Energy balance as a determinant of two-phase growth in cephalopods
Eric P. M. Grist A C and George D. Jackson BA CSIRO Marine Research, GPO Box 1538, Hobart, Tas. 7001, Australia.
B Institute of Antarctic and Southern Ocean Studies, University of Tasmania, PO Box 252-77, Hobart, Tas. 7001, Australia.
C Corresponding author. Email: ericg@stams.strath.ac.uk
Marine and Freshwater Research 55(4) 395-401 https://doi.org/10.1071/MF03154
Submitted: 7 October 2003 Accepted: 30 March 2004 Published: 22 June 2004
Abstract
Many cephalopods exhibit early exponential growth, which abruptly shifts to a much slower rate. Using a simple model of the energy balance between intake from food and expenditure in growth plus metabolism, we consider how the two-phase growth pattern may be explained in terms of energy conservation. We determine the post-hatch size and age at which exponential growth would be expected to terminate. The model is tested with laboratory hatchling data obtained for the giant Australian cuttlefish Sepia apama. Together with growth data obtained for a related species, Sepia officinalis, model projections for critical transition size and age interestingly suggest that the metabolism of S. apama in the natural habitat may be three to four times higher than in captivity. A sensitivity analysis indicates that the critical transition size is in general more sensitive than critical transition time to any invoked changes in metabolic rate.
Extra keywords: critical transition size, critical transition time, cuttlefish, energy conservation, growth, metabolism.
Acknowledgments
We are extremely grateful to Ron O’Dor for correspondence and discussions on the energy balance ‘checksum’ concept and to Jill Aitken for data collected on Sepia apama.
Aitken, J. P. (2001). The Bioenergetics of the Giant Australian Cuttlefish Sepia apama. MSc Thesis. (Dalhousie University: Halifax, Nova Scotia, Canada)
DeRusha, R. H. , Forsythe, J. W. , and Hanlon, R. T. (1987). Laboratory growth, reproduction and life span of the Pacific pygmy octopus Octopus digueti. Pacific Science 41, 104–121.

Forsythe, J. W. (1993). A working hypothesis of how seasonal temperature change may impact the field growth of young cephalopods. In
Forsythe, J. W. (2004). Accounting for the effect of temperature on squid growth in nature: from hypothesis to practice. Marine and Freshwater Research 55, 331–339.

Forsythe, J. W. , and Hanlon, R. T. (1988). Effect of temperature on laboratory growth, reproduction and life span of Octopus bimaculoides. Marine Biology 98, 369–379.

Forsythe, J. W. , and Hanlon, R. T. (1989). Growth of the Eastern Atlantic squid, Loligo forbesi Steenstrup (Mollusca: Cephalopoda). Aquaculture and Fisheries Management 20, 1–14.

Forsythe, J. W. , DeRusha, R. H. , and Hanlon, R. T. (1994). Growth, reproduction and lifespan of Sepia officinalis (Cephalopoda: Mollusca) cultured through seven consecutive generations. Journal of Zoology 233, 175–192.

Forsythe, J. W. , Walsh, L. S. , Turk, P. E. , and Lee, P. G. (2001). Impact of temperature on juvenile growth and age at first egg-laying of the Pacific reef squid Sepioteuthis lessoniana reared in captivity. Marine Biology 138, 103–112..
| Crossref | GoogleScholarGoogle Scholar |

Grist, E. P. M. , and des Clers, S. (1998). How seasonal temperature variations may influence the structure of annual squid populations. Institute of Mathematics and its Applications. Journal of Mathematic Applied to Medicine and Biology 14, 1–22.

Grist, E. P. M. , and des Clers, S. (1999). Seasonal and genotypic influences on life cycle synchronisation: further insights from annual squid. Ecological Modelling 115, 149–163..
| Crossref | GoogleScholarGoogle Scholar |

Hatfield, E. M. C. (2000). Do some like it hot? Temperature as a possible determinant of variability in the growth of the Patagonian squid, Loligo gahi (Cephalopoda: Loliginidae). Fisheries Research 47, 27–40..
| Crossref | GoogleScholarGoogle Scholar |

Hatfield, E. M. C. , Hanlon, R. T. , Forsythe, J. W. , and Grist, E. P. M. (2001). Laboratory testing of a growth hypothesis for juvenile squid Loligo pealeii (Cephalopoda: Loliginidae). Canadian Journal of Fisheries and Aquatic Sciences 58, 845–857..
| Crossref | GoogleScholarGoogle Scholar |

Jackson, G. D. (1994). Application and future potential of statolith increment analysis in squids and sepioids. Canadian Journal of Fisheries and Aquatic Sciences 51, 2612–2625.

Jackson, G. D. , and Domeier, M. L. (2003). The effects of an extraordinary El niño/La niña event on the size and growth of the squid Loligo opalescens off Southern California. Marine Biology 142, 925–935.

Jackson, G. D. , and Moltschaniwskyj, N. A. (2001). The influence of ration level on growth and statolith increment width of the tropical squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae): an experimental approach. Marine Biology 138, 819–825..
| Crossref | GoogleScholarGoogle Scholar |

Jackson, G. D. , and O’Dor, R. K. (2001). Time, space and the ecophysiology of squid growth, life in the fast lane. Vie et Milieu 51, 205–215.

Jackson, G. D. , Forsythe, J. W. , Hixon, R. F. , and Hanlon, R. T. (1997). Age, growth and maturation of Lolliguncula brevis (Cephalopoda: Loliginidae) in the Northwestern Gulf of Mexico with a comparison of length-frequency vs. statolith age analysis. Canadian Journal of Fisheries and Aquatic Sciences 54, 2907–2919..
| Crossref | GoogleScholarGoogle Scholar |

Kleiber, M. (1932). Body size and animal metabolism. Hilgardia 6, 315–353.

Kleiber, M. (1947). Body size and metabolic rate. Physiological Reviews 27, 511–541.

Minton, J. W. (2004). The pattern of growth in the early life-cycle of individual Sepia pharaonis. Marine and Freshwater Research 55, 415–422.

Norman, M. (2000).
O’Dor, R. K. , and Hoar, J. A. (2000). Does geometry limit squid growth? ICES Journal of Marine Science 57, 8–14..
| Crossref | GoogleScholarGoogle Scholar |

O’Dor, R. K. , and Webber, D. M. (1986). The constraints on cephalopods: why squid aren’t fish. Canadian Journal of Zoology 64, 1591–1605.

O’Dor, R. K. , Forsythe, J. , Webber, D. M. , Wells, J. , and Wells, M. J. (1993). The cost of living for Nautilus in the wild. Nature 362, 626–628..
| Crossref | GoogleScholarGoogle Scholar |

O’Dor, R. K. , Hoar, J. A. , Webber, D. M. , Carey, F. G. , Tanaka, S. , Martins, H. , and Porteiro, F. M. (1994). Squid (Loligo forbesi) performance and metabolic rates in nature. Marine and Freshwater Behaviour and Physiology 25, 163–177.

Pauly, D. , Christensen, V. , Dalsgaard, J. , Froese, R. , and Torres, F. (1998). Fishing down marine food webs. Science 279, 860–863..
| Crossref | GoogleScholarGoogle Scholar |

Pörtner, H. O. , and Zielinski, S. (1998). Environmental constraints and the physiology of performance in squids. South African Journal of Marine Science 20, 207–221.

Soofiani, N. M. and Hawkins, A. D. (1985). Field studies and energy budgets. In
Wells, M. J. , O’Dor, R. K. , Mangold, K. , and Wells, J. (1983). Feeding and metabolic rate in Octopus. Marine Behaviour and Physiology 9, 305–317.

Withers, P. C. (1992).
Appendix 1. Growth regression coefficient
Body mass B at time t is given by
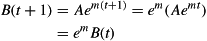
Hence, body mass increases by a factor em each day and since for small m:
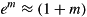
the growth regression coefficient m also represents percentage increase in body mass per day for small daily percentage increases.


