George Ernest Rogers 1927–2021
Racheline (Lynn) Rogers A *
A *
A Department of Molecular and Biomedical Science, School of Biological Sciences, The University of Adelaide, SA 5005, Australia.
Historical Records of Australian Science 34(2) 144-157 https://doi.org/10.1071/HR23004
Published: 22 June 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Australian Academy of Science. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
George Rogers (1927–2021) was elected to Fellowship of the Academy in 1977 for his outstanding contributions to our knowledge of the molecular structure of keratins and the biochemistry of keratinisation. He was a pioneer in the application of electron microscopy to hair and wool ultrastructure and to that of the hair follicle. He discovered citrulline in keratin proteins, and the enzymes, roles, and histochemical localisation of transglutaminase and peptidylarginine deiminase (PAD). He was the first to demonstrate ribosomal-dependent keratin protein synthesis in 1965 followed by detailed studies of the biosynthesis of hair keratin proteins. His research initiated studies on the molecular events in the development of the feather follicle and later led to the cloning and characterisation of the clustered genes of feather and related avian keratins. He also cloned and characterised genes for the three classes of wool keratin proteins and studied the transcription of keratin genes. In 1977, he was awarded a DSc by the University of Adelaide in recognition for his work. In 2013, he was made an Officer in the Order of Australia.
Keywords: biochemistry, hair and wool structure, historical article, keratin, microscope, molecular biology, wool synthesis.
Early days1
George Rogers was born at the family home in Prahran, Melbourne, on 27 October 1927 (Fig. 1).
His parents Bertha and Percy Rogers had moved from England in 1923 together with their infant daughter Sylvia, his only sibling, who was six years older. His father Percy left school at the age of fourteen and thereafter was largely self-educated. Percy served in World War 1 as a transport driver in the Royal Naval Air Service. After the war, he was employed as a motor car buyer for a prominent export company in Fenchurch, London, with whom he maintained a business connection from Australia for the rest of his life. George’s mother, Bertha Baxter, came from Reading in Berkshire. Her father, an Englishman, died when she was an infant and she was raised with her older sister Annie and older brother George by their mother who was born on the Isle of Wight. Bertha had minimal education and worked as a shop assistant in Oxford St, London, during World War 1.
Rogers’ parents were married in London in 1916, a year remembered for frequent bombing raids on London by German Zeppelins. His father used to relate that one of his duties was to go to sites where Zeppelins had been shot down and help to clear the wreckage. Typical of many families in the United Kingdom, his parents were devastated by the loss of many relatives including their only brothers George, who was killed in France, and Ernest, who was lost at sea. When they moved to Australia in 1923, it was intended to be for a few years only, not an emigration. The economic depression of 1929 resulted in them settling in Melbourne permanently and they did not visit England again until after World War 2. In Australia Rogers’ father continued to be involved in the motor car business and was the first to establish second-hand car auctions in Melbourne. Later he was made a partner in the establishment of Southern Motors and then Managing Director of Devon Motors, an agency for Studebaker cars. In the post-war period he was for some time President of the Automotive Chamber of Commerce in Melbourne.
Rogers grew up in the suburb of Caulfield, in a family whose few relatives all still lived in England. His upbringing was typical of the 1930s when families were recovering from the effects of the Depression. It was strict, but caring, with an underlying kindly and supportive encouragement from his parents to achieve more than they had been able to do in their own lives.
Early education
The family’s difficult situation during the Depression eventually improved and Rogers was enrolled for private education at Caulfield Grammar School in 1936. He enjoyed school, did reasonably well scholastically, and took part in numerous sporting activities. He had no desire to follow his father’s occupation in the business world and became completely fascinated by chemistry when he was about ten years old, a circumstance that he could not fully explain because neither parent had much knowledge of science in general, but probably resulted from his mother having bought him a Lott’s chemistry set. As a hobby, chemistry occupied many lonely hours that he spent quarantined at home during the poliomyelitis epidemic of 1937 and during his own childhood illnesses. Fortunately these did not include poliomyelitis, but on two occasions he suffered severe complications from measles and anaemia. His acquisition of chemicals and equipment was mainly through H. B. Selby2 in Swanston Street, Melbourne, where he was always given the best of assistance, Rogers often related, ‘because of a marvellous man Mr Still who worked at the counter of the retail side of their business’. It is amazing these days to consider that back then he could purchase dangerous materials that were poisonous, or potentially explosive, like iodine crystals, 0.880 ammonia, mercury, and more.
Rogers’ life in private school came to an abrupt end when his father’s car business encountered difficulties because of World War 2. This forced his early departure after only completing his Intermediate Certificate. Prior to moving to a state school in 1942, he and his father were given advice by the headmaster at Caulfield Grammar, that Rogers should become an apprentice to a ‘chemical-type job’. In retrospect, this seemed unwise because he was so young, but the war led to further reinforcement of Rogers’ interest in chemistry and he had decided to become an industrial chemist. Working for the war effort in a job that involved chemistry held considerable attraction for him. He was able to take up part-time study for a diploma in chemistry at the Royal Melbourne Technical College (‘Melbourne Tech’, now RMIT University).
In February 1943, at the age of fifteen, he started work as a laboratory assistant to Dr F. G. (Gordon) Lennox (1912–98),3 then Section Leader of the Leather and Fellmongery Section of the Division of Industrial Chemistry, Council for Scientific and Industrial Research (CSIR), headed by Dr Ian (later Sir Ian) Wark. Lennox later became prominent in Australian science and in wool research as Chief of the Division of Protein Chemistry in the Commonwealth Scientific and Industrial Research Organisation (CSIRO), successor to CSIR. In the war years, the division was mainly located at Fishermen’s Bend, but Lennox’s section had laboratories on the site of the Division of Animal Health, in Parkville. The war years were difficult, with materials in short supply and everyone working long hours (a 44-hour week was the norm). In addition, Rogers attended night classes for the Diploma in Chemistry at the tech. The research introduced him to the mysteries of skin and wool that became the basis of his long career. Lennox necessarily spent much of his time at a desk, but under his instruction Rogers carried out experiments on the industrial processes used to depilate wool from sheepskins for the manufacture of leather. It was a responsible job and an important influence in his life, with Lennox playing the roles of both research director and mentor. It was he who very early in Rogers’ three years in his laboratory, suggested that he should aim to matriculate (that is, complete his secondary school education) with a view to gaining university entry rather than pursuing the technical college diploma course. This was sound advice and during the ensuing two years Rogers first obtained his Leaving Certificate, and in 1945, the newly introduced Matriculation Certificate.
The following year he resigned his laboratory job with Lennox and began the science course at the University of Melbourne, majoring in biochemistry and chemistry. It was an interesting time although difficult because the university was faced with burgeoning numbers of ex-service undergraduates in first-year subjects. The pressure lessened in later years as some decided not to continue, and those who remained probably brought a more serious emphasis to university student life. Rogers found biochemistry stimulating and exciting as new discoveries in metabolism and structure were flowing from the rapid growth of research post-war, and yet the discovery of DNA structure, the tertiary structure of proteins, and the mechanisms of their synthesis were many years away! Lecturers such as Jack Legge and Hugh (later Sir Hugh) Ennor, fresh from their association with scientists such as Hans Krebs in Oxford and David Green in Wisconsin, gave advanced lectures in the third year.
Graduating in 1949 and sharing the biochemistry prize, Rogers decided to pursue a master’s degree in biochemistry, and was offered a scholarship by the professor of biochemistry Victor Trikojus (1902–85),4 a Foundation Fellow of the Australian Academy of Science who was busy developing biochemistry as a major discipline in the University of Melbourne, with an increasing emphasis on research. Rogers had always regretted leaving school and having to work hard at such a young age so he seized the opportunity for postgraduate study and his early experience of research with Lennox no doubt influenced his decision to take up the offer. By that time chemistry had less personal appeal—his background in mathematics not strong as a result of his secondary education—and he preferred biological chemistry to physical or organic chemistry.
University of Melbourne and CSIRO
Trikojus offered Rogers a place with Jack Legge who had been appointed to his staff in 1947. At the time, Legge was collaborating with R. D. Lemberg in writing their seminal work, Haematin Compounds. Legge, who later became a reader in biochemistry, was a stimulating lecturer and supervisor who decided to give Rogers a research topic that was distant from his own prime interest of oxidation–reduction mechanisms and the generation of energy in the cell.
Legge had an agile mind, and interests beyond the scientific, being markedly active politically,5 but those extracurricular activities did not reduce his effectiveness as a supervisor of Rogers’ efforts to purify the gastrointestinal hormone secretin. He made significant progress in the purification of this hormone but not to the stage for the sequencing that became possible at that time by the new degradative method developed by Pehr Edman.
Rogers graduated MSc with first class honours in 1951, but rather than pursuing a PhD he returned to CSIRO, joining the recently-established Biochemistry Unit of the Wool Research Laboratories in Parkville, Melbourne, resuming an association with research on keratins and keratin fibres that continued for the majority of his career. The Parkville laboratory became the Division of Protein Chemistry in 1959 and he remained there until 1963.6 They were productive years during which he became interested in wool/hair structure and the biochemistry of wool/hair growth, combining techniques of chemistry and cell biology.
Rogers became convinced that because the follicle was such a small structure, a combination of chemical methods at the electron microscopic level could be a productive approach and therefore decided to learn the electron microscopy (EM) methods that were finding new applications in biology in the early 1950s. He acknowledged his good fortune in getting some training in electron microscopy with Alan Hodge, a biologist who had trained at the California Institute of Technology with the structural biologist F. O. Schmitt. Alan Hodge was using an RCA transmission electron microscope (TEM) that had been purchased and installed just after the war at the CSIRO Division of Chemical Physics. This microscope had been ‘souped up’ to high resolution and was undoubtedly one of the best in the world. It was an exciting period in the Wool Research Laboratories but it also became apparent to Rogers that advancement in his career would benefit from gaining a PhD.
Cambridge
In 1954, Rogers was awarded a CSIRO Overseas Research Scholarship and travelled by sea to England, visiting Italy on the way. He was admitted to Trinity College, Cambridge, as a PhD candidate, relieved that some knowledge of Latin was no longer a requirement for admission.
George Rogers at a family function in 2014 (Rogers family collection, reproduced with the permission of the family).

Originally his supervisor was to be Dr J. R. G. Bradfield, who had published in the area of histochemical electron microscopy, but Bradfield had just taken up the junior bursarship at Trinity so Rogers’ supervisor became Dr A. F. W. Hughes, a distinguished embryologist and cell biologist who among other achievements had taken an active role in the introduction of Francis Crick into matters biological after World War 2. Rogers had also been seconded to the EM group at the Cavendish Laboratories and he was given a room in the anatomy school by the Regius Professor of Anatomy, J. Dixon Boyd. He spent much of his time in the electron microscopy unit of the Cavendish laboratories headed by Dr V. E. Cosslett, a physicist, distinguished by his contributions to the development of the EM and its applications. His ‘salad days’ at Cambridge were no less than idyllic, both socially and scientifically, the latter on account of the combination of physics and biology at this new and exciting Cavendish laboratory (Fig. 2). Amongst many opportunities he was able to attend in lectures given by leading scientists of the time such as Perutz, Kendrew, Sanger, Crick and many visiting biochemists. He also had links with the Strangeways laboratory, still famous in cell culture and headed by (later Dame) Honor B. Fell (1900–86).
The Leitz light microscope George was using in this picture in 1954 was the best available in the 1950s and the only one in the laboratory (Rogers family collection, reproduced with the permission of the family).

Rogers’ collaborator in his work there was Dr Audrey Glauert. In conjunction with her brother Richard Glauert, then an organic chemist with Ciba-Geigy, they formulated a new epoxy resin embedding medium, Araldite, for EM work. This material was especially useful for studying hair and wool fibres in sections and the development was published in a short paper in Nature that was for a time the most read paper.7
Rogers completed his PhD in 1956 on studies by Transmission Electron Microscopy (TEM) of the structure of the hair follicles and skins of animals, mainly rats and mice. He was also one of the early users of scanning electron microscopy (SEM) and collaborated with C. E. W. (later Professor Sir Charles) Oatley who developed the first practical SEM. They took the first pictures of the wool fibre surface with that instrument but did not further pursue that avenue because Rogers’ emphasis was on internal structure and not the surface cuticle. This was an unfortunate choice because it meant that he missed the chance of making findings that came later by others, relevant for the practical application to human hair cosmeceuticals.
CSIRO 1957–63
After his return to the Parkville laboratories in late 1956, Rogers published some short papers on the follicle work8 (while they waited for the arrival of a new Siemens Elmiskop TEM) since he had obtained excellent images of the hair surface. This instrument was delivered on 4 October 1957, a date that Rogers often remarked that he would never forget because it was the day that Sputnik was launched by the Russians.9 While waiting for the delivery of the instrument, he had taken up an allied interest in the analysis of the proteins of the inner root sheath (IRS) of hair follicles. This layer surrounds the cuticle of the growing fibre and consists of three distinct cellular layers: the inner root sheath cuticle, Huxley layer, and Henle layer.10
There were several properties of the IRS that distinguished it from the cortex and cuticle of the hair but Rogers was keen to understand the origin of these properties. Protein methods were improving but it was still necessary to have milligrams of protein. For this he needed large follicles from which to dissect the IRS free of cuticle or cortex, so he chose vibrissae hairs of the rat snout that had large actively growing follicles. The whole IRS could be peeled from the hair root with watchmaker forceps under a dissecting microscope. After washing with water the tissue was hydrolysed, and chromatograms run on the hydrolysate were compared with the amino acid spots obtained from the fibre. This was one of the memorable moments in his research career.
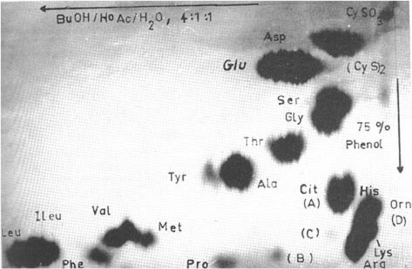
A prominent spot appeared that was distinct from those in a keratin chromatogram (Moore and Stein analysis). Subsequent studies revealed the amino acid citrulline, the first time this amino acid had been identified and defined in the composition of a protein (Fig. 3).11 The years that followed required confirmation of its identity and later definition of how it was formed, and its function.12 Those studies proceeded over the following two decades and saw the discovery of peptidylarginine deiminase (PAD) as a new enzyme, distinct from the arginase that carries out a post-translational event on the natural substrate in the IRS called trichohyalin.
This graphic is of the paper chromatogram Rogers obtained of the amino acids (dark spots) present in the proteins of the inner root sheath, a layer of cells in wool and hair follicles. The spot intensities are markedly different from those of hair or wool keratin and the spot marked ‘Cit’ in the bottom right hand corner was later identified as citrulline, the first time that this amino acid was found in a protein (Rogers family collection, reproduced with the permission of the family).
In those days the molecular make-up of wool and hair keratin was still to be better defined and the organisation of the filaments was not known in detail, especially in the orthocortical and paracortical segments that constitute the bilateral structure of the cortex in wool and play a role in the waviness or curliness of fibres. In his earlier work, Rogers had used histochemical techniques to follow oxidative pathways in the wool follicle and he was the first in the hair follicle field to use tetrazolium salts as indicators of oxidative processes in that tissue. He had also experimented with embryonic mouse skin development in organ culture but this was discontinued in favour of beginning several years of investigating the structure of wool fibre. This work had begun with examining its microscopic components, namely cortex, cuticle, and medulla at the light microscopic level including the confirmation of the bilateral structure of the cortex first reported by Mercer that is revealed through the selective uptake of basic dyes. Much of this aspect was undertaken in collaboration with physicist colleague R. D. B. (Bruce) Fraser.13
In 1957, Rogers was able to pursue those further studies of wool fibre structure and properties using the new Siemens Elmiskop TEM to progress the understanding of keratin structure at the macromolecular level, but improved preparative techniques of fibres were necessary to reveal the internal structure at high resolution. An ultramicrotome was brought into service for sectioning and a new staining protocol was designed that involved partial reduction of disulfide bonds to increase the internal deposition of osmium and of lead atoms.
In addition, the epoxy embedding medium devised in Cambridge proved to be invaluable for producing ultrathin sections about 60 nm thick for high resolution work. One of the central findings was the internal structure of keratin (‘intermediate’) filaments and the organisation of the filaments with matrix proteins in relation to the bilateral structure of the fibre cortex and fibre curl (‘crimp’), and structural changes produced after industrial processes.14 Some of this work was integrated with X-ray diffraction investigations Bruce Fraser and T. P. MacRae including a book on keratins.15 Concurrent with these studies Rogers began work on protein synthesis in the follicles of guinea pigs and sheep.
Eventually his group was the first to isolate polysomes and to obtain the synthesis of specific keratin proteins in reconstituted systems in the laboratory.16 At the higher magnification his work showed the different organisations of the filaments in the orthocortical and paracortical segments of the bilateral wool cortex and the presence of a protein matrix between them was resolved. Small ‘islands’ of non-keratin material between the bundles of fibrils were also visualised and helped to explain some of the properties of the wool fibre such as the penetration of dyestuffs.
A clearer picture of the internal structure of the cuticle of the wool fibre was also obtained including a cysteine-rich outer layer that Rogers named the A-layer. The EM work blended nicely with the X-ray studies of Bruce Fraser, for the keratin structure at the tip of an Echidna quill. High resolution EM images of the identical material were obtained and revealed a beautiful almost crystalline, arrangement of filaments, just as Fraser and others had found by low angle X-ray diffraction. In collaboration with other scientists, research extended to EM studies of different wool types and other keratinous tissues including feather and the effect of certain chemical treatments of wool. The feather studies revealed filaments that were half the diameter of those of mammalian keratin. In later years Rogers investigated the proteins of feather by applying gene cloning to the problem.
The move to academia in Adelaide
Rogers maintained his interest in the macromolecular structure of fibrous proteins but had a desire to investigate fibre differentiation at the biochemical level and this required an environment of active research into protein synthesis mechanisms and gene expression, fields that were beginning to blossom rapidly. In 1961, he received an offer from R. K. Morton (1920–63),17 then Professor of Agricultural Biochemistry at the University of Adelaide located at the Waite Institute. Morton needed to set up biological EM work on cell mechanisms related to cancer and wondered if Rogers would be interested in joining his department. Rogers turned down the offer but a year later Morton asked him again and raised the position to reader, so he applied and was appointed early in 1963.
In his usual humble way, Rogers said he was rather lucky, but he was one of a few people with a biochemical background who had knowledge of biological ultrastructure, as it was termed in those days, and he was skilled in the techniques of electron microscopy. He had given a few talks on the microstructure of cell components such as mitochondria, endoplasmic reticulum, and Golgi apparatus that were the subject of much discussion in cell biology.
By the time Rogers moved to Adelaide, Morton had taken up the Chair of Biochemistry at the main campus on North Terrace where he was initiating a new era of biochemical research and teaching in Adelaide. The changes included extensive refurbishment of the Darling Building to raise the quality of facilities in both teaching and research areas. He wanted Rogers to initiate a program on embryonic development using the frog embryo. However, the techniques available at that time were inadequate compared to the molecular biological ones that are now used for studying development in terms of regulated gene activity, and as Rogers was more passionate to broaden his interest in hair growth, Morton finally agreed.
Henceforth cellular and molecular events in the growth of hair were the centre of his research interests. Rogers often said he was fortunate to have research grants from the Australian Wool Corporation and the Australian Research Council. This enabled him to have a PhD student, an honours student, and two technicians for his studies of keratinisation in wool follicles, the process of keratin protein synthesis, and the hardening of the fibre contents by the formation of disulfide bonds. The electron microscope was used for parallel studies of morphological events in the formation of hair. Several papers were published that reported on structural features of hair follicle cells, some of which were later followed up by other workers.18 Some main features of a follicle are indicated in the diagrams below (Fig. 4).
This diagram shows all the cellular layers of a wool or hair follicle. The different events that occur as the growing fibre moves up the follicle are indicated on the right-hand side of the graphic (Rogers family collection, reproduced with the permission of the family).

Morton tragically lost his life in September 1963 as the result of a laboratory explosion and the department’s new era of development was seriously affected.
As the most senior appointment in the department, Rogers became acting head, but a consequence for him was a hiatus in his own research because of the intense activity needed to salvage what was possible from Morton’s revamping of the teaching and research directions of the department. This was carried on in difficult circumstances because there was an acute shortage of staff after Morton died. There were only four staff in the department who had to share the load of second- and third-year teaching for the undergraduate science degree, but with the generously contributed skills of colleagues Drs R. H. (Bob) Symons, Bruce Keech, and Beth Neville, courses and lectures were rapidly developed. In second year, Rogers gave lectures on amino acid metabolism, while third-year lectures were designed to cover the rapidly changing field of protein chemistry. Although he enjoyed the challenge of teaching and put considerable effort into it, he always felt that he never achieved the consummate skills of an excellent lecturer.
During the next few years, the publication of X-ray crystallographic solutions of protein tertiary structure was becoming frequent. Later, in one of his practical exercises in third-year teaching, he included the construction of a spoke model of lysozyme, the first enzyme to have its tertiary structure solved by Phillips in Oxford.19
Rogers obtained the coordinates from the Oxford group and the model components from Cambridge, and then Bruce Fraser helped him by mail and telephone with the coordinates to finally put it together. The now fluorescence-illuminated model has been on display in the foyer of the Molecular Life Sciences building at the University of Adelaide for many years (Fig. 5).
The picture of the lysozyme molecular model that was used in protein chemistry lectures in the 1960s and 1970s and was built as part of a practical course from coordinates supplied by the Oxford group of Phillips where the tertiary structure was solved by X-ray crystallography (Rogers family collection, reproduced with the permission of the family).

In 1965, the excellent choice of W. H. (Bill) Elliott was made as the new professor of biochemistry and a new phase of development took place in the department. Under Elliott’s leadership the department became one of the earliest in Australia to turn its interests towards molecular biology and to embrace the application of molecular cloning techniques to eukaryotic biology whilst retaining a strong biochemical base. Rogers was always the first to acknowledge that his research benefitted because his group pursued keratin gene discovery in both the avian and mammalian keratin systems.
Over the next twenty years they delved into the origin of citrulline in some proteins, the details of keratin gene sequences, and the organisation of the genes in the genome. Citrulline was obviously formed from arginine but the challenge was searching for the converting enzyme.20 They used homogenates of follicles from guinea pig skin after importing a colony into the department for dedication to this work.
They developed a colorimetric assay for citrulline using histones as substrates (rich in arginine) and succeeded in finding the enzyme, calling it arginine deiminase. The most likely reason for this activity was a post-translational modification. They further discovered that its activity was dependent on the presence of calcium.
Within a few years Japanese workers demonstrated that the enzyme activity was ubiquitous in body tissues and gave it a better name: peptidylarginine deiminase (PAD). Later Rogers and his group devised a more sensitive radioactive-based assay for PAD and in the 1990s purified a few micrograms of the enzyme and from this they were able to obtain the sequence of six or seven amino acid residues at the N-terminus of the protein.21 From that result they turned to gene techniques and found the gene from which they were able to decipher the total amino acid sequence.
The follicle enzyme, peptidylarginine deiminase, was finally sequenced in 1997 by means of recombinant DNA techniques and its location in the hair follicle was described, as below. The remarkable finding after all this is that PAD can denature proteins because its activity converts positively charged arginine residues in a protein to citrullines that have no charge. The result is a major shift in the charged profile of the protein and consequent marked conformational changes and denaturation.
Understanding this enzyme explained events that occur in the inner root sheath cells of the follicle during hair growth. An arginine-rich protein called trichohyalin, a rod-like molecule, is synthesised in the cells and is acted on by PAD. Consequently the trichohyalin denatures and spreads amongst intermediate-type filaments that are also made by the cells during the upward growth of the hair. This assembly of filaments and a matrix is finally cross-linked by isopeptide bonds through the activity of another enzyme, transglutaminase, that was also found to be present in the follicle.22 Structurally the changes are like those that occur in the cortex of the fibre but the chemistry is different.
Many papers on this topic were published with postgraduate students and postdoctorals named in footnotes that follow. Other workers entered the field and as part of their research found that the PAD enzyme is ubiquitous in the body and participates in protein degradation by introducing molecular conformation changes. Fifty years later, the biochemical and physiological functions of the enzyme are still being uncovered, including its role in rheumatoid arthritis.
At that time there were many excellent students wanting to proceed to higher degrees so it became possible to broaden the keratin studies to include the largely unknown feather keratins, although it was alleged that they were a simpler group than mammalian keratins and to some extent that was confirmed by subsequent studies. Rogers showed that the keratin genes of the chick were tandemly aligned in the genome, and his groups sequenced some fifteen out of a likely fifty in the genome giving the derived amino acid sequences.23 There was only one intron, located in the non-coding region of the genes and Anna Koltunow, a PhD student,24 showed that the sequence was a potential control element for gene expression.
During those busy research years, Rogers was also consulted on the Thornton murder investigation (Sydney 1960s) and in 1972 he was involved with a six-month investigation of fibre evidence in the van Beelen murder case (Adelaide 1972), and several similar murder cases in following years.25
In 1977, Rogers took study leave in France at the University of Grenoble (Fig. 6) to work with the developmental biologists Philippe Sengel and Danielle Dhouailly who were experts in the field of dermal induction of epidermal differentiation. Using facilities at the European Molecular Biology Laboratory in Grenoble, headed by Andrew Miller, they had a successful collaboration, investigating the keratins expressed when dermal layers from different body regions were switched.26 For example, scale skin can be induced by feather dermis to make a feather and a feather dermis can be produced from a feather.
This photograph of George Rogers was taken in the laboratory at the European Molecular Biology lab in Grenoble, France in 1977 when he was on study leave in the laboratory of the developmental biologists Philippe Sengel and Danielle Dhouailly (Rogers family collection reproduced with the permission of the family).

Back in Adelaide, amino acid sequences derived from the DNA sequencing of other keratinising tissues of the chick such as the scale, claw, and beak were found to contain the basic feather sequence, but with insertions of increasing numbers of a thirteen amino acid sequence.27 A similarity in the sequences with that of a lizard keratin accorded with the ancestral origin of feather keratin. Rogers was always impressed that many of his students who did their PhDs in that area became leaders in their field,28 and several others, senior scientists at CSIRO,29 and at other universities.30
The mammalian keratin work was focused on wool because the main funding came from that direction. In the course of these studies, Rogers’ groups sequenced many genes encoding the filament families and the also gene families of the fibre matrix.
Along the way, gene probes were used to establish the order in which the different families are expressed during fibre growth.31
In his typical way, Rogers described the use of sheep and wool follicles in his research as both fortunate and unfortunate: fortunate, because of the greater certainty of funding (some of the feather keratin research was even supported by the Wool Corporation, a circumstance that could not happen now!), and unfortunate, in that his pioneering molecular studies of wool fibres could only be applied indirectly to human skin diseases that affect hair growth, thus having a limited impact in the biomedical field where hair growth research became extraordinarily active. However, he was able to compare the sheep wool keratin with human keratins through his regular attendance over the years at Gordon conferences in the United States. These conferences were excellent in that besides the more formal sessions, they enabled him to meet and link up with American and other overseas scientists. Some of his students and postdoctoral fellows were also able to attend through the availability of special funding (Fig. 7).
Photograph taken in 1991 with colleagues at a Gordon conference in USA on keratinisation. The group includes from left Beverley Dale Washington University, Joseph (Joe) Rothnagel (a former PhD student then doing a Postdoc at Baylor College of Medicine, Houston, and later became a senior academic at Queensland University), George Rogers, Pam Hawlet-Nelson (NIH), and Bob Goldman (Northwestern University) (Rogers family collection, reproduced with the permission of the family).

Many honours, postgraduate students, and postdoctoral fellows passed through Rogers’ laboratory over the years from 1965 to 1992, most of them maintained contact, and became colleagues and close friends of Rogers and his family. All these years of teaching, administration, and research in the Adelaide department were enjoyable and exciting times. Over the years from 1967 until Bill Elliott’s early retirement in 1988, the teaching staff numbers grew to eight (lecturers and above) and there were two or three tutors who mainly ran the practical classes. There was always a strong complement of postdoctoral fellows, and PhD and honours students spread through the various research teams and the department had the good fortune that its staff members were complementary in function and personality. This circumstance of compatibility played an important role in the University of Adelaide biochemistry department’s advancement to a leading position in Australia of being recognised as one of the most active and progressive.
One of the department's achievements at the university was the establishment of a commonwealth funded Special Research Centre for Gene Technology in 1982, which attracted a high level of research funding. Four of the staff made a combined application involving molecular biology in projects on porphyric diseases, histones in gene expression, structure and function of plant viruses and viroids, and sheep wool genes, wool growth and transgenesis. The application was successfully defended before a panel and the centre, that began in 1982 lasted until 1991. Support for the centre was on a five-year cycle and meant that they could each expand the scope of their projects beyond that normally possible, being able to take on more postgraduate students, and appoint postdoctoral fellows, and research assistants.
Rogers often remarked that he was fortunate to have groups that were well matched in personality and enthusiasm so that he never really had any personnel problems. As laboratory members moved on, others came in. The family had frequent pool parties at their home. He found being involved with younger people pursuing their careers was a very satisfying way in which to spend one’s working life.
Over many years in his laboratory there were two traditions: one was that if there was a significant experimental result then they had either cake or champagne or even both, the other was ‘Monty Python Lumberjack Day’ every year in June. Most members of the laboratory were Monty Python fans and pictures were taken of the event as the song was sung.32
In 1988, Bill Elliott decided to retire early, leading to Rogers having a second stint as head of the department that was not much better than his first because the university was under financial stress, and university teaching was changing from terms to a semester-based system. The Research Centre for Gene Technology was well underway by this time and despite the extra duties, Rogers still found it an exciting time. Under its auspices, his group had been able to undertake an extensive study of the genes encoding the structural proteins of wool with significant results. However, funding for the centre was not renewed at the end of the second round and ceased in 1991.
Once problems of DNA recombination and gene cloning had been sorted out internationally and locally, facilities for this technology were developed in the Department of Biochemistry and protein synthesis studies progressed to characterisation of sheep genes encoding filament and matrix proteins of the wool fibre.33
Those advances constituted a forerunner to an enhanced study by German workers several years later of the equivalent human genes. The gene studies by this group extended further into the possibility of using the genes and efficient promoters to engineer the follicles to alter the expression, and hence abundance, of some of the filament and matrix structural proteins.
Officially, Rogers had to retire at the end of 1992, because retirement was mandatory at sixty-five. The university recognised his thirty years of service—fifteen as reader/associate professor and fifteen as professor—with the title of Emeritus Professor, but he was not ready to cease work and his funding from the Wool Corporation was still current.
Rogers had also become interested in the methods of gene transfer that were becoming possible at this time and with senior postdoc Barry Powell demonstrated that transgenic mice produced hair with changes in composition and properties when they were carrying specific genes targeted to overexpress wool proteins during fibre growth.34
It was inevitable that they should consider similar studies on the production of transgenic sheep, provided there was sufficient funding. With funds from the Centre for Gene Technology and the Australian Wool Corporation, his group had initiated studies on transgenic sheep by linking up with reproductive biologists of the South Australian Research and Development Institute (SARDI), who had initiated the microinjection of DNA into fertilised sheep ova and implanting the injected ova into the sheep uterus. The laboratories where this work was pursued were located at the research station at Turretfield some sixty km from Adelaide where special quarantine paddocks surrounded by high double fencing were constructed to prevent escape of the engineered sheep.
Rogers’ group was aiming to change the protein composition of wool by targeting expression of novel genes to the wool follicle, thereby introducing new physical properties that would be advantageous for the wool producer (Fig. 8).
The transgenic wool produced by the sheep expressing the extra keratin filament protein on the right) had less crimp and more lustre than the control on the left (Rogers family collection reproduced with the permission of the family).

In 1995, the group joined with a CSIRO group in Sydney that was part of the Cooperative Research Centre (CRC) for Premium Quality Wool that had begun in 1993, and for the next few years Rogers managed the transgenesis program in that CRC while located in the Department of Animal Science at the Waite Institute. SARDI was a major participant in the program.
In addition to altering wool structure, they also investigated the possibility of inserting into sheep a biochemical pathway for the synthesis of cysteine, the sulfur containing amino acid that can be rate limiting to the yield of wool. This was a tough project because of the many aspects that had to be considered. They had to isolate bacterial genes for the two enzymes involved and to engineer them so they would be targeted to express in the wall of the sheep rumen. The idea was that the cells of the rumen would capture the hydrogen sulfide that is prevalent in the rumen and normally lost as sulfate. The cysteine synthesised by the inserted pathway would be expected to pass into the blood circulation.
They had some successes,35 (Fig. 9) and were able to gain additional support from the Wool Corporation, although Rogers was never on salary. The results obtained were important, but costs of sheep transgenesis were enormously high, and over the years the work consumed around $2 million. Despite the progress and the fact that the process was patented,36 funding discontinued around 2000. There were two main problems: one was, a useful wool product was not produced, and another was that genetically modified sheep were perceived by their funders, the wool growers, CRC and SARDI, to be unacceptable by the consumer public.
The transgenic lambs shown in this picture are being held by members of the lab (from left to right: Paul Verma, Simon Bawden, Shiv Sivaprasad, and George Rogers). The genes for a novel biochemical pathway to synthesise cysteine had been incorporated into their genome but only low levels of activity were obtained at that stage of the research (Rogers family collection reproduced with the permission of the family).

In 2002, Rogers was provided with the use of an office and some laboratory space in the new Molecular Life Sciences (MLS) building, the new home for the biochemistry department on the North Terrace Campus and at the same time he was also fortunate to gain some contract research both locally and from overseas until about 2010.
In 2006, space in the MLS building was at a premium due to increases in staff and funds, but through the auspices of its then head, Rogers was able move to the medical school. That was very successful because most of the equipment he needed for the gene discovery work was in the one building, and in the following five years he wrote several papers and book chapters, and delivered lectures. This allowed him over subsequent years to continue his research interests in molecular aspects of hair growth and to conduct contractual research with University of Adelaide’s Department of Animal Science, with CSIRO, and with commercial firms in Germany and Japan. He found it very satisfying to continue working at the bench and to maintain contacts with colleagues nationally and internationally until 2017.
In 2013, Rogers was made an Officer in the Order of Australia ‘for distinguished service to biochemistry through contributions to tertiary education and leadership in research into the molecular structure and growth processes of wool and hair’.
Conclusion
George Rogers was the epitome of a gentleman, a selfless and humble man. He treated everyone thoughtfully and with respect. Following the breakdown of his first marriage, he met Racheline (known as Lynn) also a biochemist, in 1971, at a Christmas party at the University of Adelaide dental school where she was doing research. They married in 1972, had two daughters, and were loving soulmates for fifty years.
Rogers was immensely proud of their two remarkable and gifted daughters, Natasha and Nicole, and they cherished him. His ethics, hard work, and mentorship set a wonderful example. Natasha is now a professor at Sydney University and runs a research lab at WIMR (Westmead Institute for Medical Research). She is also a senior staff specialist in nephrology and head of the transplant unit at Westmead Hospital. Nicole is a senior executive for a leading global film and television production company. She has won numerous awards for programs she has produced.
There are three wonderful grandchildren whom Rogers adored and he was adored in return.
Rogers’ thoughtfulness and humility were always evident (as the quotations show in this memoir). He always thought he was fortunate when he achieved remarkable science through long, hard work or even when he received grants. In fact, Bill Elliott jokingly called him ‘Nellie Melba’,37 because whenever he obtained a grant from the Australian Wool Corporation he would say ‘this is probably the last one’, as Melba would say for every performance she gave before she ‘retired’.
Rogers outlived many of his close friends and biochemistry colleagues. He wrote biographical memoirs for R. H. (Bob) Symons and W. H. (Bill) Elliott38 for the Academy and although his eyesight was failing, also co-authored Bruce Fraser’s.39
He retained friendships with most of his past students and would organise the ‘Keratin Korna get together lunch’ at the end of every year. In the last few years the lunch was held at the University of Adelaide Staff Club, the last one, in 2019 (Fig. 10). Many students who lived and worked interstate would attend the lunch, some with their partners. Those who worked overseas and were coming to visit family in Adelaide for Christmas would arrange their itinerary to be present. They were all very fond of him and regarded him as their mentor and friend.
The last Keratin Korna lunch that George Rogers was able to organise in the University of Adelaide Staff Club in 2019 (Rogers family collection, reproduced with the permission of the family).

When Rogers turned ninety in 2017, one of his past students anonymously donated a generous sum to the university to set up a postgraduate scholarship in his name—it became the George Rogers Molecular Biology Scholarship since the Department of Biochemistry no longer existed (Fig. 11).
Lynn, George, Nicole and Natasha on his 90th birthday at the celebration for the announcement of the George Rogers Postgraduate scholarship in 2017 (Rogers family collection, reproduced with the permission of the family).

Rogers’ main hobbies were family and science although when younger he had enjoyed sea fishing and a little sailing. When he retired, he treated himself to a small fishing boat, and would go fishing occasionally with a friend, or sometimes with a student and enjoyed spending a day on the water. Apart from that, he kept the pool and house garden in order when he was not in the lab, but the highlight of his week for many years was the Friday lunch with special colleagues. It had started at the Waite but in the last few years moved to the staff club at the University of Adelaide.
George Rogers died on 3 November 2021 after a long illness, his wife Lynn holding his hand. His daughters could only say goodbye on FaceTime because it was during one of the peaks of the COVID epidemic when the South Australian borders were closed so they were unable to attend his funeral. Lynn, her brother, sister-in-law, and a close family friend were the only people present. On 11 November 2022, Nicole and Natasha organised a private memorial for close friends, colleagues, and past students at the staff club at the university and many past students and colleagues from interstate attended.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Acknowledgements
Must go to Professors Chris Dickman, Murray Whitelaw, and Ian Rae for their time, advice, patience, and corrections, in the final drafts of this Memoir.
References
Blake, C. C. F., Koenig, D. F., Mair, G. A., North, A. C. T., Phillips, D. C., and Sarma, V. R. (1965) Structure of hen egg-white lysozyme: a three-dimensional Fourier synthesis at 2 Å resolution, Nature, 206(4986), 757-761.
| Crossref | Google Scholar |
Clarke, R. M., and Rogers, G. E. (1965b) Keratin protofilaments and ribosomes from hair follicles, Nature, 205, 77-78.
| Crossref | Google Scholar |
D’Andrea, R. J., Sivaprasad, A. V., Bawden, S., Kuczek, E. S., Whitbread, L. A., and Rogers, G. E. (1987) ‘Isolation of microbiol genes for cysteine synthesis and prospects for their use in increasing wool growth’, in The Biology of Wool and Hair, eds G. E. Rogers, P. J. Reiss, K. A. Ward, and R. C. Marshall, Chapman and Hall, London/New York, pp. 447–463.
Dhouailly, D., Rogers, G. E., and Sengel, P. (1978) The specification of feather and scale protein synthesis in epidermal-dermal recombination, Developmental Biology, 65, 58-68.
| Crossref | Google Scholar |
Downes, A. M., Sharry, L. F., and Rogers, G. E. (1963) Separate synthesis of fibrillar and matrix proteins in the formation of keratin, Nature, 199, 1059-1061.
| Crossref | Google Scholar |
Filshie, B. K., and Rogers, G. E. (1962) An electron microscope study of the fine structure of feather keratin, Journal of Cell Biology, 13, 1-12.
| Crossref | Google Scholar |
Fraser, B. (2008) ‘Professor George Rogers, biochemist’, https://www.science.org.au/learning/general-audience/history/interviews-australian-scientists/professor-george-rogers#12, viewed January 2023.
Fraser, R. D. B., MacRae, T. P., and Rogers, G. E. (1960) 33—Recent observations on the structure of α-keratin, Journal of the Textile Institute Transactions, 51, T497-T505.
| Crossref | Google Scholar |
Frenkel, M. J., Powell, B. C., Ward, K. A., Sleigh, M. J., and Rogers, G. E. (1989) The keratin BIIIB gene family: isolation of cDNA clones and structure of a gene and a related pseudogene, Genomics, 4, 182-191.
| Crossref | Google Scholar |
Glauert, A. M., Rogers, G. E., and Glauert, R. H. (1956) A new embedding medium for electron microscopy, Nature, 178, 803.
| Crossref | Google Scholar |
Harding, H. W. J., and Rogers, G. E. (1971) ε-(γ-glutamyl)lysine cross-linkage in citrulline-containing protein fractions from hair, Biochemistry, 10, 624-630.
| Crossref | Google Scholar |
Harding, H. W. J., and Rogers, G. E. (1972a) The occurrence of the ε-(γ-glutamyl)lysine cross-link in the medulla of hair and quill, Biochimica et Biophysica Acta, 257, 37-39.
| Crossref | Google Scholar |
Harding, H. W. J., and Rogers, G. E. (1972b) Formation of the ε-(γ-glutamyl)lysine crosslink in hair proteins. Investigation of transamidases in hair follicles, Biochemistry, 11, 2858-2863.
| Crossref | Google Scholar |
Harding, H. W. J., and Rogers, G. E. (1976) Isolation of peptides containing citrulline and the crosslink ε-(γ-glutamyl)lysine, from hair medulla protein, Biochimica et Biophysica Acta, 427, 315-324.
| Crossref | Google Scholar |
Keough, R., Powell, B., and Rogers, G. (1995) Targeted expression of SV40 T antigen in the hair follicle of transgenic mice produces an aberrant hair phenotype, Journal of Cell Science, 108, 957-966.
| Crossref | Google Scholar |
Koltunow, A. M., Gregg, K., and Rogers, G. E. (1986) Intron sequences modulate feather keratin gene transcription in Xenopus oocytes, Nucleic Acids Research, 14(16), 6375-6392.
| Crossref | Google Scholar |
Lockett, T. J., Kemp, D. J., and Rogers, G. E. (1979) Organization of the unique and repetitive sequences in feather keratin messenger ribonucleic acid, Biochemistry, 18, 5654-5663.
| Crossref | Google Scholar |
Morris, C. P., and Rogers, G. E. (1979) The terminal structures of feather keratin mRNA, Molecular Biology Reports, 5, 145-149.
| Crossref | Google Scholar |
Powell, B. C., and Rogers, G. E. (1979) Isolation of messenger RNA coding for the “fast” protein of embryonic chick feathers, Nucleic Acids Research, 7, 2165-2176.
| Crossref | Google Scholar |
Powell, B. C., and Rogers, G. E. (1990) Cyclic hair-loss and regrowth in transgenic mice overexpressing an intermediate filament gene, The EMBO Journal, 9, 1485-1493.
| Crossref | Google Scholar |
Rogers, G. E. (1957a) Electron microscope observations on the structure of sebaceous glands, Experimental Cell Research, 13, 517-520.
| Crossref | Google Scholar |
Rogers, G. E. (1957b) Electron microscope observations on the glassy layer of the hair follicle, Experimental Cell Research, 13, 521-528.
| Crossref | Google Scholar |
Rogers, G. E. (1958a) Some observations on the proteins of the inner root sheath cells of hair follicles, Biochimica et Biophysica Acta, 29(1), 33-43.
| Crossref | Google Scholar |
Rogers, G. E. (1958b) Some aspects of the structure of the inner root sheath of hair follicles revealed by light and electron microscopy, Experimental Cell Research, 14, 378-387.
| Crossref | Google Scholar |
Rogers, G. E. (1959a) Electron microscope studies of hair and wool, Annals of New York Academy of Sciences, 83(3), 378-399.
| Crossref | Google Scholar |
Rogers, G. E. (1959b) Newer findings on the enzymes and proteins of hair follicles, Annals of New York Academy of Sciences, 83(3), 408-428.
| Crossref | Google Scholar |
Rogers, G. E. (1966) Structure and biosynthesis in the hair follicle and related structures in symposium on electron microscope studies on the biosynthesis and assembly of fibrous proteins, Proceedings of the Royal Microscope Society, 1, 67.
| Google Scholar |
Rogers, G. E. (1990) Improvement of wool production through genetic engineering, Trends in Biotechnology, 8, 6-11.
| Crossref | Google Scholar |
Rogers, G. E. (2003) Memoir for Dr. P. M. Steinert former PhD student, Journal of Investigative Dermatology, 121(8), iv.
| Google Scholar |
Rogers, G. E. (2013) William Herdman Elliott 1925–2012, Historical Records of Australian Science, 24, 80-95.
| Crossref | Google Scholar |
Rogers, G. E., and Elliott, W. H. (2008a) Robert Henry Symons, Biographical Memoirs of Fellows of the Royal Society, 54, 383-400.
| Crossref | Google Scholar |
Rogers, G. E., and Elliott, W. H. (2008b) Robert Henry Symons 1934–2006, Historical Records of Australian Science, 19, 191-213.
| Crossref | Google Scholar |
Rogers, G. E., and Simmonds, D. H. (1958) Content of citrulline and other amino acids in a protein of hair follicles, Nature, 182, 186-187.
| Crossref | Google Scholar |
Rogers, G. E., Bawden, C. S., Cam, G. R., D’Andrea, R. J., Kuczek, E. S., Powell, B. C., and Sivaprasad, A. V. (1987) ‘A review of the genes for wool keratin and for cysteine biosynthesis: their significance for studies directed towards modified wool production’, in Merino Improvement Programs in Australia, ed. B. J. McGuirk, Australian Wool Corporation, pp. 511–518.
Rogers, G. E., Miller, A., and Parry, D. A. D. (2020) Robert Donald Bruce Fraser 1924–2019, Historical Records of Australian Science, 31, 157-168.
| Crossref | Google Scholar |
Rothnagel, J. A. and Rogers, G. E. (1983) ‘A sensitive assay for the enzyme activity in hair follicles and epidermis that catalyses the peptidyl-arginine-citrulline post-translation modification’, in Normal and Abnormal Epidermal Keratinization, eds I. A. Bernstein and M. Seiji, University of Tokyo Press, pp. 171–184.
Rothnagel, J. A., and Rogers, G. E. (1986) Trichohyalin, an intermediate filament-associated protein of the hair follicle, Journal of Cell Biology, 102(4), 1419-1429.
| Crossref | Google Scholar |
Steinert, P. M., and Rogers, G. E. (1971) The synthesis of hair keratin proteins in vitro, Biochimica et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis, 238, 150-155.
| Crossref | Google Scholar |
Steinert, P. M., Harding, H. W. J., and Rogers, G. E. (1969) The characterisation of protein-bound citrulline, Biochimica et Biophysica Acta (BBA) - Protein Structure, 175, 1-9.
| Crossref | Google Scholar |
Walker, S. K., Heard, T. M., Verma, P. J., Rogers, G. E., Bawden, C. S., Sivaprasad, A. V., McLaughlin, K. J., and Seamark, R. F. (1990) In vitro assessment of the viability of sheep zygotes after pronuclear microinjection, Reproduction, Fertility and Development, 2, 633-640.
| Crossref | Google Scholar |
Ward, K. A., Sleigh, M. J., Powell, B. C., and Rogers, G. E. (1982) The isolation and analysis of the major wool keratin gene families, Proceedings of the Second World Congress on Genetics Applied to Livestock Production, 6, 146-156.
| Google Scholar |
Wilton, S. D., Crocker, L. A., and Rogers, G. E. (1985) Isolation and characterisation of keratin mRNA from the scale epidermis of the embryonic chick, Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression, 824, 201-208.
| Crossref | Google Scholar |
Footnotes
1 This memoir draws on discussions with family and former students, on a draft biographical memoir prepared by George Rogers before he died, and on the transcript of an interview with Rogers conducted by Bruce Fraser for the Australian Academy of Science in 2008. Fraser (2008).
3 https://csiropedia.csiro.au/lennox-Francis-Gordon, viewed April 2023.
5 Legge was a pacifist, a charter member of the Australian Peace Council, and a long-term member of the Communist Party of Australia: J. W. Legge explained, https://www.awm.gov.au/collection/C1147994, viewed June 2023.
8 Rogers (1957a, 1957b, 1958a, 1958b). Further papers are listed in the Bibliography in the Supplementary Material.
9 Sputnik and the dawn of the space age, https://history.nasa.gov/sputnik.html, viewed April 2023.
10 Rogers (1959a, 1959b).
12 Steinert and others (1969). Harding and Rogers (1976b). Rothnagel and Rogers (1984). Further papers are listed in the Bibliography in the Supplementary Material.
13 Fraser and Rogers (1956). Further papers are listed in the Bibliography in the Supplementary Material.
14 Early examples are described in Rogers (1959a). Fraser and others (1960). Filshie and Rogers (1962). Further papers are listed in the Bibliography in the Supplementary Material.
15 Fraser and others (1972). Further papers are listed in the Bibliography in the Supplementary Material.
16 Early examples are described in Downes and others (1963). Clarke and Rogers (1965a). Steinert and Rogers (1971). Further papers on this subject were later published with students and colleagues and are listed in the Bibliography in the Supplementary Material.
17 Robert Kerford Morton (1920–63), https://www.eoas.info/biogs/P000659b.htm, viewed April 2023.
18 Clarke and Rogers (1965b). Rogers (1966). Further papers are listed in the Bibliography in the Supplementary Material.
20 Steinert and others (1969). Harding and Rogers (1971) and further papers are listed in the Bibliography in the Supplementary Material. Peter Steinert and Harry Harding were PhD students at the time.
21 Rothnagel and Rogers (1983, 1984) and further papers are listed in the Bibliography in the Supplementary Material. Joseph (Joe) Rothnagel was a PhD student .in the late 1970s.
22 Harding and Rogers (1972a,1972b). Further papers are listed in the Bibliography in the Supplementary Material.
23 Morris and Rogers (1979). Powell and Rogers (1979). Rogers and others (1981) and further papers are listed in the Bibliography in the Supplementary Material.
25 This is when and how Forensic Science began in South Australia. Rogers later encouraged H. W. J. (Harry) Harding, then a postdoctoral in the Rogers’ group, to continue as Officer-in-Charge of the Forensic Biology Laboratory in the IMVS (The Institute of Medical and Veterinary Science) from 1975.
27 Lockett and others (1979) and further papers are listed in the Bibliography in the Supplementary Material. David Lockett was a PhD student. In later years he became Group leader, Personalised Health, CSIRO Health and Biosecurity Business Unit until 2017.
28 Steve Wilton, a PhD student in the early 1980s, Wilton and others (1985), became Director (and on the board) of the Perron Institute for Neurological and Translational Science, Perth’s oldest private medical institute. He is also Director of the Centre for Molecular Medicine and Innovative Therapeutics. He received an AO in 2021.
29 Anna Koltunow, now a Fellow of both the Australian Academy of Science and the Australian Academy of Technology and Engineering, became a Chief Research Scientist at CSIRO, a Deputy Chief of the Division Chief of the Division of Plant Industry overseeing many CSIRO sites, and is now the leader of a Bill and Melinda Gates funded international program to improve crop yield in sub-Saharan Africa.
30 Joe Rothnagel later became an associate professor the University of QueenslandUniversity until his retirement. Rothnagel and Rogers (1986).
31 Frenkel and others (1989) and further papers are listed in the Bibliography in the Supplementary Material. Morry Frenkel was a PhD student from 1978 to 1982. He became Principle Research Scientist at CSIRO until his retirement in 2011.
32 George often related how a visiting postdoctoral fellow from China was completely dismayed by all these antics that related to a British comedy group.
33 Early examples are described in Rogers (1982). Ward and others (1982). Further papers are listed in the Bibliography in the Supplementary Material.
34 Powell and Rogers (1990) who were the first to target transgene expression to the hair follicle and show that it was capable of altering the properties of the hair fibre. The mouse made the cover of the EMBO Journal. Barry Powell was a PhD student from 1974 and remained with the Rogers research group as a senior postdoctoral for over twenty years.
35 Early examples are described in Rogers (1990). Keough and others (1995). Walker and others (1990). Further papers are listed in the Bibliography in the Supplementary Material. Rebecca Keough was a PhD student at the time. Currently she is the Manager, Flinders Health and Medical Research Institute, Flinders University.
36 Rogers and others (1987). D’Andrea and others (1987). Further papers are listed in the Bibliography in the Supplementary Material.


