Seasonal flooding, instream habitat structure and fish assemblages in the Mulgrave River, north-east Queensland: towards a new conceptual framework for understanding fish-habitat dynamics in small tropical rivers
Thomas S. Rayner A C D , Bradley J. Pusey B and Richard G. Pearson AA School of Marine and Tropical Biology, James Cook University, Townsville, Queensland 4811, Australia.
B Australian Rivers Institute, Griffith University, Brisbane, Queensland 4111, Australia.
C Present address: School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia.
D Corresponding author. Email: thomas.rayner@unsw.edu.au
Marine and Freshwater Research 59(2) 97-116 https://doi.org/10.1071/MF07129
Submitted: 6 July 2007 Accepted: 19 December 2007 Published: 27 February 2008
Abstract
Strong relationships between seasonal flooding, instream habitat structure and fish assemblages have been well documented in large tropical rivers (e.g. the flood pulse concept). However, the mechanics of these relationships are likely to differ substantially in smaller coastal rivers, such as those in Costa Rica, south-east Brazil and Australia’s Wet Tropics. These systems typically feature steep upland streams with short, deeply incised lowland channels and poorly connected floodplains. This hypothesis was investigated by documenting spatial and temporal variation in fish-habitat relationships in the Mulgrave River, north-east Queensland. Sampling was conducted at four lowland sites under a range of flow conditions, from dry-season baseflows to a one-in-ten-year flood. Longitudinal environmental gradients and fine-scale habitat patches were important in regulating fish assemblage structure during the dry season. However, high wet-season flows, constrained by the deep channel, acted as disturbances rather than gentle flood-pulses. In particular, the mobilisation of bed sediments led to scouring of aquatic vegetation and a dramatic reduction in habitat heterogeneity. Seasonal movements of fish led to significant changes in assemblage structure – from a community dominated by Neosilurus ater, Hypseleotris compressa, Awaous acritosus and Redigobius bikolanus during the dry season, to one dominated by Nematalosa erebi, Ambassis agrammus and Glossamia aprion during the wet season. Based on these observations, together with information from the literature, a conceptual model of fish-habitat dynamics is presented that is better suited to small tropical rivers than those developed in larger systems with expansive floodplains.
Introduction
Tropical rivers are characterised by predictable wet season flow events that play a major role in regulating the dynamics of instream habitats and freshwater fish communities (Lowe-McConnell 1975; Goulding et al. 1988; Winemiller 1990, 1996; Jepsen 1997). In large systems, such as the Amazon River, these flows take the form of a flood pulse, which dramatically increases the extent of aquatic habitats (Junk et al. 1989; Ward 1989; Amoros and Bronette 2002; Galacatos et al. 2004) and favours the development of life history adaptations that exploit the associated increases in food and habitat resources (Junk et al. 1989; Winemiller 1989a, 1989b; Bunn and Arthington 2002). In smaller tropical rivers, however, freshwater fish are forced to rely more heavily on habitats within the main channel and its tributaries – not only because the surrounding floodplain is relatively poorly connected, but also because it is less expansive and, in many cases, anthropogenically disturbed (i.e. of limited value to freshwater fish; Pusey et al. 1995a; Pusey and Kennard 1996; Russell et al. 1996).
Here, we define small tropical rivers as those with catchment areas less than 2500 km2, a mean discharge of ~25 m3 s–1, perennial flows (with a maximum wet-season discharge <5000 m3 s–1) and low interannual flow variability (annual coefficient of variation (CV) value <40%). These systems typically feature steep upper catchments with short lowland channels that are deeply incised relative to the surrounding floodplain (Willmott and Stephenson 1989; Russell et al. 1996; Sattler and Williams 1999; Nott et al. 2001; Nott 2003; Veitch and Sawynok 2005). Wet season flooding is usually brief, lasting days to weeks rather than months, and only the largest floods escape the main channel.
Given these unique characteristics, a conceptual framework incorporating longitudinal gradients, patch dynamics and disturbance hypotheses (e.g. the riverine ecosystem synthesis (Thorp et al. 2006)) may more accurately describe the influence of seasonal flows on fish-habitat relationships than models adapted from larger systems (e.g. the flood-pulse concept (Junk et al. 1989)). For example, many studies in small tropical systems have documented strong influences of habitat diversity and fine-scale habitat variables (e.g. width, depth, cover, substrate diversity, etc.; Gorman and Karr 1978; Winemiller 1983; Angermeier and Schlosser 1989; Martin-Smith 1998), whereas others have identified the over-riding importance of distance from the ocean and the presence of instream barriers to fish movement (Lyons and Schneider 1990; Pusey et al. 1995a; Pusey and Kennard 1996; Russell et al. 1996, 2003; Martin-Smith and Laird 1998).
We tested the hypothesis that seasonal, flow-mediated change in habitat structure is the principal factor affecting fish community composition in the Mulgrave River, north-eastern Queensland, by describing spatial and temporal changes in habitat structure and lowland fish assemblages under a range of flow conditions and investigating the habitat preferences of individual fish species. Our overall goal was to construct a conceptual framework of these dynamics that can be: (i) widely applied across relatively small tropical rivers with steep gradients, short lowland reaches and poorly connected floodplains; and (ii) used as a template for the future assessment of the relative importance of other factors likely to influence fish assemblage structure in these systems, including dietary requirements, reproductive activity and movement dynamics.
Materials and methods
Study area and study sites
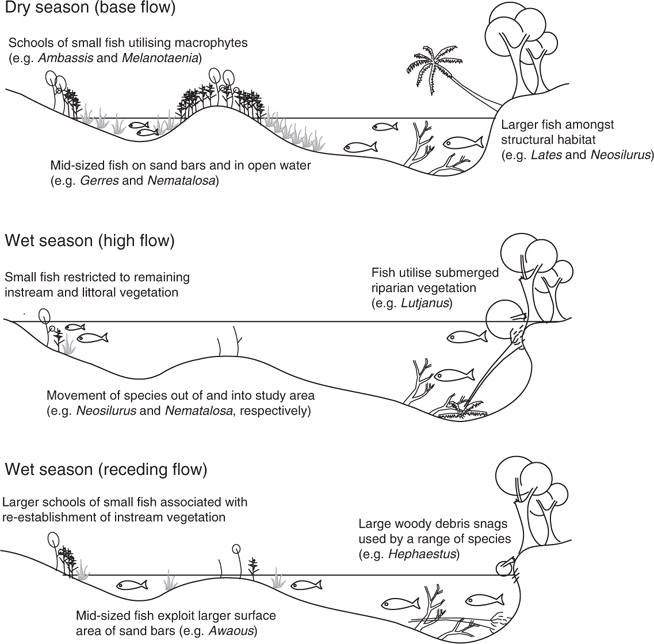
The Wet Tropics region extends through four degrees of latitude in a narrow strip (~100 km wide) of Australia’s north-eastern coast (Pusey et al. 1995b). The Mulgrave River catchment lies at the centre of the region (17°7′S, 145°51′E) and covers ~810 km2 (Russell et al. 1996; Fig. 1). Like most Wet Tropics catchments, it is characterised by forested mountain ranges in the upper reaches and cleared alluvial floodplains in the lowlands (Russell et al. 1996). Although the pattern of discharge is highly seasonal, reflecting the seasonal pattern of rainfall, inter-annual flow variability in the region is among the lowest in Australia (Pusey et al. 2000, 2004).
|
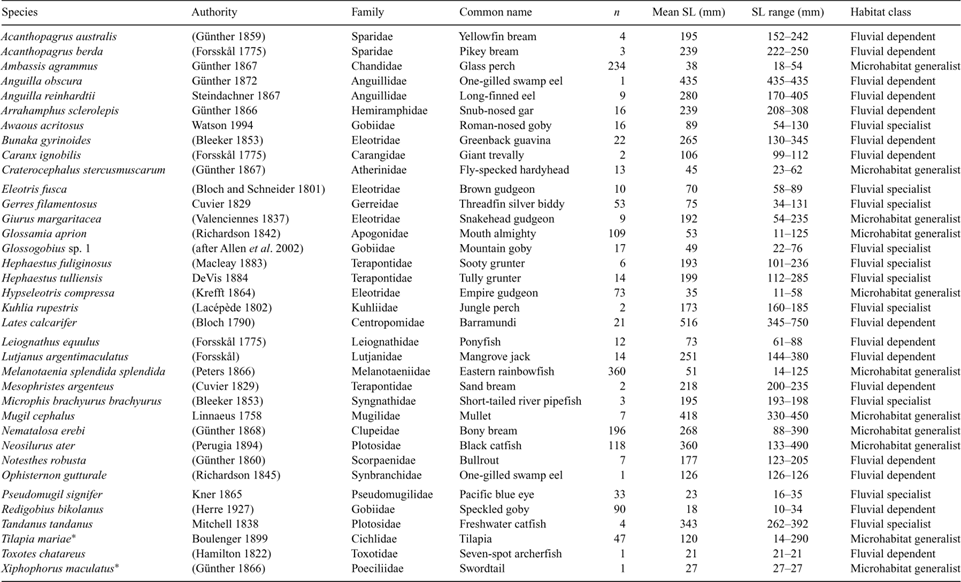
A total of 107 freshwater fish species, representing 37 families, have been recorded in the Wet Tropics region – most being restricted to lowlands by the presence of natural barriers to upstream fish movement (waterfalls, etc.; Unmack 2001; Pusey et al. 2004, 2008). The Mulgrave River has a particularly rich fish fauna, owing partly to the presence of several Wet Tropics endemics (e.g. Cairnsichthys rhombosomoides, Glossogobius sp. 1 and sp. 4, and Tandanus sp.). Pusey et al. (1995a) collected 36 species from 12 sites using backpack electrofishing. Subsequent sampling has increased this number to over 70 species, if main-channel surveys and estuarine vagrants are included (B. Pusey, unpubl. data; Halliday et al. 2001).
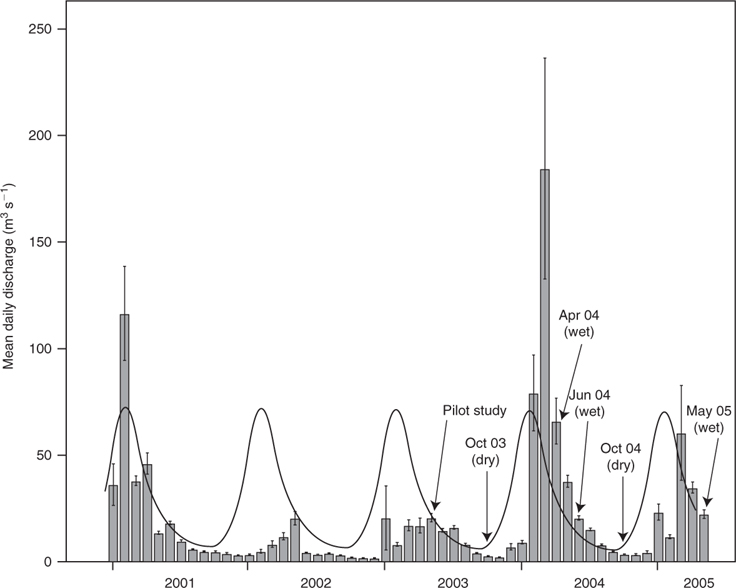
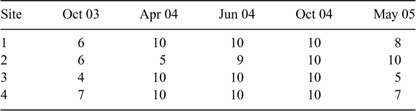
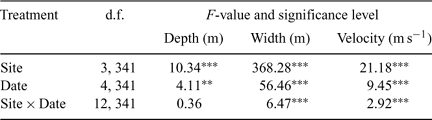
The present study focussed on four main-channel sites on the lowland floodplain reach. These sites were selected in order to encompass the full range of habitat features likely to be available to fishes throughout main channels of lowland Wet Tropics rivers, although access and the longitudinal spread of sites along the river reach were also considered. Each site was ~200 m in length, 50–80 m wide and up to 6 m deep. Sampling took place under a range of flow conditions (Fig. 2), from dry-season baseflows to immediately following a one-in-ten-year wet season flood with a peak instantaneous discharge of 2445 m3s–1 (MacNamara 1985). Dry-season samples were collected in October 2003 and October 2004, whereas wet-season samples were collected in April 2004, June 2004 and May 2005. All sites were sampled on all occasions and all were located above the tidal extent. However, high tides during the dry season (when discharge was relatively low) did cause freshwater to ‘back-up’ on the incoming salt-wedge at sites 2–4.
Fish surveys
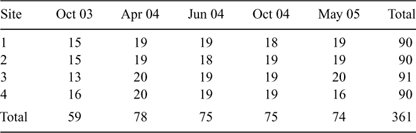
A combination of methods was used to sample fish assemblages: over 5 h of electrofishing ‘power-on’ time, 245 h of gill-netting and 728 h of bait trapping were undertaken. Electrofishing was conducted using a 2.5 kVA Smith-Root Generator Powered Pulsator (GPP) (Smith-Root, Vancouver, WA, USA) boat-mounted unit (500–1000 V, 60 pulses per second, 60–100 duty cycle range and 3–4 A). Six shots, each 5 min in duration, were undertaken at each site during each round of sampling (three on each bank in an upstream direction). Three multi-panel gill-nets (three 4 × 10 m panels with mesh sizes of 45, 90 and 120 mm) were set in open water habitats, at each site on each sampling occasion, for 4 h (from 1200 to 1600 h). These nets were set parallel to the bank to prevent them from being drowned out, pushed flat and clogged with mobile debris and floating aquatic plants. Additionally, up to 10 small unbaited traps (40 cm × 20 cm × 20 cm, 3 mm mesh; five on each bank) were used to sample cryptic fish species in shallow littoral habitats (i.e. less than 1 m deep) for the same time period (Table 1). The standard length of every fish caught was measured. Most fish were then returned to the water alive, except for non-indigenous species and a sub-sample of specimens that were euthanased in Benzocaine (Ethyl p-aminobenzoate, 100 mg L–1), or an icy slurry, and preserved in 10% formalin (37% aqueous solution of formaldehyde, diluted in water) for later calculation of length–weight relationships and investigation of diets (results to be reported elsewhere). Although long-term preservation in formalin and ethanol is known to cause length and weight loss (Kelly et al. 1975), the impact of such biases on the conclusions drawn in the present study are likely to be minimal because: (i) samples were processed soon after collection; and (ii) the differences between samples in terms of biomass per unit effort were driven primarily by large differences in the abundances of several key species.
|
Habitat assessment
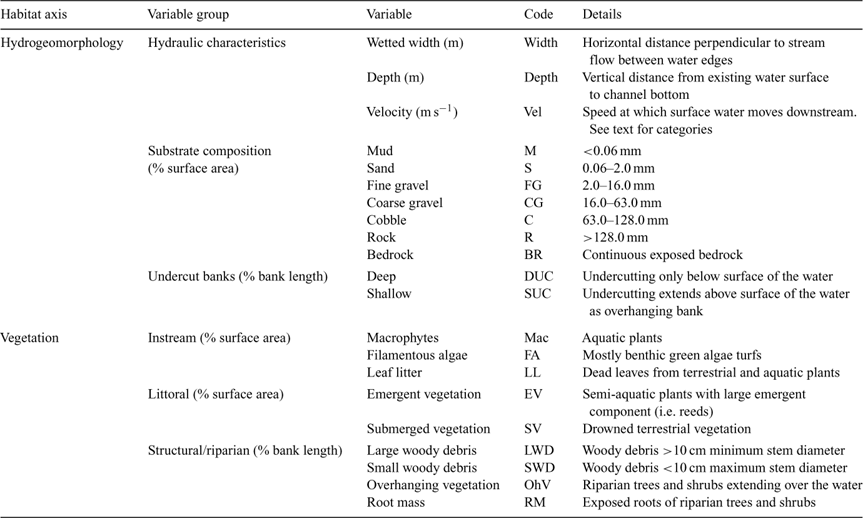
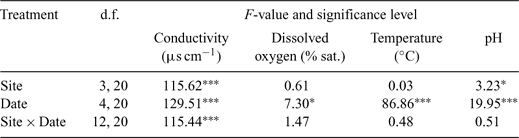
Hydrogeomorphologic, vegetation and water quality variables were estimated for each shot of fish sampling effort, at each site, on each sampling occasion, using a methodology similar to that of Pusey et al. (2004) and Kennard (2005) (Tables 2 and 3). Hydrogeomorphologic and vegetation variables were estimated in one of two ways: those habitat features that varied across the river bed were estimated as the percentage of total surface area sampled (which varied between 10 and 750 m2), whereas microhabitat structures confined to the river margins (e.g. undercut banks) were estimated as the linear portion/percentage of total bank length sampled (Kennard 2005). Ambient water quality variables (temperature, pH, dissolved oxygen and conductivity) were measured using multi-probe instruments (Hydrolab DS4 and DS3 – HachR Environmental, Loveland, CO, USA; or YSI 556 MPS – YSI Environmental, Yellow Springs, OH, USA). Areal and vertical variation of these variables within sites was minimal (T. Rayner and P. Godfrey, unpubl. data), so three replicates were collected at each site during each sampling date, at a depth of 0.5 m.
|
|
Data analysis
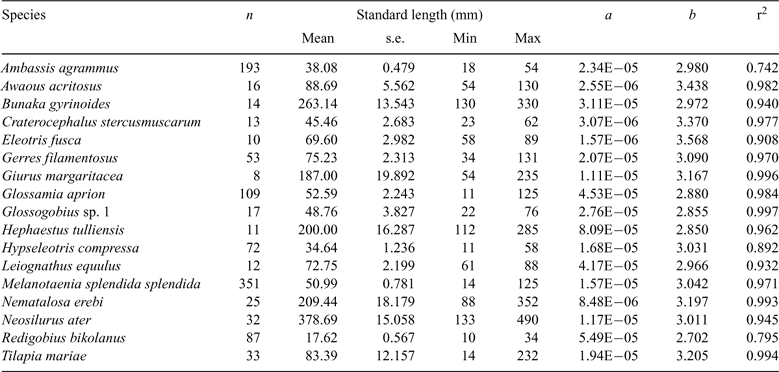
Length–weight relationships from the present study and from the literature (Table 4) were used to estimate the biomass of fish that were collected. The total number of individuals and biomass of each species caught at each site on each sampling date were then standardised by sampling effort (i.e. number of seconds of boat electrofishing ‘power-on’ time, gill-net hours and bait-trap hours expended). Non-parametric Kruskal–Wallis tests were used to test for differences in catch per unit effort (CPUE) and biomass per unit effort (BPUE) among sites (averaged across dates) and dates (averaged across sites) for each method, as data did not conform to analysis of variances (ANOVA) assumptions of normality or homogeneity of variance, even after transformation. ANOVA was, however, used to test for differences in richness and evenness of catch data.
|
Temporal and spatial differences in habitat characteristics were investigated using multivariate analysis of variance (MANOVA) of width, depth, velocity, conductivity, temperature, dissolved oxygen and pH. Tukey’s Honestly Significant Difference (HSD) post-hoc test was used to identify homogeneous subsets of sites and sampling dates. Other vegetation and substrate variables (i.e. per cent cover and per cent bank length) did not conform to the assumptions of normality for ANOVA testing because values were usually either 0% or 100% within microhabitats. Consequently, these variables were not statistically tested. Repeated-measures were not applied in any tests because habitat variables were assessed at random points within each site on each sampling date (as part of a stratified design), rather than tracking dynamics at specific locations within each site through time.
Three-dimensional semi-strong hybrid multidimensional scaling (SSHMDS – PATN Version 3.03, Belbin (1991)) was used to explore relationships among the four sites and the wet and dry season samples on the basis of: (i) instream habitat structure (mean value of each habitat variable at each site on each sampling date); (ii) fish abundance (standardised CPUE of each species summed across methods for each site on each sampling date); and (iii) fish biomass (standardised BPUE of each species summed across methods for each site on each sampling date). Combining catch data from different gear types is an effective way to negate biases caused by gear selectivity (Weaver et al. 1993). The Gower metric association measure was used for analysis of habitat data (Gower 1971; Gower and Legendre 1986), whereas the Bray–Curtis association measure was used for analysis of CPUE and BPUE (Bray and Curtis 1957). BPUE and CPUE datasets were log10(x + 1) transformed before analysis. In all cases, differences between a priori site and seasonal groups were tested using analysis of similarity (anosim; Clarke and Green 1988).
In order to identify the likely important variables in determining spatial and temporal variation between sites and seasons, principal component correlation (PCC) was applied to the relevant variables (i.e. those used in the ordination or separate covariates) and tested using the Monte-Carlo attributes in ordination (MCAO) permutation test. PCC vectors were plotted on ordination figures if the percentage of MCAO permutation r-squared values that exceeded the real r-squared value was less than or equal to 5%, and coded as follows: *** = 0%, ** = 1%, * = 2–5%. Although these percentages approximate P-values of <0.001, <0.01 and <0.05, respectively (L. Belbin, pers. comm., 2005), because actual ‘P-values’ are not produced by MCAO testing, we describe PCC vectors with ‘significant’ results as ‘strongly correlated’ with the distribution of sites in ordination space. Some fish samples from the electrofishing survey of site 2 in October 2003 were lost in transit from the field to the laboratory – this sample was removed from univariate and multivariate analyses of CPUE and BPUE that included electrofishing data.
The general importance of fluvial main-channel habitats to freshwater fishes in the Mulgrave River was assessed by assigning species to one of three habitat groups, using data from Pusey et al. (1995a, 2004) and the present study. The habitat groups (following Galat and Zweimuller 2001, after Kinsolving and Bain 1993) were: (i) fluvial specialists, which are species that are almost always found only in streams and rivers or use flowing water habitats throughout life; (ii) fluvial dependent species, which are found in a variety of habitats including estuaries, but require flowing water at some stage in their life cycle; and (iii) microhabitat generalists, which are commonly found in lakes, floodplain water bodies, streams and rivers, but are capable of completing their life cycle in lentic systems.
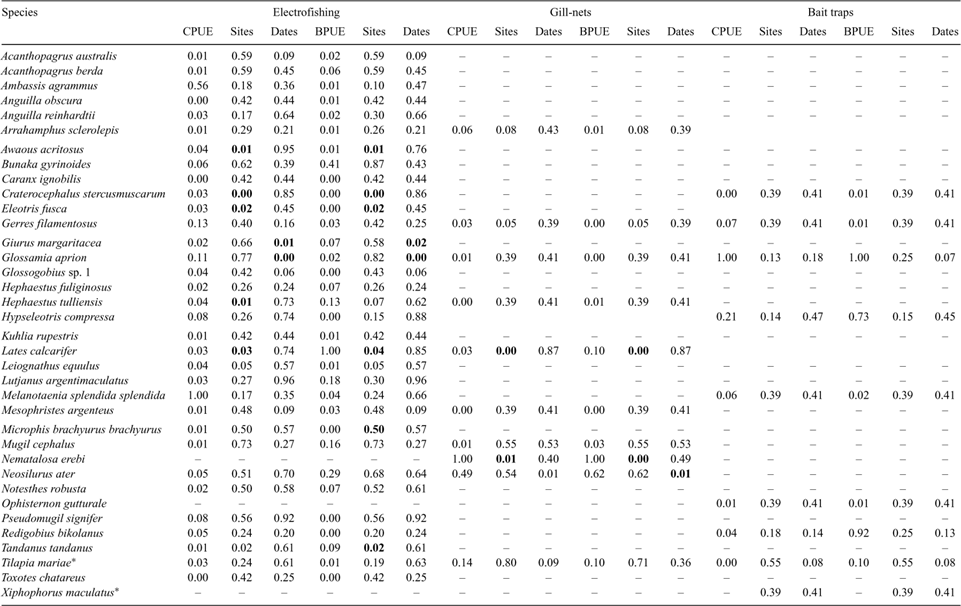
Seasonal habitat preferences of fish species were investigated by dividing the habitat use of each species by habitat availability during wet and dry seasons. Habitat ‘use’ was defined as the mean value of each habitat variable recorded at the locations where the individual fish of each species were captured using electrofishing (Table 2). Habitat ‘availability’ was defined as the mean value of each habitat variable across all 24 electrofishing shots (calculated separately for wet and dry seasons). The flexible unweighted pair-group method using arithmetic averages (UPGMA) was then carried out (using PATN) on the resulting matrix of species’ habitat-preference values, in order to classify seasonal species samples into habitat guilds.
Results
Spatial and temporal habitat variability
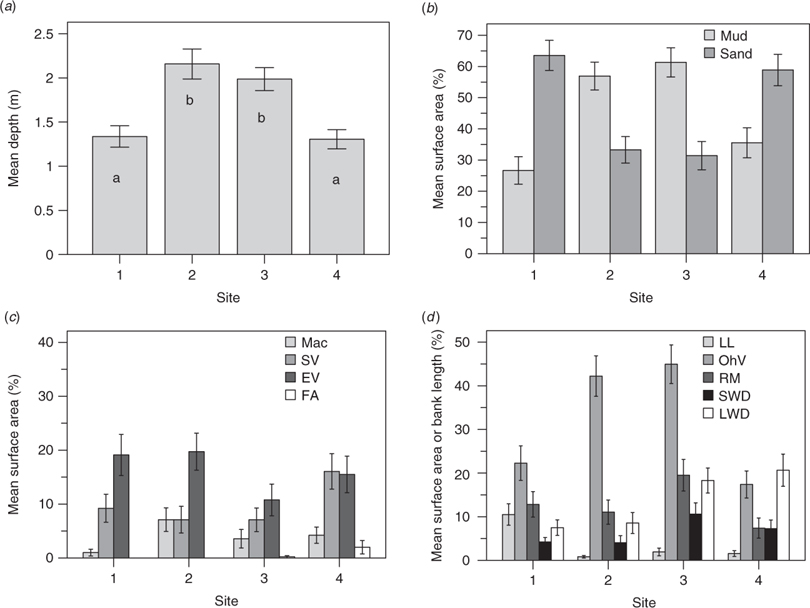
The distribution of study sites in ordination space reflected their main habitat features (Fig. 3a). Site 1 was characterised by an abundance of deep undercut banks and leaf litter debris on sand substrate (Fig. 4). Site 4 featured woody debris, patches of filamentous algae and higher conductivity (during the dry season; Tables 5 and 6, Fig. 4). These sites were similar to one another, despite being located at the upper and lower limits of the study area, respectively, as both sites were influenced by the presence of large, mobile point bars. ANOSIM testing identified a significant difference between sites 1 and 4, and sites 2 and 3 in ordination space (real F = 1.187, best F = 1.114, % randomised F > real F = 0). The latter sites were significantly wider and deeper, with mud substrates, overhanging vegetation, exposed root masses and an abundance of instream vegetation (macrophytes, submerged vegetation and emergent vegetation; Tables 7 and 8, Fig. 4).
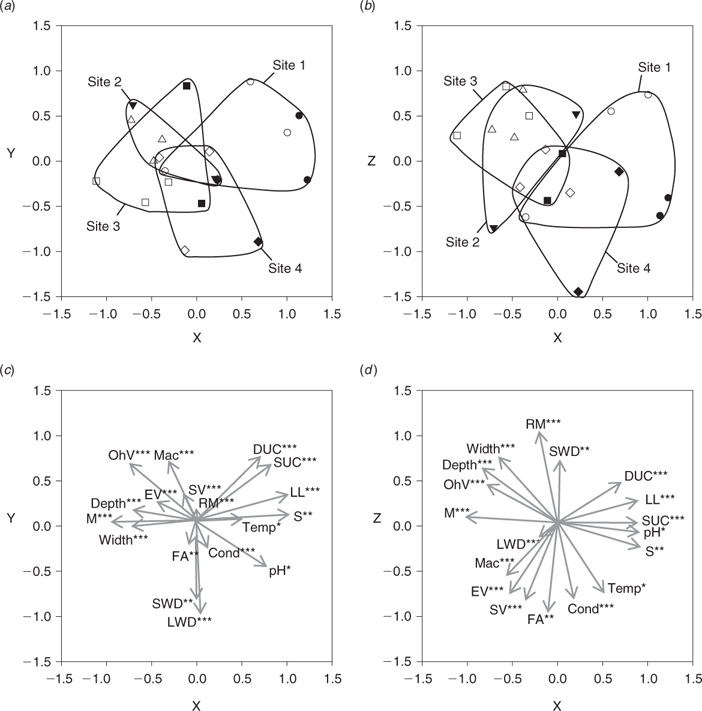
|

|
|

|
|
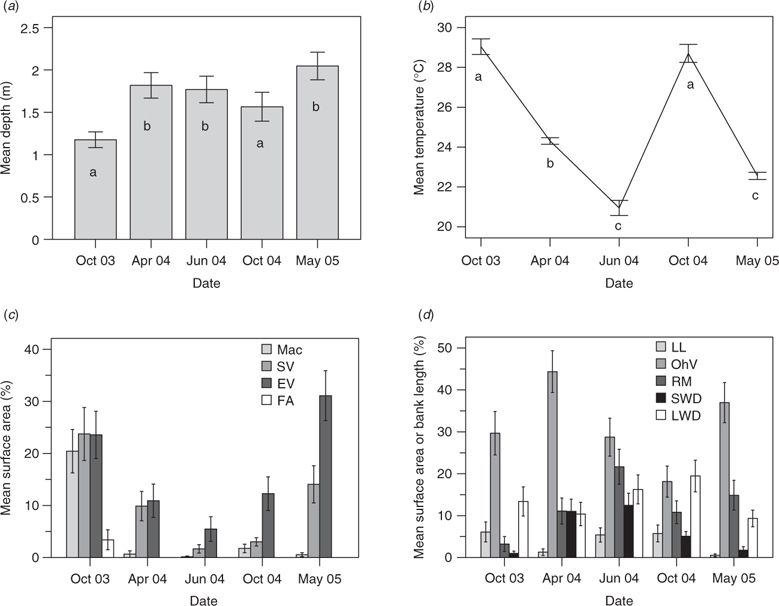
All sites exhibited a similar response to high flows relative to their condition at the start of the study (Fig. 3b). Dry season samples were strongly correlated with the abundance of various vegetation types (macrophytes, emergent vegetation, submerged vegetation and filamentous algae) and higher water temperatures (Fig. 5a, c, d; tidal penetration was also greater at this time). However, wet-season flow events significantly increased width, depth and flow velocity at all sites (Table 8). Substrate mobilisation during the wet season scoured instream vegetation from mid-channel bars and led to a greater portion of riparian vegetation overhanging the main channel (Fig. 5d). Associated bank erosion and mass movement recruited large woody debris to the channel and exposed root masses. ANOSIM testing revealed a significant difference between wet- and dry-season samples in ordination space (Fig. 3b; real F = 1.206, best F = 1.167, % randomised F > real F = 0).
Throughout the study, a wide range of habitats were available across the lowland reach of the Mulgrave River. Although hydrogeomorphologic variables were most important in determining spatial variability between sites, a combination of vegetation and water quality variables accounted for much of the temporal variability between sampling dates. In general, water quality variables were well within the tolerance ranges of the species that were caught (Pusey et al. 2004) and no ‘poor’ water quality conditions (i.e. those that may be found in a badly degraded system) were encountered. Mean temperature, conductivity and pH were higher in the dry season than in the wet season, but dissolved oxygen concentration did not show a distinct seasonal pattern.
Fish community composition
A total of 1530 individual fish, representing 36 species (including two exotic species), 33 genera and 26 families, were collected during the study (Table 9). Electrofishing was the most effective sampling method used, collecting 33 of the 36 species. Melanotaenia splendida splendida and Ambassis agrammus dominated the electrofishing catch and were three to five times more abundant than the next most abundant species, Gerres filamentosus and Glossamia aprion (Table 10). Other abundant species included Pseudomugil signifer, Hypseleotris compressa, Bunaka gyrinoides, Neosilurus ater, Redigobius bikolanus and Awaous acritosus. Hephaestus tulliensis was more abundant than the closely related Hephaestus fuliginosus. The biomass of the electrofishing catch was dominated by larger species, such as Lates calcarifer, B. gyrinoides, N. ater and Lutjanus argentimaculatus, with smaller species such as M. s. splendida and A. agrammus making limited contributions, despite their numerical abundance (Table 10). There were no significant differences in species richness (F3, 15 = 1.81, P > 0.1; F4, 15 = 1.04, P > 0.1) or evenness (F3, 15 = 1.24, P > 0.1; F4, 15 = 1.7, P > 0.1) of the electrofishing catch between sites or sampling dates respectively.
There were significant differences in mean electrofishing CPUE and BPUE between sites for some species (Table 10). CPUE of A. acritosus differed significantly between sites, whereas G. aprion differed significantly between sampling dates. The former species was more abundant at sites 1 and 4, whereas the latter was more abundant during the wet season than during the dry season. Of the 10 species providing the greatest contribution to overall electrofishing biomass, two showed significant differences in BPUE between sites: Tandanus tandanus and L. calcarifer were more abundant at upstream and downstream sites respectively. Giurus margaritacea was the only one of these 10 species to exhibit a significant difference in BPUE between sampling dates, being consistently more abundant during the wet season.
Ten species were collected using gill-netting. Nematalosa erebi and Neosilurus ater dominated the catch (Table 10), although Tilapia mariae, Arrhamphus sclerolepis, G. filamentosus and L. calcarifer were also abundant. Nematalosa erebi and N. ater also dominated gill-net BPUE, with T. mariae and L. calcarifer making smaller contributions. As was the case for electrofishing, the CPUE and BPUE of species caught using gill-nets did not differ significantly between sites or sampling dates in most cases. L. calcarifer and N. erebi were significantly more abundant (CPUE and BPUE) at sites 2 and 3, whereas N. ater was significantly more abundant during the dry season, but contributed a greater biomass during the wet season.
Glossamia aprion, R. bikolanus and H. compressa comprised most of the bait trap catch. Seven other species were infrequently encountered. Ophisternon gutturale and Xiphophorus maculatus (the second exotic species) were the only species not caught using the other methods. Glossamia aprion dominated the total biomass collected using bait-traps, with smaller contributions made by H. compressa, G. filamentosus, M. s. splendida and R. bikolanus. There were no significant differences in CPUE or BPUE between sites or sampling dates for any of the species captured using bait-traps (Table 10).
Ordination of combined CPUE data confirmed the patterns described above (Fig. 6). Temporal variability in fish community composition was slightly stronger than spatial variability, although significant ANOSIM results were obtained for both comparisons (sites: real F = 1.167, best F = 1.194, % randomised F > real F = 1.01%; seasons: real F = 1.157, best F = 1.101, % randomised F > real F = 0). PCC vectors representing N. ater, H. compressa, A. acritosus and R. bikolanus were correlated with dry-season samples, whereas those representing N. erebi, G. aprion and Craterocephalus stercusmuscarum were correlated with wet season samples (Fig. 6). Hephaestus tulliensis, Anguilla reinhardtii and T. mariae PCC vectors were strongly correlated with site 1. Those representing habitat variables reflected the results presented previously: overhanging vegetation, mud, depth and width were correlated with wet-season samples, whereas variables such as pH, temperature and submerged vegetation were correlated with dry-season samples (Fig. 6d).
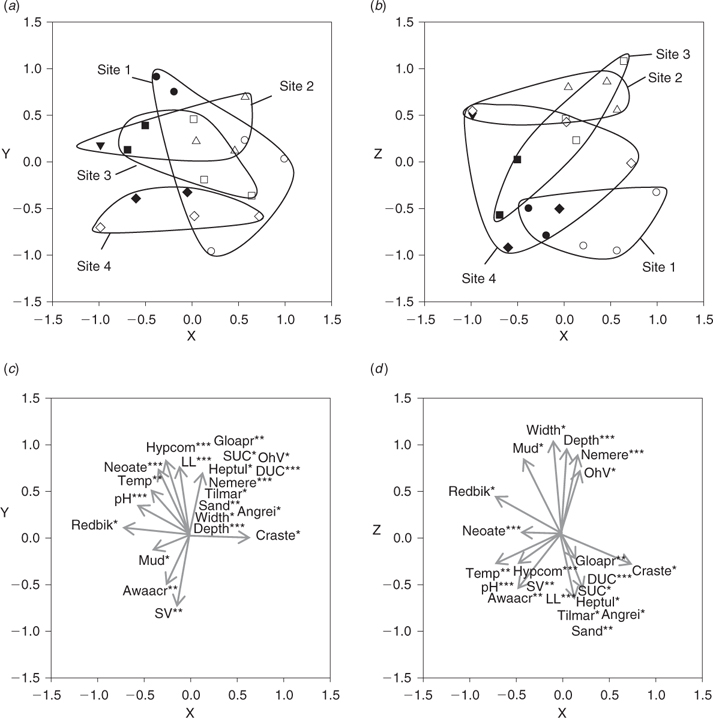
|
Unlike CPUE, ordination of BPUE data did not reveal clear patterns of temporal variability in fish community structure (ANOSIM; real F = 1.035, best F = 1.316, % randomised F > real F = 26.263%), although significant differences were identified between sites (ANOSIM; real F = 1.161, best F = 1.168, % randomised F > real F = 1.01% – Fig. 7). PCC vectors representing T. mariae, T. tandanus, A. acritosus and C. stercusmuscarum were correlated with sites 1 and 4, which were positioned to the left of the ordination biplots, whereas those representing N. erebi and N. ater were strongly correlated with sites 2 and 3, which were positioned to the right (Fig. 7). As was the case for CPUE data, PCC vectors representing habitat variables reflected the results of the analysis of habitat structure presented above.
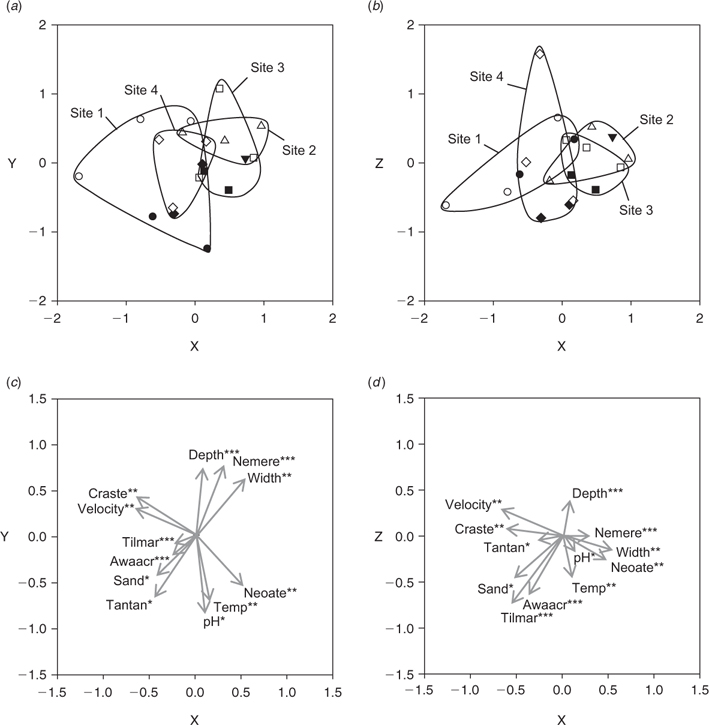
|
Habitat use by individual species
When classified into the broad habitat groups of Galat and Zweimuller (2001), 70% of the 36 species caught during the study were associated with fluvial habitats (Table 9). A total of 10 species (28%) were fluvial specialists, known to favour faster-flowing shallow water habitats (e.g. A. acritosus, Glossogobius sp. 1, P. signifer, T. tandanus – Pusey et al. 2004). Fifteen (42%) fluvial-dependent species together constituted the largest group, with a diverse taxonomic range of families represented, including some species with estuarine populations (e.g. Mesophristes argenteus, Acanthopagrus australis and Acanthopagrus berda). There were 11 generalist species (30%) that occurred in a variety of microhabitats. These species, with the exception of G. margaritacea and X. maculatus, were mostly ubiquitous across sites and sampling dates.
Habitat use by fishes varied seasonally. Eight habitat guilds were identified for species caught using electrofishing, on the basis of the mean habitat use by each species relative to habitat availability during wet and dry seasons (Fig. 8). Guild 1 included a diverse taxonomic spectrum (from gobies to a larger lutjanid species) with a variety of habitat preferences, but with a general trend towards wide, deep sections of the river, with mud substrates and large woody debris elements. Most of the members of this first guild were collected during the wet-season. Guild 2 was composed of root mass specialists – A. reinhardtii, H. fuliginosus and Notesthes robusta – from dry season samples. Overhanging vegetation appeared to be important to P. signifer, the sole member of guild 3, although just three individuals of this species were caught using electrofishing and the result should be treated with caution.
The two members of guild 4, H. tulliensis and T. tandanus, were associated with deep undercut banks, whereas the two goby species in guild 5, A. acritosus and Glossogobius sp. 1, preferred high velocity areas with fine gravel substrates and/or abundant leaf litter. All of these habitat types were abundant at site 1, the most upstream site. All members of guild 7 were from dry-season samples and favoured emergent vegetation, filamentous algae, submerged vegetation and aquatic macrophytes (e.g. G. filamentosus and H. compressa). Guild 8 comprised a single species, L. calcarifer from dry season samples, which was associated with large woody debris and macrophyte beds.
There was a high degree of seasonal variability in habitat guild membership (Fig. 8). Most species that were caught in both seasons moved between guilds, particularly from guilds 7 and 8 (instream vegetation) during the dry season to guild 1 (wide, deep habitats with mud substrate and large woody debris) during the wet season. Exceptions to this trend were N. ater and B. gyrinoides, which favoured the latter habitats in both seasons, and Glossogobius sp. 1 and A. acritosus, which both remained in guild 5 at all times. In general, fish species collected during the dry season belonged to guilds with more specific habitat preferences (i.e. guilds 2–8, but not guild 6) than those species collected during the wet season, most of which belonged to guild 1.
Discussion
Spatial variability in fish assemblage structure
Spatial variability in fish assemblages has been well documented in a range of river systems from the temperate zone (Koehn et al. 1994; Gehrke et al. 1999; King 2004), the sub-tropics (Pusey et al. 1993; Arthington et al. 2005) and the tropics (Gorman and Karr 1978; Martin-Smith 1998; Pusey et al. 1998; Bishop et al. 2001). These studies typically demonstrate that fish assemblages are influenced simultaneously by a variety of factors operating at various scales and along longitudinal gradients (Sheldon 1968; Schlosser 1982; Angermeier and Schlosser 1989; Harvey 1991; Arunachalam 2000). Pusey et al. (1995a) identified distance upstream as an important regulator of assemblage structure in the Mulgrave River, with instream barriers to fish movement limiting species richness in upstream areas. This finding was repeated in the present study, with Melanotaenia splendida splendida, Tandanus tandanus and Hephaestus fuliginosus more common at upstream sites (1 and 2), and Ambassis agrammus, Redigobius bikolanus, Lates calcarifer and Lutjanus argentimaculatus dominating numerical abundance and biomass at downstream sites (3 and 4). However, because the study area was situated downstream of any such barriers, this longitudinal gradient was less pronounced than reported by Pusey et al. (1995a), who considered the entire catchment.
As habitat availability varies spatially across the riverine landscape, so too does the abundance of individual species and the composition of species assemblages because fish seek areas that best suit their ecological requirements (Davies 1989; Arthington et al. 2005; Rice 2005). In the present study, habitat use by fish species was consistent with their published habitat preferences (Pusey et al. 2004) – some species were ubiquitous across sites (e.g. Tilapia mariae and Hypseleotris compressa), whereas others were strongly correlated with the presence of specific micro-habitat features. For example, Bunaka gyrinoides and Notesthes robusta were associated with root masses, whereas Awaous acritosus was found on shallow sand/gravel bars. Overall, 70% of the native species caught were fluvial specialists or fluvial dependents (Table 9), a higher percentage than reported for rivers in North America (45–67%) and Europe (41–54%; Galat and Zweimuller 2001), and almost certainly a result of the near-absence of non-fluvial habitats in the main channels of Wet Tropics rivers. Within the Mulgrave catchment, over 50% of off-channel habitats have been destroyed for agricultural development (Russell et al. 1996), effectively limiting the overall diversity of habitats available to fish species to what is essentially a main-channel habitat envelope – dominated by deeper, slower-flowing habitats with sand substrates and small areas of shallow, faster-flowing littoral habitats – within which present day fish-habitat dynamics occur.
Temporal variability in fish community structure
In most large, undisturbed tropical rivers, highly predictable floods inundate floodplains and increase habitat availability during the wet season (Junk et al. 1989). However, in Australia’s Wet Tropics region, the relatively deep incision of main channels increases the probability that high flow events will be erosive rather than expansive. Unlike expansive floods, which typically inundate floodplains for several months, erosive floods are characterised by a short pulse of fast-moving, turbulent water, with the power to entrain substrates and debris, scour vegetation, erode banks, in-fill pools and expose root masses (Matthews 1998). Such an event (a one-in-ten-year flood (MacNamara 1985)) occurred in the Mulgrave River during March 2004, triggering a series of habitat changes that lasted for more than 12 months. Instream vegetation, for example, was almost completely removed from the study area and, by May 2005, only emergent vegetation, consisting mostly of highly flood-tolerant para grass (Urochloa mutica (Forssk.) T.Q. Nguyen (Bunn et al. 1998)), had recovered to pre-flood levels.
The response of fish communities to seasonal habitat changes has not been previously assessed in the Wet Tropics. However, these dynamics are well documented for other regions of Australia (e.g. Koehn et al. 1994; Pollino et al. 2004). Arthington et al. (2005) described seasonal change in the fish assemblages of Cooper Creek, an arid-zone floodplain river. They found that marked changes in fish community structure between the early (April) and late (September) dry season were related to habitat loss: as water levels receded and pools dried up, within-waterhole features, such as boulders, root masses and large woody debris, were exposed and the number of sheltered places where fish could rest and forage was reduced (Arthington et al. 2005). Unlike systems in these drier areas, perennial rivers in the Wet Tropics remain connected throughout the dry season, and the extreme habitat shortages described by Arthington et al. (2005) are not encountered. Although the latter conditions could lead to relatively stable fish assemblages, seasonal habitat preferences of fish species in the present study matched variations in habitat availability within the main channel. Most species used a range of microhabitats and moved between habitat guilds on a seasonal basis. Kennard (1995) reported similar results in floodplain lagoons of the Normanby River. There, most species used the most common microhabitat type within each lagoon and temporal patterns in habitat use were dependent on seasonal patterns of habitat availability, rather than competition for space per se (Kennard 1995). In the present study, microhabitats in the Mulgrave River appeared to be more diverse and distinct during the dry season than during the wet season. When high-flow events removed instream and littoral vegetation, the heterogeneity of habitats within sites was greatly reduced. Habitat guild membership reflected these changes, with most species belonging to guild 1 (wide, deep habitats with mud substrate and large woody debris) during the wet season.
The structure of fish communities varied with this changing habitat use of individual species. During the dry season, the lowland assemblage was dominated by Neosilurus ater, H. compressa, A. acritosus and R. bikolanus, whereas Nematalosa erebi, A. agrammus and Glossamia aprion were more important during the wet season. In general, benthic species were important in determining assemblage structure during the dry season, whereas water column species were more important during the wet season. There are several ecologically plausible explanations for this pattern including: fish moved out of the study area to seek refuge from high wet season flows (Ross and Baker 1983; Winemiller and Jepsen 1998); reproductive activity was stimulated by flooding and species migrated out of the study area to find suitable habitats for spawning and recruitment (Orr and Milward 1984; Pusey et al. 1995b); food availability was reduced by wet season habitat disturbance and fish moved in order to target their preferred prey items (particularly aquatic invertebrates, which are highly sensitive to substrate disturbance (Rabeni and Minshall 1977; Pusey et al. 1995b; Winemiller and Jepsen 1998; Bishop et al. 2001; Kennard et al. 2001)); and/or biotic controls partly determined community structure (e.g. predation or competition for habitat, especially during the dry season (Hoeinghaus et al. 2003)). These possibilities are discussed in more detail below.
It should also be noted that our small boat-mounted electrofishing unit (2.5 kVA) was underpowered in deep (>3.5 m), fast-flowing (>1 m s–1) habitats (i.e. under wet season flow conditions), where it failed to stun some benthic species completely. Although it is possible that lower catch efficiency may have introduced methodological bias to our results, there was no significant difference in the mean proportional contribution of electrofishing samples to summed CPUE values between wet (mean = 0.22) and dry (mean = 0.21) seasons’ samples (t = –0.276, d.f. = 251, P = 0.783). Summing catch data across gear types may also increase the risk of committing type II errors in multivariate analyses by reducing the spread of sites in ordination space (i.e. Figs 6 and 7), but thoughtful combining of data from multiple gears can both preserve information and enhance detection of differences among sites (Hinch et al. 1991; Weaver et al. 1993). The conclusions reached in the present study match those drawn from ordination plots based on catch data from separate gear types (Rayner 2007) and we feel the dataset presented here is an accurate representation of the fish-habitat dynamics we observed in the field.
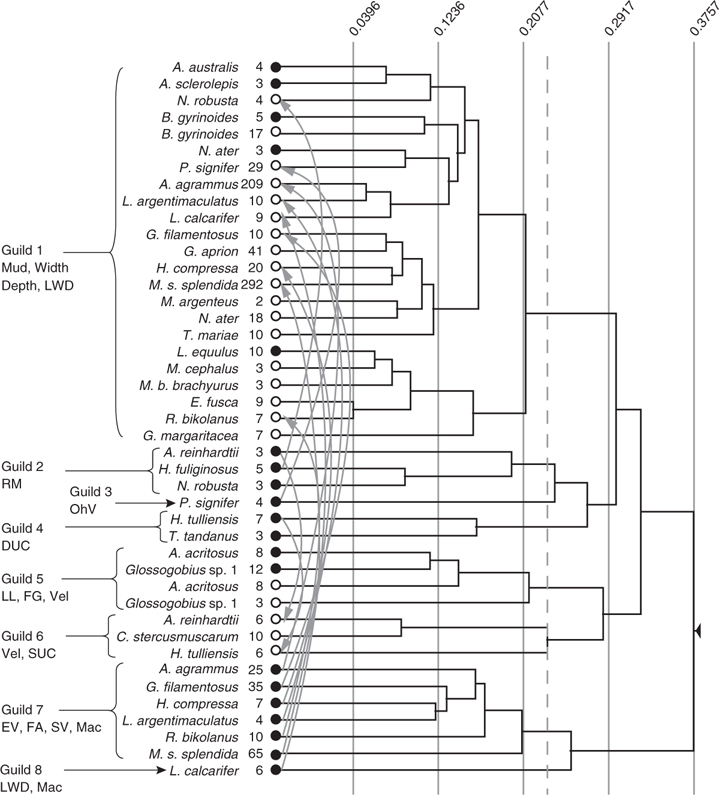
Conceptual model of fish-habitat dynamics for lowland Wet Tropics rivers
Various elements of existing conceptual frameworks of fish-habitat dynamics apply to the lowland rivers of Australia’s Wet Tropics. However, the unique geomorphology and hydrology of these rivers warrant a model that more clearly represents their spatial organisation and, particularly, their temporal function. We have developed a conceptual framework that depicts the fluvial main-channel habitats used by Wet Tropics fish species under different flow conditions (Fig. 9). During the dry season, a combination of the hierarchical patch dynamics model (Townsend 1989; Wu and Loucks 1995; Thorp et al. 2006) and the river continuum concept (Vannote et al. 1980) apply. Habitat patches, ranging in size from ~10 to 1000 m2 and sharing similar abiotic characteristics, occur repeatedly along the lowland reach of the river (i.e. within a single functional process zone (see Thorp et al. 2006)). Overlaying these habitat patches are longitudinal gradients of habitat change, such as increasing depth and conductivity – the latter related to greater tidal penetration under low flow conditions – from upstream to downstream. This assortment of habitat patches and gradients is reflected in the distribution of individual fish species and, therefore, the composition of the fish assemblage present. For example, the goby A. acritosus is found on shallow point bars, with gravel/sand substrate and some leaf litter, at the upper and lower extremes of the study area (i.e. irrespective of the position within the functional process zone), whereas L. calcarifer, a large-bodied piscivore that uses estuarine habitats for spawning, is found in relatively wide and deep downstream habitats, but not in upstream areas.
Variability in microhabitat patches and the relative importance of longitudinal gradients in determining fish assemblage structure appears to be regulated by wet season flooding. However, one could ask, given that Wet Tropics rivers experience predictable flooding, what constitutes a disturbance in these systems? Is the magnitude of flooding linearly related to the degree of disturbance, or are there thresholds of response for different variables (e.g. habitat characteristics, benthic or littoral invertebrate communities, fish assemblages)? In the present study, we documented a reduction in the type and number of distinct habitat types following floods with return periods of one-in-five- to one-in-ten-years (i.e. all habitats were characterised by relatively deep water over bare sand substrates). Unlike larger tropical systems, the flash-like nature of these events meant that there was little opportunity for the build-up of huge stocks of water hyacinths, grasses or other macrophytes during the wet season, as predicted by the flood pulse concept (Junk et al. 1989; Winemiller 2004). Instead, the reverse was true: instream and littoral vegetation accumulated in the main channel under base-flow conditions before being removed by the erosive action of wet-season floods. Smaller floods (annual to one-in-three year events) had similar effects, but on more limited spatial and temporal scales – microhabitats, rather than the entire channel, and weeks, rather than months.
Seasonal changes in fish community structure are driven primarily by changes in the abundances of key species. In the present study, main-channel habitats were used by a diverse fish community at all times of the year, but because the habitat preferences of individual species varied seasonally in most cases and fish obviously moved around the riverine landscape, factors other than habitat diversity and availability must also affect fish communities. Further research is required to assess the relative merit of the four ecological explanations mentioned above. However, we hypothesise that movements associated with reproduction and feeding are most likely responsible for the observed patterns. Many Wet Tropics fish species spawn during the wet season. Pusey et al. (2004) and Bishop et al. (2001) described longitudinal movements by species such as T. tandanus, L. calcarifer and N. erebi, which they suggested were associated with the need for these species to access suitable spawning habitats. In addition, Pusey et al. (1995b) identified five feeding guilds in the Mulgrave and South Johnstone Rivers, noting the importance of fish size in determining guild membership. However, as no direct analyses of temporal dynamics or food availability were included, the authors had difficulty accounting for some of the more complex feeding patterns. They concluded by asking: is dietary partitioning in lowland habitats related to differences in microhabitat usage; does the overall pattern of resource partitioning change seasonally; and if dietary overlap is high, but food does not appear to be limited, what other factors act to restrict fish abundance? Clearly there is a need for further research into the dynamics of fish movements in response to flow events in Wet Tropics rivers.
Following the flood peak, habitat variables and fish communities in the main-channel enter a transition period, during which deterministic factors related to biotic interactions become more important in structuring fish assemblages. As flood waters recede, wetted width, water depth and flow velocity are reduced along the entire lowland reach. Deposition of sand and organic matter, together with the expansion of remnant patches of aquatic plants, occurs across the channel, particularly on and around mid-channel bars. Although habitat space is slightly reduced, the diversity of microhabitat types increases during this period. As conditions settle, fish quickly recolonise the lowland reach (at periods ranging from days to weeks). This resilience of fish communities to flooding is not surprising, given their high dispersal rates and the lack of physical barriers to recolonisation within lowland reaches (Meffe and Sheldon 1990; Kennard 2005). We would also expect the resilience of fish assemblages to be greater than that of other taxonomic groups – epibenthic invertebrates, for example, are highly sensitive to the degree of substrate disturbance at individual sites (Townsend et al. 1997) and may take much longer (i.e. up to and exceeding 12 months) to recover.
Applicability to other small tropical rivers
Research in small tropical rivers worldwide has repeatedly identified four key factors that regulate fish community structure. These are: the distance of a study site from the river mouth (Lyons and Schneider 1990; Winemiller and Leslie 1992; Pusey and Kennard 1996; Hoeinghaus et al. 2003; Ibanez et al. 2007); the biological characteristics of the individual species present, including their feeding morphology and dietary composition, life-history requirements and water quality tolerances (notably salinity and pH); the duration and intensity of wet season flooding (Pearsons and Li 1992; Agostinho and Zalewski 1995; Hoeinghaus et al. 2003); and the complexity and diversity of micro- and mesohabitat features (Gorman and Karr 1978; Pearsons and Li 1992). In general, authors report distinct longitudinal changes in fish assemblage structure from upstream to downstream (owing to addition or replacement of species according to their salinity tolerance; Winemiller and Leslie 1992; Pusey et al. 1995a), with a relatively stochastic redistribution of fish within the catchment following wet season flooding (Rodriguez and Lewis 1994; Rodriguez and Lewis 1997). The duration and intensity of flooding then influences the shift from stochastic to deterministic assemblage controls during the transition from wet to dry seasons as microhabitat patches recover from disturbance and interspecific interactions (particularly predation) control recolonisation of individual sites or tributaries (Zaret and Rand 1971; Rodriguez and Lewis 1994). Our aim here has been to take the first steps towards developing a model that embraces these large-scale dynamics, but provides a more specific framework for the ongoing assessment of fine-scale dynamics that control fish distributions within individual river reaches. Too often authors are left pondering the mechanics driving fish-habitat relationships, but are not armed with a conceptual framework for more specific hypothesis testing. The dynamics we observed in the Mulgrave River also provide support for tenets 1 and 5 proposed by Thorp et al. (2006) in their Riverine Ecosystem Synthesis. These tenets stress the importance of habitat patches and a hierarchical habitat template, combined with short- and long-term flows, in regulating community dynamics and species interactions. As Thorp et al. (2006) suggest, any observed biocomplexity in river networks is possibly a response to both the characteristic variability and the probable mean state of the environment in any given functional process zone.
We have demonstrated: (i) the high importance of the main channel in river systems with poorly connected floodplains, particularly those that have been anthropogenically degraded; and (ii) the role of erosive (rather than expansive) wet season floods in regulating the structure and ecological function of fish assemblages and their habitats. In this sense, small tropical rivers could be said to act like tributaries of larger tropical systems or even temperate systems (albeit with highly predictable flow regimes). However, many questions remain unanswered and we believe, especially given that the response of biotic communities to disturbance can vary markedly between taxonomic groups and river systems (Ward and Stanford 1983; Lake 2000), expansion of the model presented, to include invertebrate communities and the associated trophic dynamics of fish, would greatly aid our understanding of the role of seasonal flows in regulating fish assemblage structure in small tropical rivers worldwide.
Acknowledgements
This project was funded by grants from the Cooperative Research Centre for Tropical Rainforest Ecology and Management (Rainforest CRC) and James Cook University (JCU). Mike Steele (JCU), Lee Belbin (Blatant Fabrications) and Mark Kennard (Griffith University) assisted with statistical analyses. Mirjam Maughan (JCU) helped prepare Fig. 1. In-kind support was provided by Queensland Department of Primary Industries – Fisheries. Alan Hooper (Queensland Department of Natural Resources and Mines) provided hydrological data for the Mulgrave River. Field assistance was provided by Colton Perna, Zoe Baker, Paul Thuesen, Paul Godfrey, Amanda Soymonoff, Mo Healy, Anne Gulliard, Megan Barnes, Cameron Crothers-Stomp, Andrew Kaus, Andrew Jones, Rusty Ligon and Michael Pusey. Access to private land and other assistance in the field was provided by the Rossi, Thomasen and Moller families. We would like to thank two anonymous reviewers for their comments on this manuscript.
Agostinho, A. A. , and Zalewski, M. (1995). The dependence of fish community structure and dynamics on floodplain and riparian ecotone zone in Parana River, Brazil. Hydrobiologia 303, 141–148.
Bray, R. J. , and Curtis, J. T. (1957). An ordination of the upland forest communities of Southern Wisconsin. Ecological Monographs 27, 325–349.
| Crossref | GoogleScholarGoogle Scholar |
Gower, J. C. (1971). A general coefficient of similarity and some of its properties. Biometrics 27, 857–874.
| Crossref | GoogleScholarGoogle Scholar |
Harvey, B. C. (1991). Interactions among stream fishes: predator-induced habitat shifts and larval survival. Oecologia 87, 29–36.
| Crossref | GoogleScholarGoogle Scholar |
Kelly, T. M. , Jones, J. D. , and Smith, G. R. (1975). Historical changes in mercury contamination in Michigan Walleyes. Journal of the Fisheries Research Board of Canada 32, 1745–1754.
King, A. J. (2004). Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river. Journal of Fish Biology 65, 1582–1603.
| Crossref | GoogleScholarGoogle Scholar |
Lyons, J. , and Schneider, D. W. (1990). Factors influencing fish distribution and community structure in a small coastal river in southwestern Costa Rica. Hydrobiologia 203, 1–14.
| Crossref | GoogleScholarGoogle Scholar |
Martin-Smith, K. M. (1998). Relationships between fishes and habitat in rainforest streams in Sabah, Malaysia. Journal of Fish Biology 52, 458–482.
Meffe, G. K. , and Sheldon, A. L. (1990). Post-defaunation recovery of fish assemblages in southeastern blackwater streams. Ecology 71, 657–667.
| Crossref | GoogleScholarGoogle Scholar |
Rabeni, C. I. , and Minshall, G. M. (1977). Factors affecting microdistribution of stream benthic insects. Oikos 29, 33–43.
| Crossref | GoogleScholarGoogle Scholar |
Rice, J. C. (2005). Understanding fish habitat ecology to achieve conservation. Journal of Fish Biology 67, 1–22.
| Crossref | GoogleScholarGoogle Scholar |
Russell, D. J. , Ryan, T. J. , McDougall, A. J. , Kistle, S. E. , and Aland, G. (2003). Species diversity and spatial variation in fish assemblage structure of streams in connected tropical catchments in northern Australia with reference to the occurrence of translocated and exotic species. Marine and Freshwater Research 54, 813–824.
| Crossref | GoogleScholarGoogle Scholar |
Schlosser, I. J. (1982). Fish community structure and function along two habitat gradients in a headwater stream. Ecological Monographs 52, 395–414.
| Crossref | GoogleScholarGoogle Scholar |
Ward, J. V. (1989). The four-dimensional nature of lotic ecosystems. Journal of the North American Benthological Society 8, 2–8.
| Crossref | GoogleScholarGoogle Scholar |
Weaver, M. J. , Magnuson, J. J. , and Clayton, M. K. (1993). Analyses for differentiating littoral fish assemblages with catch data from multiple sampling gears. Transactions of the American Fisheries Society 122, 1111–1119.
| Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O. (1983). An introduction to the freshwater fish communities of Corcovado National Park, Costa Rica. Brenesia 21, 47–66.
Winemiller, K. O. , and Jepsen, D. B. (1998). Effects of seasonality and fish movement on tropical river food webs. Journal of Fish Biology 53, 267–296.

Winemiller, K. O. , and Leslie, M. A. (1992). Fish assemblages across a complex, tropical fresh-water marine ecotone. Environmental Biology of Fishes 34, 29–50.
| Crossref | GoogleScholarGoogle Scholar |

Wu, J. , and Loucks, O. L. (1995). From balance of nature to hierarchical patch dynamics: A paradigm shift in ecology. The Quarterly Review of Biology 70, 439–466.
| Crossref | GoogleScholarGoogle Scholar |

Zaret, T. M. , and Rand, A. S. (1971). Competition in tropical stream fishes: support for the competitive exclusion principle. Ecology 52, 336–342.
| Crossref | GoogleScholarGoogle Scholar |