy, C., and Pilditch, C. (2008). Sediment and nutrient accumulation rates in sediments of twelve New Zealand lakes: influence of lake morphology, catchment characteristics and trophic state. Marine and Freshwater Research 59, 1067–1078.
| Sediment and nutrient accumulation rates in sediments of twelve New Zealand lakes: influence of lake morphology, catchment characteristics and trophic state.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFWgu7fP&md5=b98f32854a938b0ddcc34ef34241a341CAS |
Trolle, D., Hamilton, D. P., and Pilditch, D. (2010). Evaluating the influence of lake morphology, trophic status and diagenesis on geochemical profiles in lake sediments. Applied Geochemistry 25, 621–632.
| Evaluating the influence of lake morphology, trophic status and diagenesis on geochemical profiles in lake sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkvFCgs7k%3D&md5=f1131f3d6884df0be84b419e44eb0d1aCAS |
Turner, B. L., Mahieu, N., and Condron, L. (2003). Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts. Soil Science Society of America 67, 497–510.
| Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkslChsLo%3D&md5=d3b1ee0e79e3a4a05038811906bf78daCAS |
Turner, B. L., Frossard, E., and Baldwin, D. S. (Eds) (2005). ‘Organic Phosphorus in the Environment.’ (CABI Publishing: London.)
Vollenweider, R. A. (1976). Advances in defining critical loading levels for phosphorus in lake eutrophication. Memorie dell’Istituto Italiano di Idrobiologia 33, 53–83.
| 1:CAS:528:DyaE1cXktFemtA%3D%3D&md5=2dee373e25b24fcde01687668d00a4dbCAS |
White, E., Don, B. J., Downes, M. T., Kemp, L. J., MacKenzie, A. L., and Payne, G. W. (1978). Sediments of Lake Rotorua as sources and sinks for plant nutrients. New Zealand Journal of Marine and Freshwater Research 12, 121–130.
| Sediments of Lake Rotorua as sources and sinks for plant nutrients.Crossref | GoogleScholarGoogle Scholar |