Pop-up archival satellite tagging of Carcharias taurus: movements and depth/temperature-related use of south-eastern Australian waters
N. M. Otway A B and M. T. Ellis AA Industry and Investment NSW, Port Stephens Fisheries Institute, Taylors Beach, NSW 2315, Australia.
B Corresponding author. Email: nick.otway@industry.nsw.gov.au
Marine and Freshwater Research 62(6) 607-620 https://doi.org/10.1071/MF10139
Submitted: 15 June 2010 Accepted: 13 December 2010 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Knowledge of migratory movements and depth/temperature-related use of coastal waters by sharks can lead to more sustainable fisheries and assist in managing the long-term conservation of those species now considered threatened. Pop-up archival satellite tags (PATs) provide an alternative to conventional tagging for documenting migratory movements. This study focussed on the migratory movements of Carcharias taurus, a critically endangered shark found along the east coast of Australia. From October 2003 to July 2008, 15 C. taurus individuals were tagged with PATs with varying deployments (60–150 days) and acoustic tags linked to an acoustic monitoring system providing accurate geo-location. Distances moved by C. taurus individuals ranged from 5 to 1550 km and varied according to sex and season. Migrations north and south were punctuated en route by occupation of sites for varying periods of time. The deepest depth recorded was 232 m off South West Rocks on the New South Wales mid-north coast. On average, C. taurus males and females spent at least 71% of their time in waters <40 m and 95% of their time in waters 17–24°C. By mainly occupying inshore waters, C. taurus is exposed to potentially adverse fishing-related interactions that may be difficult to mitigate.
Additional keywords: acoustic tagging, tag performance.
Introduction
Knowledge of migratory movements is fundamental to understanding a species’ large-scale spatial and temporal patterns of abundance, reproduction, demography and capacity to withstand exploitation through directed fisheries or when captured as by-catch in another fishery. This is particularly true for many sharks, whose life-history characteristics, comprising slow growth, late onset of sexual maturity and low fecundity (Hoenig and Gruber 1990; Cortés 2000; Mollet and Cailliet 2002), make them extremely susceptible to targeted fishing (McAuley et al. 2007) and incidental capture in non-elasmobranch fisheries (e.g. Marín et al. 1998; Moyes et al. 2006; Campana et al. 2009). Fishing mortality, even at relatively low levels, can have severe consequences and results in dramatic population declines (Baum et al. 2003; Myers and Worm 2003; Baum and Myers 2004), especially for species with extremely low fecundity such as the grey nurse shark, Carcharias taurus Rafinesque, 1810.
In the past, C. taurus had a broad, albeit disjunct, distribution on inshore continental shelves in cool temperate to subtropical waters off the main continental landmasses (Compagno 2002; Last and Stevens 2009). With extensive overfishing and resulting population declines over much of its global range (Musick et al. 1993; Lucifora et al. 2002; Otway et al. 2004), it is doubtful that C. taurus still exists in the Mediterranean Sea (Fergusson et al. 2002). Its main remaining populations are now likely restricted to the east coasts of North and South America, South Africa and the east and west coasts of Australia. Globally, C. taurus is listed as ‘Vulnerable’ in the IUCN Red List of Threatened Species (Cavanagh et al. 2003).
In Australia, C. taurus occur as two separate and genetically distinct populations on the east and west coasts (Cavanagh et al. 2003; Stow et al. 2006). The species has been caught occasionally in the Arafura Sea, but has not been found in Tasmanian waters (Read and Ward 1986; Last and Stevens 2009). The range of the population off west Australia is less well known, but analysis of by-catch of commercial shark fisheries indicates that C. taurus may occupy sites from North-West Cape (21°46.00′S, 114°09.00′E) to the coastal waters off Cocklebiddy (32°15.00′S, 126°12.00′E) in the Great Australian Bight (Cavanagh et al. 2003). In contrast, the eastern Australian population has, over the past century, been subjected to many fisheries (Caldwell and Ellison 1939; Cropp 1964; Pepperell 1992; Reid and Krogh 1992), causing a markedly reduced population that extends from Yeppoon, Queensland (Qld) to Eden, New South Wales (NSW) (Fig. 1). C. taurus is now listed as critically endangered along the east coast of Australia under the IUCN (Cavanagh et al. 2003), NSW, Qld and Federal legislation.
|
The species is a relatively placid, strong-swimming shark that aggregates in rocky gutters, caves or under overhangs around inshore rocky reefs and islands. The shark is mainly piscivorous (Bass et al. 1975; Smale 2005) and has been recorded to depths of 191 m (Pollard et al. 1996; Compagno 2002). Like many elasmobranchs, C. taurus exhibits late onset of sexual maturity (USA, males: 6–7 years, females: 9–10 years), low fecundity (two pups born biennially after intrauterine cannibalism and oophagy – Springer 1948; Gilmore et al. 1983) and longevity of ≈35 years (Branstetter and Musick 1994; Goldman et al. 2006). Their biology and specific habitats make them extremely vulnerable to over-exploitation, with reduced populations requiring decades to recover (Smith et al. 1998; Mollet and Cailliet 2002; Otway et al. 2004).
An understanding of the migratory movements of C. taurus and its associated reproductive cycle (Bass et al. 1975; Gilmore et al. 1983; Smale 2002) has emanated from conventional tagging in the USA (Kohler et al. 1998) and South Africa (Davies and Joubert 1966; Dicken et al. 2007). However, the emergence of patterns from these studies has required tagging over decades and the long-term commitment of the recreational anglers involved. In contrast, preliminary data on the migration of east Australian C. taurus have been obtained using underwater visual censuses (Otway et al. 2003), conventional (cattle-ear) tagging (Otway and Burke 2004) and acoustic tagging (Bruce et al. 2005). These studies all inferred a northerly migration over autumn–winter months and a southerly migration during spring–summer months.
In 2003, conventional tagging of C. taurus off the east Australian coast was stopped by the Federal government over concerns that tag bio-fouling and associated abrasions could be detrimental to the sharks (Department of the Environment and Heritage 2003). Pop-up archival satellite tags (PATs) provide an alternative to conventional tagging and have yielded detailed information on the migratory movements and post-release survival of a range of sharks (e.g. Weng et al. 2007; Campana et al. 2009; Stevens et al. 2010). As fishing is a key threatening process for C. taurus, it is important to identify the frequency and spatial extent of fishing-related interactions. To do so requires an understanding of the shark’s migratory movements and depth/temperature-related use of coastal waters. Moreover, demographic modelling (Otway et al. 2004) has shown that mitigating these anthropogenic threats, particularly with immature females, is critical to the recovery and long-term survival of the east coast C. taurus population. Hence, this study used PATs to quantify the migratory routes, distances travelled and the depth/temperature-related occupation of east Australian waters by C. taurus. A serendipitous event also enabled an assessment of the survival and movements of a sexually mature female after the removal of a fishing gaff embedded in the animal’s oesophagus.
Materials and methods
Study sites
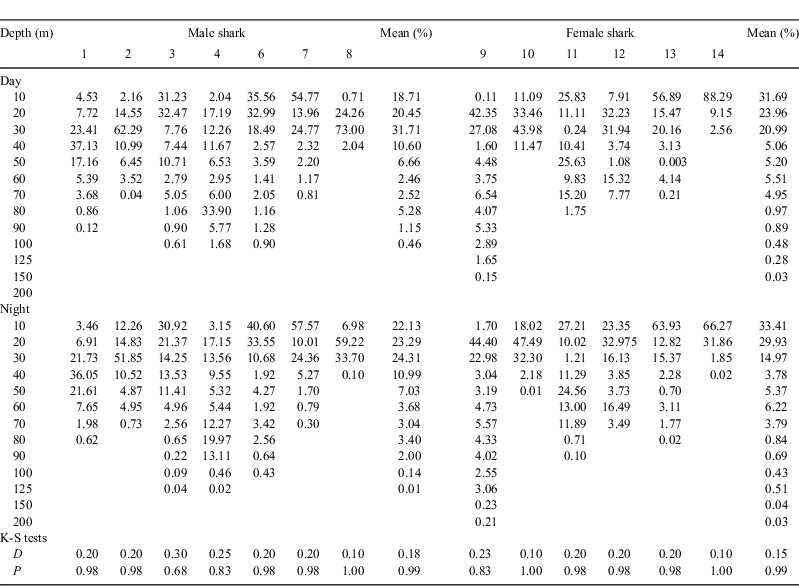
Tagging was done at Fish Rock off South West Rocks (30°56.25′S, 153°06.05′E) and Julian Rocks off Byron Bay (28°37.00′S, 153°37.75′E) between October 2003 and July 2008 (Fig. 1). Fish Rock lies in 20–40 m of water, 2.1 km offshore of Smoky Cape, and the surrounding reef supports aggregations of C. taurus and a diverse temperate fish community with some tropical species present during the warmer months (Otway et al. 2003). In contrast, Julian Rocks is 3.5 km offshore of Byron Bay in 20 m of water. The site supports a diverse tropical and temperate fish community throughout the year (Harriott et al. 1997), with C. taurus aggregating in the gutters to the north of the site during winter months. Males are generally more abundant in June, while putatively pregnant females are present from July (Hayward 2003; Otway et al. 2003). Both sites are influenced by the East Australian Current (EAC) (Tranter et al. 1986), onshore winds and a prevailing 1–2-m south-easterly swell. Mean seawater temperatures range from ≈19.0°C in winter to ≈25.0°C in summer, with fluctuations occurring with EAC reversals, internal waves, upwellings and weather systems. The resulting daily variation in seawater temperature at both sites is greatest in summer and least in winter (N. M. Otway and M. T. Ellis, unpubl. data).
Pop-up archival satellite tags and geo-location of sharks
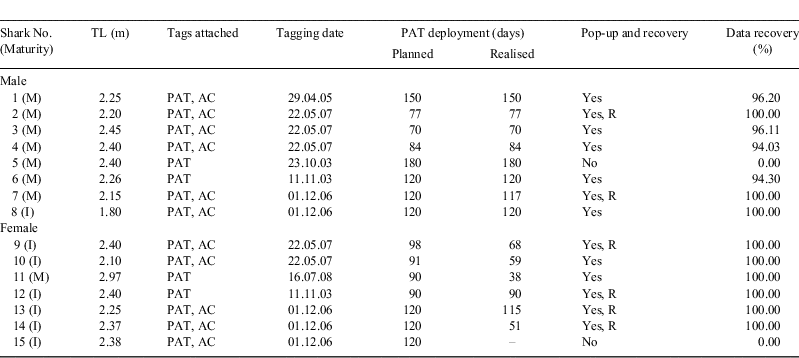
The pop-up archival satellite tags used included four PTT 100 PATs (Microwave Telemetry, Columbia, MD, USA) and eleven Mk 10 PATs (Wildlife Computers, Redmond, WA, USA). These tags archive data on temperature, pressure (depth) and light levels and transmit this information in different formats via the ARGOS system following release. Microwave Telemetry PATs transmit hourly readings, whereas Wildlife Computers PATs transmit summarised data comprising time at depth, time at temperature histograms and depth temperature profiles for 12 user-defined bins. If released tags are recovered, the archived data can be retrieved.
PATs were programmed with a range of deployment durations (Table 1) for two reasons. First, rough seas can greatly affect the transmission of archived data to the ARGOS system and differing deployment periods can help ensure that PATs deployed contemporaneously are not exposed to the same adverse sea conditions following release, thus enhancing data recovery. Second, using differing deployment periods hopefully permits the staggering of PAT releases along the route, enabling a better determination of the overall migratory path.
Sharks were also tagged with Vemco V16TP, R-coded, 69-kHz acoustic tags (Amirix Systems Inc., Nova Scotia, Canada) with randomised transmission delays of 60–183 s. The tags were detected on the SE Australian coastal acoustic monitoring system (SEACAMS), comprising a network of ≈60 Vemco VR2W acoustic listening stations deployed at numerous sites along the NSW coast (Fig. 1).
The locations of PAT-tagged C. taurus individuals during their migrations were determined using a variety of methods. In order of decreasing accuracy, these included: SEACAMS; observations by recreational SCUBA divers with the shark’s sex, total length (TL), tag position (left or right) and date sighted used for identification; and the geo-positioning algorithms associated with the light curves obtained from each PAT together with comparisons of measured depths and water temperature to charted bathymetry and sea-surface temperatures.
The final (pop-up) locations of PAT-tagged C. taurus individuals were determined using either SEACAMS or position fixes derived from the ARGOS system. When acoustic detections were obtained on SEACAMS at the time of the PAT’s release, the known position of the particular listening station was used. Alternatively, the shark’s final position was determined using the time of the first PAT transmission combined with positional fixes of the released PAT provided by the ARGOS system. The reliability of these satellite fixes is coded in location classes (LC) with increasing accuracy from B through A, 0, 1, 2 and 3, with the most accurate position (LC 3) having a root mean square error of <150 m ARGOS (2008). The time of the first PAT transmission, the first satellite fix classified as LC 3 or LC 2 following PAT release and the post-release surface track were then used to back-calculate the position of a PAT when released.
Tagging procedures
Prior to deployment, each tag was attached to ≈10 cm of monofilament line (100 kg breaking strain), which was, in turn, attached to an intra-muscular dart made of surgical plastic. The acoustic tags were then inserted into small floats to provide buoyancy and prevent abrasions of the shark’s skin. As fouling organisms can reduce a PAT’s buoyancy and prevent surfacing following release (Hays et al. 2007) and conventional tags on C. taurus have become fouled (Dicken et al. 2006; N. M. Otway, unpubl. data), the PAT and acoustic tags were painted with two coats of antifouling paint.
C. taurus individuals were captured at Fish Rock and Julian Rocks using a similar method that was modified at each site because of the different vessels used. While detailed descriptions can be found in Smith (1992) and Otway et al. (2009), briefly, the chosen C. taurus individual was caught by SCUBA divers using a lasso, taken to the surface and placed in a partially submerged stretcher on or next to the boat. This process took no longer than 10 min and resulted in minimal struggling by C. taurus. Each shark was then placed in dorsal recumbency to induce tonic immobility (Watsky and Gruber 1990; Henningsen 1994) and examined for evidence of injuries associated with its capture or prior fishing interactions. This was done as previous studies have shown that many C. taurus have hooks and associated nylon line and/or wire trace embedded in and around their jaws, buccal cavity and gills (Otway et al. 2003; Otway 2004; Bansemer and Bennett 2009). Immediately before tagging, the shark was rolled into left or right lateral recumbency to provide easier access to the tagging site. The PAT and acoustic tags were attached on either side of the first dorsal fin by inserting the plastic dart through a 5-mm incision in the skin covering the epaxial muscle mass using a stainless-steel applicator. The TL of each shark, with caudal fin in the depressed position (Francis 2006), was then measured to the nearest cm. Reproductive data from the east Australian C. taurus population obtained via tagging and necropsies (n = 211) over the past 10 years have shown that 50% of males and females are sexually mature at 2.101 (s.e. = 0.063) m TL and 2.587 (s.e. = 0.027) m TL, respectively (Otway et al. 2009). Consequently, the sex of the shark was determined via the presence of claspers in males, the length and calcification of which were used in combination with TL to determine sexual maturity. The sexual maturity of captured females was determined using TL together with the presence/absence of hymen. Finally, any embedded fishing gear was removed and the shark released.
Data processing and statistical analyses
Comparisons of the planned (programmed) and realised periods of PAT deployment and similar comparisons between the sexes were done using t-tests (Snedecor and Cochran 1967). Depth and temperature data from the PATs were examined and 6-hourly means calculated. The means were linked to known positions and used to provide depth and temperature profiles for individual legs in each migratory path. Kolmogorov–Smirnov (K-S) tests (Sokal and Rohlf 1969) were used to examine the percentages of time that C. taurus spent in waters of various depths during the hours of daylight and darkness for individual sharks and between the sexes using means for males and females. Similarly, the mean percentages of time that C. taurus spent in waters of various temperatures were compared between the sexes using K-S tests after pooling across individual males and females.
Results
Tagging
Fifteen C. taurus individuals were tagged with PATs between October 2003 and July 2008, with 11 individuals also tagged with an acoustic tag (Table 1). Fourteen C. taurus individuals were tagged at Fish Rock, with 87.5% of males sexually mature and 100% of females sexually immature. Despite this, the mean (±s.e.) TL of males (2.24 ± 0.073) and females (2.32 ± 0.049) did not differ significantly between the sexes (t12 = –0.89, P = 0.39) when pooled over the autumn–winter and spring–summer tagging periods. The last C. taurus tagged was captured at Julian Rocks to remove a 1.07-m long fishing gaff embedded in the animal’s oesophagus. Following the gaff’s successful removal, this sexually mature, 2.97-m TL female was tagged with a PAT to assess the shark’s survival and subsequent movements.
None of the sharks caught using the lasso became overly stressed following capture and restraint, tagging, the associated measurements and the removal of fishing gear. Diver observations indicated that within minutes of release, the swimming behaviour of each tagged shark was indistinguishable from those of the surrounding C. taurus individuals. Additional reports including video footage of PAT-tagged sharks were provided by recreational SCUBA divers on numerous occasions throughout the tag deployments and also indicated that the sharks were swimming normally and unaffected by the presence of the tags.
Performance of PATs
Two PATs failed to transmit any data via the ARGOS system (Table 1). The first tag, deployed on a C. taurus male, was observed by SCUBA divers at Big Seal Rock on two occasions. On the first occasion (a week before the PAT’s release), the tag had several barnacles, with basal diameters of ≈15 mm, growing on the float. On the second occasion (10 days after the tag’s scheduled release), the PAT was absent. The second tag, deployed on a C. taurus female, was not detected on SEACAMS or observed by divers. A further seven PATs were washed ashore over a wide geographic range from Main Beach, North Stradbroke Island (Qld) to Cookies Beach, Durras (southern NSW) and six were recovered, enabling 100% of the archived data to be obtained (Table 1). Transmissions from the remaining PATs enabled varying amounts of data to be obtained (Table 1).
While the planned PAT deployment periods in autumn–winter and spring–summer varied (Table 1), the mean (±s.e.) planned deployment periods of C. taurus males (115.13 ± 13.33 days, n = 8) and females (101.50 ± 5.98 days, n = 6) did not differ significantly (t12 = 0.93, P = 0.37). In contrast, the mean realised PAT deployment period of C. taurus males was significantly greater than that of females (t12 = 2.54, P = 0.026) and this difference appeared to be unaffected by the timing of tag deployment (Table 1). The planned and realised PAT deployment periods also varied with sex. With males, the mean (±s.e.) planned (115.13 ± 13.33 days) and realised (114.75 ± 13.32 days) deployment periods did not differ significantly (t7 =1.00, P = 0.35). However, for females, the mean (±s.e.) planned (101.50 ± 5.98 days) and realised (70.17 ± 11.45 days) deployment periods differed significantly (t5 = 2.89, P = 0.034), with the realised PAT deployment period reduced by 39%, on average.
Migratory movements
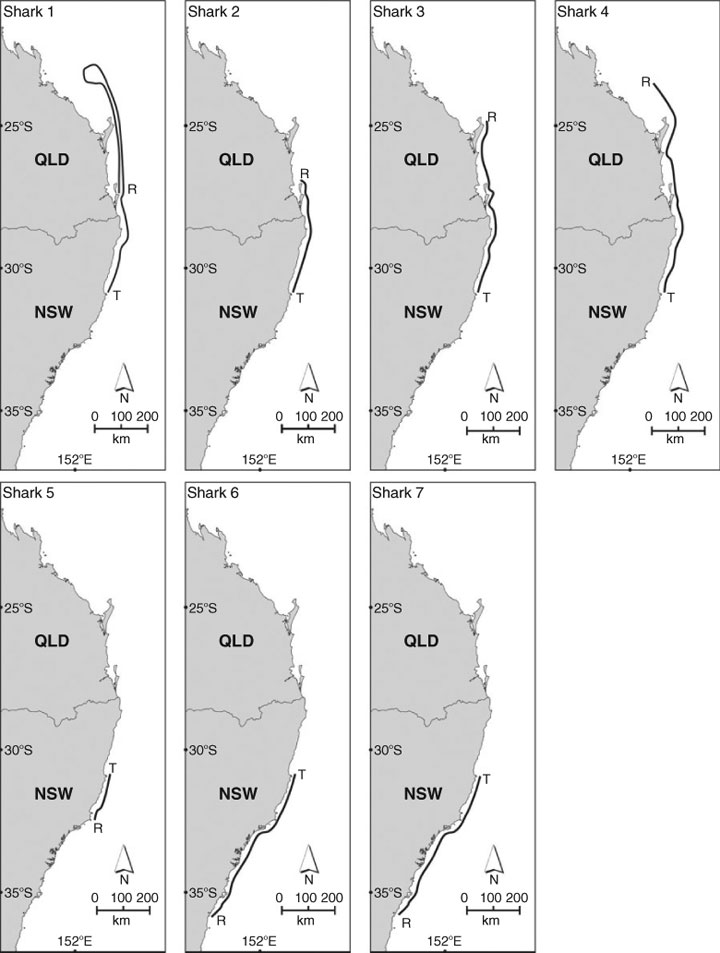
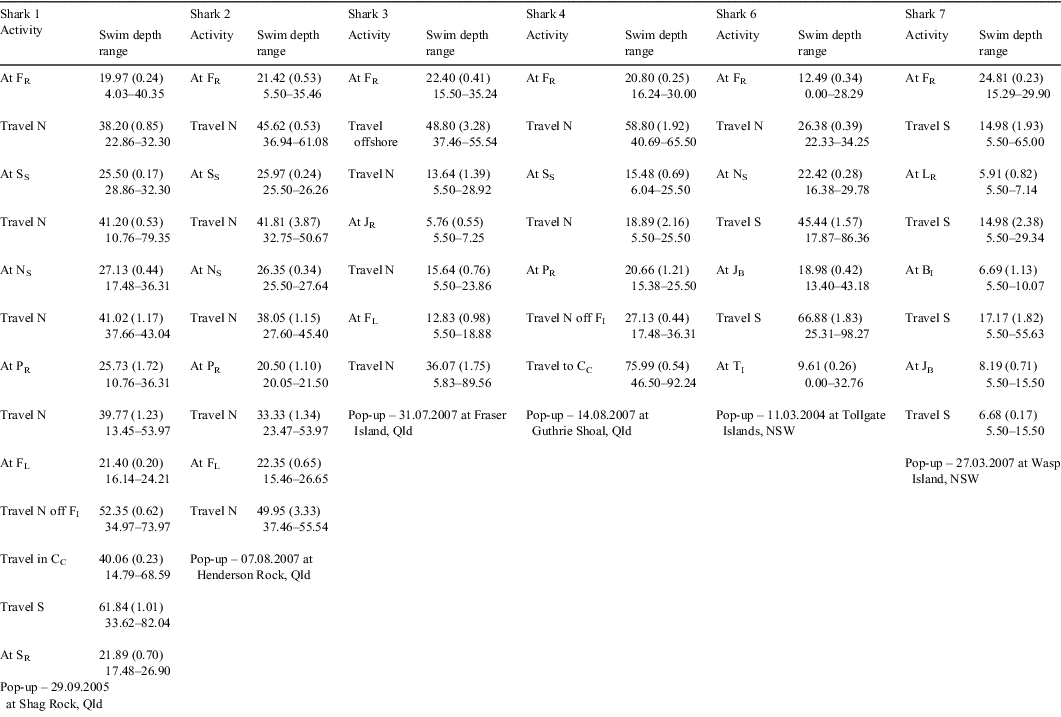
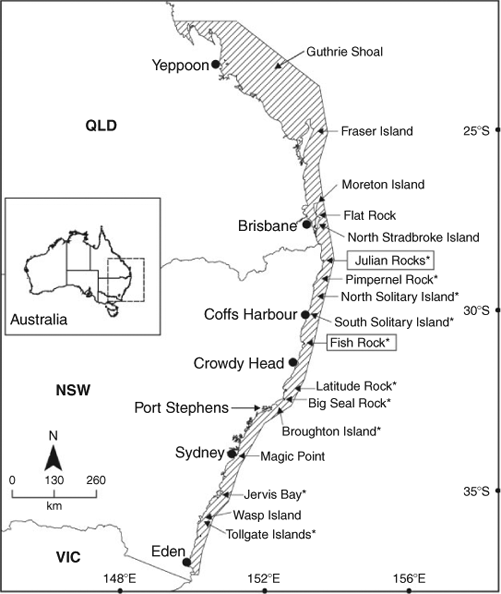
Sexually mature C. taurus males tagged at Fish Rock in autumn–winter (Sharks 1–4) migrated north (Fig. 2). Their northerly migration was punctuated via the occupation of various sites en route (Table 2). Whilst at these rocky reefs, the sharks were observed and photographed by SCUBA divers and detected by SEACAMS. The duration of occupation of these sites varied among sharks and sites, and ranged from less than a day to 2 weeks. Whilst at these sites, the sharks swam at shallower depths, on average, compared with when migrating north between sites (Table 2). The PATs on two C. taurus males (Sharks 2 and 3) were released near Henderson Rock (off Moreton Is., Qld) and Fraser Is. (Qld), having travelled at least 450 km and 700 km, respectively, from Fish Rock. Over the period of monitoring, both sharks spent at least 90% of their time in water depths <60 m and did not exceed a depth of 128 m. While seawater temperatures ranged from 18 to 23.6°C, the sharks spent ≈95% of their time in waters 19–23°C. The two remaining sharks (Sharks 1 and 4) swam north to Fraser Is. (Qld), then east of the coral reefs comprising the Capricorn and Bunker groups (Great Barrier Reef, Qld) and entered the Capricorn Channel (Table 2). After ≈14 days in the Capricorn Channel, the PAT on Shark 4 was released ≈6 km to the east of Guthrie Shoal (Table 2) The remaining shark (Shark 1) spent ≈42 days in the Capricorn Channel before its southward journey to Shag Rock (off North Stradbroke Is., Qld), where its PAT was released (Table 2). This shark then migrated further south and was detected at Fish Rock over 17 days in November 2005 by SEACAMS. During their northerly migration, Sharks 1 and 4 travelled at least 1000 km and 1550 km, respectively, and did not exceed a depth of 136 m. They spent ≈49% and ≈91% of their time, respectively in water depths <60 m and mainly occupied deeper waters when swimming north of Fraser Island and in the Capricorn Channel (Table 2). While seawater temperatures ranged from 17.4 to 25.2°C, both sharks spent at least 90% of their time in waters 20–24°C.
Sexually mature C. taurus males tagged at Fish Rock in spring–summer (Sharks 5–7) migrated south (Fig. 2). As discussed earlier, one PAT (on Shark 5) failed to surface. However, SCUBA divers observed the animal for 7 days before leaving Fish Rock, then at Big Seal Rock just before the PAT’s scheduled release. Assuming the PAT was released at Big Seal Rock, this shark would have travelled at least 180 km during its migration south. The southerly migration of the two remaining sharks (Sharks 6 and 7) was punctuated by the occupation of various sites en route for varying durations where they swam at shallower depths, on average, compared with when migrating south between sites (Table 2). Whilst at these sites, the sharks were observed and photographed by SCUBA divers and/or detected by SEACAMS. After tagging, one shark (Shark 6) remained at Fish Rock for ≈4 weeks, then swam to North Solitary Island, where it remained for 9 days. The shark then swam south to a site off Jervis Bay and then to the Tollgate Islands where it remained until its PAT was released (Table 2). The other shark (Shark 7) left Fish Rock, swam south and occupied at least three sites for short periods before reaching Wasp Island where its PAT was released (Table 2). During their migration south, Sharks 6 and 7 travelled at least 720 km and 640 km, respectively, spent over 90% of their time in water depths <60 m and did not exceed a depth of 125 m. While both sharks occupied waters with a wide range of temperatures (i.e. 13.8–25°C), they spent at least 84% of their time in waters 18–25°C.
The single sexually immature C. taurus male (Shark 8) tagged at Fish Rock in spring–summer remained at the site until the PAT was released on 2 April 2007. While at Fish Rock, the shark swam at an overall mean (±s.e.) depth of 21.61 (0.41) m (range = 0.00–41.70 m), was detected almost continuously by the four SEACAMS listening stations and was regularly observed by SCUBA divers. During its occupation of the site, it spent 100% of its time in water depths <45 m and 100% of its time in waters 18–25°C.
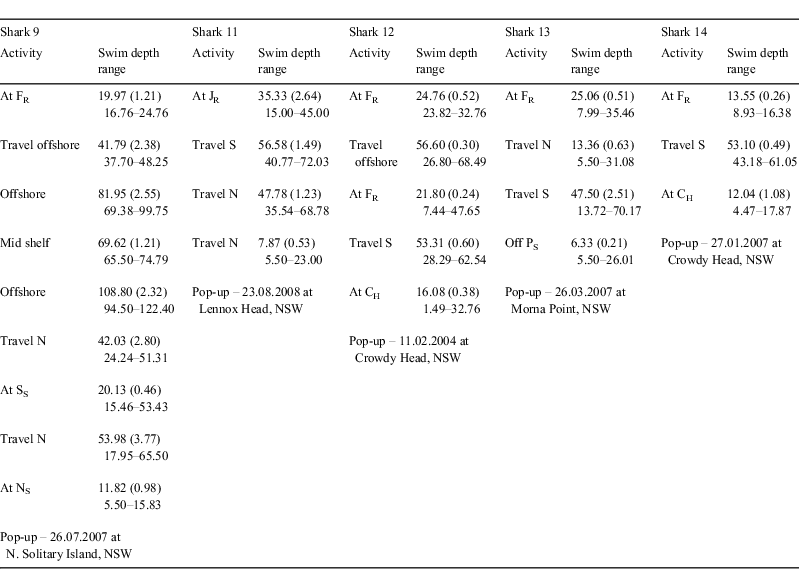
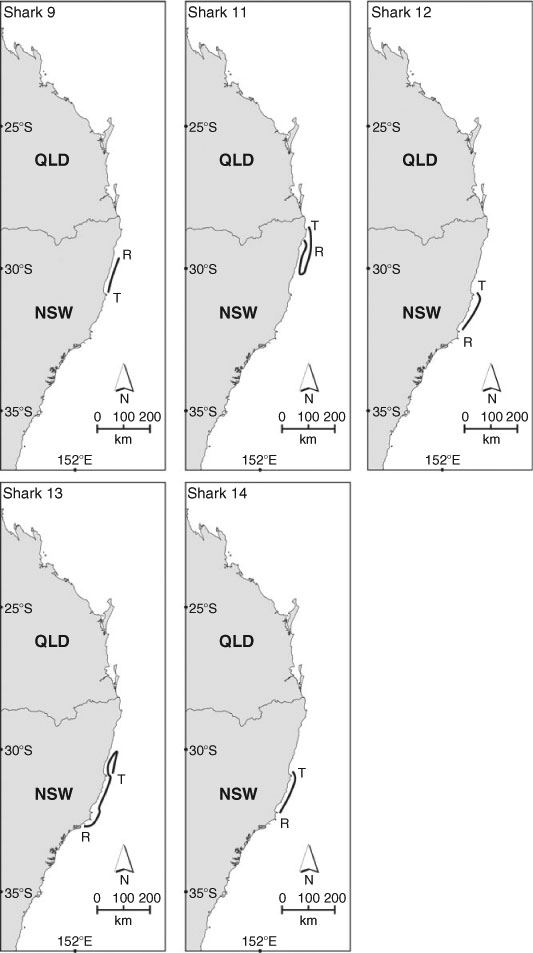
The single sexually mature C. taurus female (Shark 11) tagged at Julian Rocks in autumn–winter survived following the removal of the embedded fishing gaff as indicated by the absence of PAT release within 96 h (via the tag’s constant depth option) and its subsequent migratory movements (Fig. 3). The shark remained at Julian Rocks for ≈6 h, swam south for 9 days and then north until reaching Lennox Head where its PAT was released (Table 3). Over the period of monitoring, the shark travelled at least 400 km and did not exceed a depth of 100 m. It spent ≈37% of its time in water depths <30 m and ≈61% of its time in waters 40–80 m in depth. While seawater temperatures ranged from 18 to 23°C, the shark spent ≈92% of its time in waters 19–22°C.
|
The two sexually immature C. taurus females tagged at Fish Rock in autumn–winter (Sharks 9 and 10) exhibited very different patterns of movement, with only one individual migrating (Fig. 3). The first shark (Shark 9) remained at Fish Rock for 1 day, was detected by SEACAMS and then spent 16 days undertaking two offshore excursions (Table 3). During the first excursion, lasting 7 days, the shark swam offshore and on one occasion (27 May 2007) spent ≈5 min in waters 150–200 m in depth and 17.6°C. During the second excursion, lasting 9 days, the shark moved further offshore and (on 3 June 2007) descended over the shelf break for ≈17 min, swimming to a maximum depth of 232 m. On returning to near-shore waters, the shark swam north to South Solitary Island where it remained for 40 days (SEACAMS detections). The shark then swam to North Solitary Island where it remained until its PAT was released. Whilst at these sites, the shark swam at shallower depths, on average, compared with when migrating between the sites (Table 3). During the period of monitoring, this C. taurus female travelled at least 150 km north and spent ≈75% of its time in water depths <60 m. While seawater temperatures ranged from 15 to 26°C, the shark spent ≈91% of its time in waters 19–24°C. The second shark (Shark 10) remained at the site for the entire PAT deployment (released 19 July 2007) and was detected almost continuously by the four SEACAMS listening stations. Whilst at Fish Rock, it swam at an overall mean (±s.e.) depth of 17.81 (0.52) m (range = 5.50–35.46 m) and was regularly observed by SCUBA divers. During its occupation of the site, it did not exceed a depth of 60 m, spent ≈94% of its time in water depths <40 m and ≈99% of its time in waters 20–24°C.
Sexually immature C. taurus females tagged at Fish Rock in spring–summer (Sharks 12–14) migrated south (Fig. 3). Two individuals (Sharks 12 and 14) spent some time at Fish Rock, with one individual also swimming offshore for a short period. Both sharks migrated south in deeper waters, then moved inshore and occupied shallower waters off Crowdy Head where their PATs were released (Table 3). During the period of monitoring, these sharks travelled at least 100 km, did not exceed 80 m depth and spent at least 78% of their time in water depths <60 m. The sharks occupied waters with a wide range of temperatures (i.e. 15–26°C) and spent at least 74% of their time in waters 18–25°C. The remaining shark (Shark 13) remained at Fish Rock for 15 days then swam north to a site off Coffs Harbour. It then swam south, reaching the waters off Port Stephens where its PAT was released (Table 3). Over the period of monitoring, this shark travelled at least 400 km (100 km north and then 300 km south). During its migration, it did not exceed a depth of 100 m, spent ≈96% of its time in water depths <60 m and ≈90% of its time in waters 19–26°C.
Depth-related occupation of coastal waters
The percentages of time spent at various depths by individual C. taurus males (Table 4) did not differ significantly between day and night (K-S tests, D = 0.100–0.300, P = 0.68–1.00). The immature C. taurus male (Shark 8) spent 100% of its time in a narrower depth range (0–45 m) compared with the sexually mature males. The percentage of time spent at various depths averaged across all C. taurus males, all females and by the individual female (Table 4) did not differ significantly between day and night (K-S tests, D = 0.182, P = 0.988; D = 0.153, P = 0.995; D = 0.100–0.231, P = 0.83–1.00, respectively). The sexually mature female (Shark 11) occupied a similar range of depths during the day and at night to the sexually immature females (Table 4).
On average, the percentages of time spent at various depths by C. taurus males and females did not differ significantly during the day (K-S test, D = 0.317, P = 0.556) or at night (K-S test, D = 0.279, P = 0.663). C. taurus males spent ≈71% of their time in waters <40 m and ≈94% of their time in waters <80 m (Table 4). On average, C. taurus females spent ≈78% of their time in waters <40 m and ≈97% of their time in waters <80 m, respectively (Table 4). Combined, both sexes spent ≈74% of their time in waters <40 m and ≈95% of their time in waters <80 m.
Temperature-related occupation of coastal waters
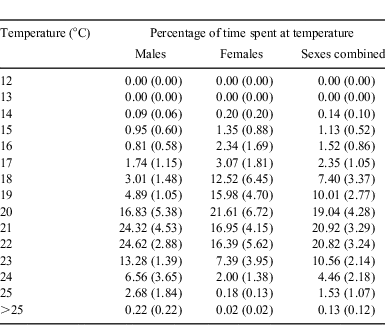
Males and females of C. taurus were similar in their occupation of waters of varying temperatures (Table 5). On average, the percentages of time spent in waters of various temperatures did not differ significantly between the sexes (K-S test, D = 0.133, P = 0.998). Combined, C. taurus males and females spent ≈96% of their time, on average, in waters 17–24°C (Table 5).
|
Discussion
This is the first study to use PATs to provide detailed information on the autumn–winter and spring–summer migratory movements of sexually mature C. taurus males and sexually immature C. taurus females, the distances travelled, and depth- and temperature-related use of continental shelf waters off eastern Australia. Double-tagging C. taurus with acoustic tags substantially enhanced positional accuracy and enabled various legs of the migratory paths to be documented.
Patterns of movement
Over autumn–winter months, sexually mature C. taurus males migrated north from Fish Rock (NSW waters) to the Capricorn Channel in the southern Great Barrier Reef, Qld and this was followed by a return journey in spring–summer. The migration north was consistent with previous studies (Otway et al. 2003; Otway and Burke 2004; Bruce et al. 2005) and punctuated by the occupation of sites en route for periods of up to 14 days. When travelling north, the males mainly swam in shallower waters (i.e. <50 m) until Fraser Island, after which they moved into deeper waters (i.e. 60–80 m). The northward distances ranged from 450 to 1550 km and represented various stages along the migratory route and included a partial return south to North Stradbroke Island by one individual. This same shark was subsequently detected by SEACAMS at Fish Rock in November 2005, having travelled an overall distance of at least 3100 km in 6 months. In contrast, the maximum distance travelled by C. taurus individuals tagged off the east coast of the USA as part of National Marine Fisheries Service Cooperative Shark Tagging Program is 1187 km (Kohler et al. 1998). In South Africa, little is known about the movements of sexually mature C. taurus males. A recent study (Dicken et al. 2007) only documented four males in 177 recaptures. The female recaptures included a pregnant individual that was tagged at a gestation site off northern KwaZulu-Natal and travelled 1897 km south to a parturition site.
Over the spring–summer months, sexually mature C. taurus males migrated to southern NSW waters, travelling unidirectional distances of 650 km over 3–4 months. When migrating south, the sharks often swam in deeper waters (i.e. 60–80 m) and their movements were likely assisted by the EAC. This southerly migration was also punctuated by the occupation of sites en route for periods lasting up to several weeks. Previous catches in the shark meshing program (Reid and Krogh 1992) and more recent underwater visual censuses (Otway et al. 2003) have shown that over the summer–autumn months, the C. taurus population south of Port Stephens is significantly biased towards females and comprises mainly sexually mature females and juveniles of both sexes. These observations suggest that on returning from their northerly migration, only some sexually mature males migrate to sites such as Wasp Island and the Tollgate Islands in southern NSW waters. It is likely that the remainder disperse over various sites between Fish Rock and Port Stephens. Combining the northerly and more extensive southerly movements, this suggests that a proportion of sexually mature males may migrate 4500 km annually.
Sexually immature C. taurus females migrated over smaller distances compared with sexually mature males. Over the spring–summer period, sexually immature females migrated south over distances ranging from 100 to 400 km and swam in deeper waters, presumably to gain assistance from the EAC. The movements south were also punctuated by the occupation of sites en route for periods lasting several days. In South Africa, C. taurus females >1.8 m TL travelled a mean distance of 324 km (Dicken et al. 2007) and these distances are similar in magnitude to the juveniles documented in this study. However, it is likely that the movements of sexually mature, gestating females are substantially greater. Evidence for this is provided from the necropsies of postpartum females caught during spring between Sydney and Port Stephens (N. M. Otway and M. T. Ellis, unpubl. data) together with a study of putatively, gestating females at Wolf Rock, Qld (Bansemer and Bennett 2009). Combined, the data suggest that a southerly migration to parturition sites in NSW waters likely occurs over winter–spring, with females covering unidirectional distances of 1000–1500 km. In contrast, C. taurus females <1.8 m TL have been estimated to travel a mean distance of only 17.3 km in South African waters (Dicken et al. 2007). However, along the east coast of Australia, an earlier tagging study using cattle ear tags (Otway and Burke 2004) showed that a sexually immature female, 1.53 m TL when tagged, migrated north from the Tollgate Islands to Fish Rock (Fig. 1) over autumn–winter and travelled 650 km over 2 months. Thus, it is likely that the distances travelled by South African C. taurus <1.8 m TL have been underestimated, primarily as a direct consequence of the very seasonal fishing effort underpinning the tagging and recapture events in the summer fishing season, a situation acknowledged by Dicken et al. (2007).
Depth and temperature related use of coastal waters
Three C. taurus individuals tagged at Fish Rock moved offshore for periods of 3–16 days and occupied mid-shelf waters with depths of 60–80 m and abnormally higher temperatures (i.e. 19–21°C). Their offshore movements were likely in response to an episodic incursion of the EAC or the intrusion of a warm-core eddy onto the continental shelf at Smoky Cape as such events have been documented in previous oceanographic studies (Rochford 1984; Tranter et al. 1986). Similar swimming behaviour associated with the Leeuwin Current, has been hypothesised for C. taurus off west Australia (McAuley 2004).
Overall, C. taurus males and females exhibited similar patterns of depth and temperature-related use of coastal waters. Combined, both sexes spent ≈95% of their time in waters 17–24°C, ≈74% of their time, on average, in waters <40 m and ≈95% of their time in waters <80 m. The occupation of deeper depths and cooler temperatures was mainly evident when migrating north or south. The large proportion of time spent in waters <40 m indicates that C. taurus mainly occupies the inshore coastal waters of the east Australian continental shelf. This same area is also used by many vessels in the commercial and recreational fishing fleets.
The similar depth-related use of the continental shelf waters, together with the extensive migrations documented here, further reinforce results of genetic studies (Stow et al. 2006; Feldheim et al. 2007) showing that the east coast C. taurus individuals comprise a single, well mixed population with very low genetic variation. Moreover, a recent study (Ahonen and Stow 2009) has confirmed the results in Stow et al. (2006) and modelled present-day genetic variation (measured as allelic richness) in the east coast population. The modelling showed that processes preventing migration can result in further erosion of genetic variation, the rate of which would accelerate as population size decreases. Importantly, the occupation of inshore waters means that C. taurus will continue to be captured, albeit accidentally, by commercial and recreational fishers (Otway et al. 2004; Bansemer and Bennett 2010) and caught in the shark meshing programs off the NSW and Qld coasts (Reid and Krogh 1992). This continuing fishing-related mortality provides the most likely process capable of causing disruptions to migratory movements and losses in genetic variation over future generations.
The variation in depth-related use of coastal waters by individual sexually mature C. taurus males during their northerly migration over autumn–winter and the contrasting, contemporaneous movements of sexually immature females obtained in this initial study demand that additional PATs are deployed to ensure that the emerging migratory patterns are truly representative. The migratory movements of sexually immature males and sexually mature females in their gestational and resting phases were not documented in this study. Such studies are needed and would likely provide information on possible parturition sites and the timing and duration of the southward migration of pregnant females. Quantifying the movements of sexually mature females during the year-long reproductive resting phase (Branstetter and Musick 1994; Smale 2002; Dicken et al. 2007) is also fundamental to understanding possible fishing-related interactions. During the resting phase, C. taurus females likely feed continuously to replace the energy reserves expended during the previous pregnancy. By doing so, the resting-phase females may increase their risk of adverse fishing-related interactions, leading to greater rates of mortality in this demographic stage and a reduction in the population growth rate (Otway et al. 2004).
More generally, studies using PATs and acoustic tags can provide substantial, much-needed data on the migratory movements of many shark species that are targeted or caught as by-catch in various fisheries of the world (e.g. Marín et al. 1998; Baum and Myers 2004; Stevens et al. 2010). The detailed information arising from the use of PATs can also substantially increase our understanding of shark mortality rates (Moyes et al. 2006; Campana et al. 2009) and ecology (Dewar et al. 2004; Weng et al. 2007), and lead to better demographic models and estimates of sustainable harvest (Cortés 2000; Mollet and Cailliet 2002; McAuley et al. 2007). The detailed information from such studies can also assist management efforts to ensure the long-term conservation of the ever-increasing list of threatened shark species in the world’s oceans and seas.
PAT performance
Of the 15 PATs deployed, one was not sighted by SCUBA divers and failed to transmit data. Of the 14 remaining PATs, eight tags reached their programmed pop-up dates and two tags attained 97.5% and 95.8% of their planned deployments, respectively. The remaining four PATs were all attached to females and realised deployments of between 42.2 and 69.3% of the programmed deployments, resulting in a significantly reduced mean deployment period on C. taurus females. In previous studies on sharks (e.g. Weng et al. 2007; Campana et al. 2009; Stevens et al. 2010), a significant proportion of PATs did not reach their programmed pop-off dates. While the performance of the PATs used in this study was generally equivalent to that reported previously, a sex-based difference has not been identified in earlier studies. This difference may be peculiar to C. taurus and more attributable to the different life-history stages (i.e. sexually mature males and sexually immature females) examined in this study. Additional PAT deployments on other life-history stages of C. taurus, including sexually immature males and sexually mature females in gestational and resting phases, will be required to identify any further differences, and possible causes of differences, in PAT performance.
Of the two PATs that failed to transmit data, one tag was released, but SCUBA divers had reported observing several barnacles attached to the tag’s float 1 week before the end of its programmed 6-month deployment. It is highly likely that the PAT failed to reach the surface owing to the reduced buoyancy attributable to the bio-fouling, a factor that has been identified in a study investigating why ARGOS satellite tags on marine organisms stop transmitting (Hays et al. 2007). It is unclear why the second tag failed to transmit and while there are several possible reasons for the failure, including aerial damage (Hays et al. 2007), scavenging by predators (Kerstetter et al. 2004) and tag destruction, bio-fouling appears to be the most likely reason given the barnacles on the other PAT and the substantial bio-fouling documented previously on conventional tags used with C. taurus in South Africa and Australia (Davies and Joubert 1966; Otway and Burke 2004; Dicken et al. 2006).
Acknowledgements
We are grateful to B. Louden, J. Gilligan, P. and K. Hitchins, T. Long and Sea World staff, M. Gray, P. and S. Byrne, J. and B. Nelson, J. Cavazzini, and the Cape Byron Marine Park staff for help with tagging. The functioning of SEACAMS would not continue without the efforts of H. Malcolm, J. and K. Duggan, R. and J. Duncan, and the staff of Sydney Aquarium and Oceanworld Manly. We thank the numerous divers who reported the locations of tagged sharks, the people who found and returned the PATs and J. Mumby, who spent many days preparing the PAT data for analysis. Comments from R. Creese, M. Lowry, the editor, guest editors and referees were constructive and improved the paper. Funds were provided by Industry and Investment NSW, the Sea World Research and Rescue Foundation Inc., the Northern Rivers CMA, and the Department of the Environment, Water, Heritage and the Arts. The work was done under scientific research permit (P01/0059[A]) and in accordance with an Animal Care and Ethics Committee approval (ACEC 99/14 Port Stephens).
References
Ahonen, H., and Stow, A. (2009). Population size and structure of grey nurse shark in east and west Australia. Final Report to Department of Environment, Water, Heritage and the Arts, Canberra. Available at http://www.environment.gov.au/coasts/publications/size-structure-grey-nurse.html (accessed 1 November 2010).ARGOS (2008). ARGOS user’s manual. Available at http://www.argossystem.org/manual/ (accessed 25 May 2010).
Bansemer, C. S., and Bennett, M. B. (2009). Reproductive periodicity, localised movements and behavioural segregation of pregnant Carcharias taurus at Wolf Rock southeast Queensland, Australia. Marine Ecology Progress Series 374, 215–227.
| Reproductive periodicity, localised movements and behavioural segregation of pregnant Carcharias taurus at Wolf Rock southeast Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Bansemer, C. S., and Bennett, M. B. (2010). Retained fishing gear and associated injuries in the east Australian grey nurse sharks (Carcharias taurus): implications for population recovery. Marine and Freshwater Research 61, 97–103.
| Retained fishing gear and associated injuries in the east Australian grey nurse sharks (Carcharias taurus): implications for population recovery.Crossref | GoogleScholarGoogle Scholar |
Bass, A. J., Aubrey, J. D. D., and Kistnasamy, N. (1975). Sharks of the east coast of southern Africa. IV. The families Odontaspididae, Scapanorhynchidae, Isuridae, Cetorhinidae, Alopiidae, Orectolobidae and Rhiniodontidae. Oceanographic Research Institute Investigational Report 39, 12–17.
Baum, J. K., and Myers, R. A. (2004). Shifting baselines and the decline of pelagic shark populations in the Gulf of Mexico. Ecology Letters 7, 135–145.
| Shifting baselines and the decline of pelagic shark populations in the Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Baum, J. K., Myers, R. A., Kelhler, D. G., Worm, B., Harley, S. J., et al. (2003). Collapse and conservation of shark populations in the northwest Atlantic. Science 299, 389–392.
| Collapse and conservation of shark populations in the northwest Atlantic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjsF2kuw%3D%3D&md5=924bb8fc3c277e1376697b925d80c33aCAS | 12532016PubMed |
Branstetter, S., and Musick, J. A. (1994). Age and growth estimates for the sand tiger shark in the northwestern Atlantic Ocean. Transactions of the American Fisheries Society 123, 242–254.
| Age and growth estimates for the sand tiger shark in the northwestern Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
Bruce, B. D., Stevens, J. D., and Bradford, R. W. (2005). Designing protected areas for grey nurse sharks off eastern Australia. CSIRO Marine and Atmospheric Research Report, Hobart. Available at http://www.environment.gov.au/coasts/publications/grey-nurse-protected-areas/index.html (accessed 1 November 2010).
Caldwell, N., and Ellison, N. (1939). ‘Fangs of the Sea.’ (Angus and Robertson: Sydney.)
Campana, S. E., Joyce, W., and Manning, M. J. (2009). Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Marine Ecology Progress Series 387, 241–253.
| Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags.Crossref | GoogleScholarGoogle Scholar |
Cavanagh, R., Kyne, P., Fowler, S. L., Musick, J. A., and Bennett, M. B. (2003). The conservation status of Australasian chondrichthyans. Report of the IUCN Shark Specialist Group Australia and Oceania Regional Red List workshop, 7–9 March 2003, University of Queensland, Brisbane, Australia. Available from http://www.uf.edu/fish/organizations/ssg/region8/Ausfinal.pdf (accessed 1 November 2010).
Compagno, L. J. V. (2002). ‘FAO Species Catalogue. Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Vol. 2. Bullhead, Mackerel and Carpet Sharks (Heterodontiformes, Lamniformes and Orectolobiformes).’ (FAO: Rome.)
Cortés, E. (2000). Life-history patterns and correlation in sharks. Reviews in Fisheries Science 8, 299–344.
Cropp, B. (1964). ‘Shark Hunters.’ (Rigby: Adelaide.)
Davies, D. H., and Joubert, L. S. (1966). Tag evaluation and shark tagging in South African waters. Oceanographic Research Institute, South African Association for Marine Biological Research, Durban Report 12, 1–36.
Department of Environment and Heritage (2003). Review of grey nurse shark tagging research. DEH, Canberra. Available at http://www.environment.gov.au/coasts/species/sharks/greynurse/tagging.html (accessed 1 June 2010).
Dewar, H., Domeier, M., and Nasby-Lucas, N. (2004). Insights into young of the year white shark, Carcharodon carcharias, behaviour in the southern California Bight. Environmental Biology of Fishes 70, 133–143.
| Insights into young of the year white shark, Carcharodon carcharias, behaviour in the southern California Bight.Crossref | GoogleScholarGoogle Scholar |
Dicken, M. L., Booth, A. J., and Smale, M. J. (2006). Preliminary investigation of tag shedding, tag reporting, tag wounds and tag biofouling for raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa. ICES Journal of Marine Science 63, 1640–1648.
| Preliminary investigation of tag shedding, tag reporting, tag wounds and tag biofouling for raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa.Crossref | GoogleScholarGoogle Scholar |
Dicken, M. L., Booth, A. J., Smale, M. J., and Cliff, G. (2007). Spatial and seasonal distribution patterns of juvenile and adult raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa. Marine and Freshwater Research 58, 127–134.
| Spatial and seasonal distribution patterns of juvenile and adult raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa.Crossref | GoogleScholarGoogle Scholar |
Feldheim, K. A., Stow, A. J., Ahonen, H., Chapman, D. D., Shivji, M., et al. (2007). Polymorphic microsatellite markers for studies of the conservation and reproductive genetics of imperilled sand tiger sharks (Carcharias taurus). Molecular Ecology 7, 1366–1368.
| Polymorphic microsatellite markers for studies of the conservation and reproductive genetics of imperilled sand tiger sharks (Carcharias taurus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlslSrsQ%3D%3D&md5=342fa44f26d47282e3700c539f68d993CAS |
Fergusson, I. K., Vacchi, M., and Serena, F. (2002). Note on the declining status of the sandtiger shark Carcharias taurus in the Mediterranean Sea. In ‘Fourth Meeting of the European Elasmobranch Association, Livorno, Italy’, 27–30 November 2002. (Eds G. Vacchi, G. La Mesa, F. Serena and B. Seret.) pp. 73–76. (Association Societe Francaise d’Ictyologie (SFI): Paris.)
Francis, M. (2006). Morphometric minefields – towards a measurement standard for chondrichthyan fishes. Environmental Biology of Fishes 77, 407–421.
| Morphometric minefields – towards a measurement standard for chondrichthyan fishes.Crossref | GoogleScholarGoogle Scholar |
Gilmore, R. G., Dodrill, J. W., and Linely, P. A. (1983). Reproduction and embryonic development of the sand tiger shark, Odontaspis taurus (Rafinesque). Fishery Bulletin 81, 201–225.
Goldman, K. J., Branstetter, S., and Musick, J. A. (2006). A re-examination of the age and growth of sand tiger sharks, Carcharias taurus, in the western North Atlantic: the importance of ageing protocols and use of multiple back-calculation techniques. Environmental Biology of Fishes 77, 241–252.
| A re-examination of the age and growth of sand tiger sharks, Carcharias taurus, in the western North Atlantic: the importance of ageing protocols and use of multiple back-calculation techniques.Crossref | GoogleScholarGoogle Scholar |
Harriott, V. J., Davis, D., and Banks, S. A. (1997). Recreational diving and its impact in marine protected areas in eastern Australia. Ambio 26, 173–179.
Hays, G. C., Bradshaw, C. J. A., James, M. C., Lovell, P., and Sims, D. W. (2007). Why do Argos satellite tags deployed on marine animals stop transmitting? Journal of Experimental Marine Biology and Ecology 349, 52–60.
| Why do Argos satellite tags deployed on marine animals stop transmitting?Crossref | GoogleScholarGoogle Scholar |
Hayward, A. (2003). Observations of grey nurse shark (Carcharias taurus) and scuba diver behaviour. Honours thesis, Southern Cross University, Lismore, NSW.
Henningsen, A. (1994). Tonic immobility in 12 elasmobranchs: use as an aid in captive husbandry. Zoo Biology 13, 325–332.
| Tonic immobility in 12 elasmobranchs: use as an aid in captive husbandry.Crossref | GoogleScholarGoogle Scholar |
Hoenig, J. M., and Gruber, S. H. (1990). Life-history patterns in the elasmobranchs: implications for fisheries management. In ‘Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics and the Status of the Fisheries. NOAA Technical Report 90’. (Eds H. L. Pratt, S. H. Gruber and T. Taniuchi.) pp. 1–16. (National Marine Fisheries Services: Washington, DC.)
Kerstetter, D. W., Polovina, J. J., and Graves, J. E. (2004). Evidence of shark predation and scavenging on fishes equipped with pop-up satellite archival tags. Fishery Bulletin 102, 750–756.
Kohler, N. E., Casey, J. G., and Turner, P. A. (1998). NMFS cooperative shark tagging program, 1962–93: an atlas of shark tag and recapture data. Marine Fisheries Review 60, 1–87.
Last, P. R., and Stevens, J. D. (2009). ‘Sharks and Rays of Australia.’ (CSIRO Publishing: Melbourne.)
Lucifora, L. O., Menni, R. C., and Escalante, A. H. (2002). Reproductive ecology and abundance of the sand tiger shark, Carcharias taurus, from the southwestern Atlantic. Journal of Marine Science 59, 553–561.
Marín, Y. H., Brum, F., Barea, L. C., and Chocca, J. F. (1998). Incidental catch associated with swordfish longline fisheries in the south-west Atlantic Ocean. Marine and Freshwater Research 49, 633–640.
| Incidental catch associated with swordfish longline fisheries in the south-west Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
McAuley, R. B. (2004). Western Australian grey nurse shark pop-up archival tag project. Final Report to Department of Environment and Heritage, Canberra.
McAuley, R. B., Simpfendorfer, C. A., and Hall, N. G. (2007). A method for evaluating the impacts of fishing mortality and stochastic influences on the demography of two long-lived shark stocks. ICES Journal of Marine Science 64, 1710–1722.
| A method for evaluating the impacts of fishing mortality and stochastic influences on the demography of two long-lived shark stocks.Crossref | GoogleScholarGoogle Scholar |
Mollet, H. F., and Cailliet, G. M. (2002). Comparative population demography of elasmobranchs using life history tables, Leslie matrices and stage-based matrix models. Marine and Freshwater Research 53, 503–516.
| Comparative population demography of elasmobranchs using life history tables, Leslie matrices and stage-based matrix models.Crossref | GoogleScholarGoogle Scholar |
Moyes, C. D., Fragoso, N., Musyl, M. K., and Brill, R. W. (2006). Predicting postrelease survival in large pelagic fish. Transactions of the American Fisheries Society 135, 1389–1397.
| Predicting postrelease survival in large pelagic fish.Crossref | GoogleScholarGoogle Scholar |
Musick, J. A., Branstetter, S., and Colvocoresses, J. A. (1993). Trends in shark abundance from 1974 to 1991 for the Chesapeake Bight region of the U.S. mid-Atlantic coast. NOAA Technical Report 115. National Marine Fisheries Services, Washington, DC. Available from http://www.spo.nmfs.noaa.gov/tr115.pdf (accessed 1 November 2010).
Myers, R. A., and Worm, B. (2003). Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283.
| Rapid worldwide depletion of predatory fish communities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjs1ynurw%3D&md5=7bea077664c6c01ae2c511e1749f403eCAS | 12748640PubMed |
Otway, N. M. (2004). A grey future for the grey nurse. In ‘Proceedings of the Australian Association of Veterinary Conservation Biologists’. Canberra, ACT, 3–5 May 2004. (Ed. R. Woods.) pp. 8–27. (Australian Veterinary Association, Canberra.)
Otway, N. M., and Burke, A. L. (2004). Mark–recapture population estimate and movements of Grey Nurse Sharks. NSW Fisheries Final Report Series No.63. NSW Fisheries, Sydney.
Otway, N. M., Burke, A. L., Morrison, N. M., and Parker, P. C. (2003). Monitoring and identification of NSW critical habitat sites for conservation of Grey Nurse Sharks. NSW Fisheries Final Report Series No.47. NSW Fisheries, Sydney.
Otway, N. M., Bradshaw, C. J. A., and Harcourt, R. G. (2004). Estimating the rate of quasi-extinction of the Australian grey nurse shark (Carcharias taurus) population using deterministic age- and stage-classified models. Biological Conservation 119, 341–350.
| Estimating the rate of quasi-extinction of the Australian grey nurse shark (Carcharias taurus) population using deterministic age- and stage-classified models.Crossref | GoogleScholarGoogle Scholar |
Otway, N. M., Ellis, M. T., Louden, B. M., and Gilligan, J. J. (2009). Documentation of depth related migratory movements, localised movements at critical habitat sites and the effects of scuba diving for the east coast grey nurse shark population. Industry and Investment NSW – Fisheries Final Report Series No.112. NSW Fisheries, Sydney. Available from http://www.environment.gov.au/coasts/publications/movements-diving.html (accessed 1 November 2010).
Pepperell, J. G. (1992). Trends in the distribution, species composition and size of sharks caught by gamefish anglers off south-eastern Australia, 1961–90. Australian Journal of Marine and Freshwater Research 43, 213–225.
| Trends in the distribution, species composition and size of sharks caught by gamefish anglers off south-eastern Australia, 1961–90.Crossref | GoogleScholarGoogle Scholar |
Pollard, D. A., Lincoln Smith, M. P., and Smith, A. K. (1996). The biology and conservation status of the grey nurse shark (Carcharias taurus Rafinesque 1810) in New South Wales, Australia. Aquatic Conservation: Marine and Freshwater Ecosystems 6, 1–20.
| The biology and conservation status of the grey nurse shark (Carcharias taurus Rafinesque 1810) in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Read, A. D., and Ward, T. (1986). Taiwanese longliners off northern Australia. Australian Fisheries 45, 6–8.
Reid, D., and Krogh, M. (1992). Assessment of catches from protective shark meshing off NSW beaches between 1950 and 1990. Australian Journal of Marine and Freshwater Research 43, 283–296.
| Assessment of catches from protective shark meshing off NSW beaches between 1950 and 1990.Crossref | GoogleScholarGoogle Scholar |
Rochford, D. J. (1984). Nitrates in eastern Australian coastal waters. Australian Journal of Marine and Freshwater Research 35, 385–397.
| Nitrates in eastern Australian coastal waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXlslKqu78%3D&md5=2d111951862a1bc4255366e5b5100322CAS |
Smale, M. J. (2002). Occurrence of Carcharias taurus in nursery areas of the Eastern and Western Cape, South Africa. Marine and Freshwater Research 53, 551–556.
| Occurrence of Carcharias taurus in nursery areas of the Eastern and Western Cape, South Africa.Crossref | GoogleScholarGoogle Scholar |
Smale, M. J. (2005). The diet of the ragged-tooth shark Carcharias taurus Rafinesque 1810 in the Eastern Cape, South Africa. African Journal of Marine Science 27, 331–335.
| The diet of the ragged-tooth shark Carcharias taurus Rafinesque 1810 in the Eastern Cape, South Africa.Crossref | GoogleScholarGoogle Scholar |
Smith, M. F. L. (1992). Capture and transportation of elasmobranchs, with emphasis on the grey nurse shark (Carcharias taurus). Australian Journal of Marine and Freshwater Research 43, 325–343.
| Capture and transportation of elasmobranchs, with emphasis on the grey nurse shark (Carcharias taurus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XkvVOhsr4%3D&md5=180e2530cba206f89a0cae697444d63fCAS |
Smith, S. E., Au, D. W., and Show, C. (1998). Intrinsic rebound potentials of 26 species of Pacific sharks. Marine and Freshwater Research 49, 663–678.
| Intrinsic rebound potentials of 26 species of Pacific sharks.Crossref | GoogleScholarGoogle Scholar |
Snedecor, G. W., and Cochran, G. W. (1967). ‘Statistical Methods.’ (Iowa State University Press: Ames, IA.)
Sokal, R. R., and Rohlf, F. J. (1969). ‘Biometry.’ (W.H. Freeman: San Francisco, CA.)
Springer, S. (1948). Oviphagous embryos of the sand shark, Carcharias taurus. Copeia 1948, 153–157.
| Oviphagous embryos of the sand shark, Carcharias taurus.Crossref | GoogleScholarGoogle Scholar |
Stevens, J. D., Bradford, R. W., and West, G. J. (2010). Satellite tagging of blue sharks (Prionace glauca) and other pelagic sharks off eastern Australia: depth behaviour, temperature experience and movements. Marine Biology 157, 575–591.
| Satellite tagging of blue sharks (Prionace glauca) and other pelagic sharks off eastern Australia: depth behaviour, temperature experience and movements.Crossref | GoogleScholarGoogle Scholar |
Stow, A., Zenger, K., Briscoe, D., Gillings, M., Peddemors, V., et al. (2006). Isolation and genetic diversity of endangered grey nurse shark (Carcharias taurus) populations. Biology Letters 2, 308–311.
| Isolation and genetic diversity of endangered grey nurse shark (Carcharias taurus) populations.Crossref | GoogleScholarGoogle Scholar | 17148390PubMed |
Tranter, D. J., Carpenter, D. J., and Leech, G. S. (1986). The coastal enrichment effect of the East Australian Current eddy field. Deep-Sea Research 33, 1705–1728.
| The coastal enrichment effect of the East Australian Current eddy field.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXhs1aitLo%3D&md5=d13fb05f47dc0db909b5de532b0ff727CAS |
Watsky, M. A., and Gruber, S. H. (1990). Induction and duration of tonic immobility in the lemon shark, Negaprion brevirostris. Fish Physiology and Biochemistry 8, 207–210.
| Induction and duration of tonic immobility in the lemon shark, Negaprion brevirostris.Crossref | GoogleScholarGoogle Scholar |
Weng, K. C., Boustany, A. M., Pyle, P., Anderson, S. D., Brown, A., et al. (2007). Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean. Marine Biology 152, 877–894.
| Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |