Geochemical factors affecting the solubility of copper in seawater
Brad M. Angel A C , Simon C. Apte A , Graeme E. Batley A and Mark Raven B
A C , Simon C. Apte A , Graeme E. Batley A and Mark Raven B
A CSIRO Land and Water, Locked Bag 2007, Kirrawee, NSW 2232, Australia.
B CSIRO Land and Water, Urrbrae, SA 5064, Australia.
C Corresponding author. Email: brad.angel@csiro.au
Environmental Chemistry 18(1) 1-11 https://doi.org/10.1071/EN20133
Submitted: 15 September 2020 Accepted: 8 November 2020 Published: 27 November 2020
Journal Compilation © CSIRO 2021 Open Access CC BY
Environmental context. Many trace metals, including copper, are only sparingly soluble in seawater and may exist in both dissolved and particulate forms (e.g. as precipitates). Aquatic organisms may experience different toxic effects from exposure to dissolved and particulate trace metals. This study investigates how concentration, reaction time and changes to precipitate composition/mineral formation affect copper solubility in seawater, thus influencing metal bioavailability and toxicity in the field and laboratory.
Abstract. A lack of knowledge on the solubility of metals such as copper affects the ability to predict the forms (dissolved and particulate) that organisms are exposed to in field and laboratory waters. Laboratory tests were conducted where copper (total concentrations of 0.5 to 20 mg L−1) was added to natural and artificial seawater (pH 8.15, 22 °C), equilibrated for 28 days and dissolved copper monitored at periodic intervals. At 0.5 mg L−1, dissolved copper concentrations remained stable over 28 days and no precipitates were detected. However, at higher total copper concentrations, an initial rapid precipitation phase was followed by the establishment of a metastable equilibrium that persisted for periods of days to weeks, and whose solubility concentrations and duration were influenced by the total copper concentration and typically in the range 0.6 to 0.9 mg L−1. After 5 to 15 days, a step change decrease in dissolved copper concentration followed by a slow decline was observed in the >2 mg L−1 total copper treatments. The minimum solubility measured after 28 days was 0.053 mg L−1. Elemental and X-ray diffraction analyses indicated that the copper precipitates comprised similar proportions of amorphous copper hydroxycarbonate and amorphous dicopper trihydroxide chloride after 1 day and transformed to predominantly mineralised dicopper trihydroxide chloride in the clinoatacamite polymorph form after 28 days. These observations have particular relevance for toxicity tests of less sensitive organisms and highlight the need to consider metal solubility, exposure to precipitates and changes in precipitate mineral phases.
Keywords: clinoatacamite, concentration, copper, duration, geochemical factors, precipitation, seawater, toxicity.
Introduction
Experimental studies of environmental systems often require the preparation of spiked solutions containing trace metals at concentrations far greater than experienced in the natural environment. Typical examples are the test solutions prepared by ecotoxicologists for use in the determination of toxicity test dose–response curves (Burton and Fisher 1990; Levy et al. 2008; Luoma and Rainbow 2008) and the metal-spiked solutions equilibrated with natural sediments for the determination of adsorption isotherms (Frimmel and Huber 1996; Wang et al. 1997; Hua et al. 2012). Frequently, the concentrations used extend over several orders of magnitude. Given that most trace metals are sparingly soluble in natural waters, an important consideration in all of these applications is the limit of solubility of the metal in solution. At spiking concentrations exceeding the solubility limit, precipitates are expected to form, which may dramatically influence the outcomes of experiments. For instance, the presence of varying concentrations of dissolved and precipitated forms of a metal over a toxicity test duration presents a problem for understanding exposure pathways to test organisms. Both dissolved and particulate metal species may be toxic but are likely to have different modes of action and relative toxicities (Golding et al. 2015).
For the non-specialist, obtaining information on the solubility of many metals in natural waters that can guide experimental design can be difficult, as there are no comprehensive compilations available in the scientific literature. Solubility limit estimates can be obtained using computer modelling programs such as Visual Minteq (Gustafsson 2015), but these require the user to assign the mineral phase controlling solubility. Often the precipitating phase is of unknown composition or there is uncertainty as to which mineral controls solubility. The situation is further complicated by the relatively short timescales of toxicity tests (hours to days) and other laboratory experiments where solutions cannot be assumed to have attained true thermodynamic equilibrium, which is a necessary assumption of the computer modelling approach. Often metastable equilibria can be established that may persist for relatively long durations (Stumm and Morgan 1996; Hidmi and Edwards 1999). Such constraints lead to a trial and error approach in experimental design where solubility limits are often not taken into account.
Metal solubility is commonly determined by equilibrating an excess of preformed precipitates or minerals with water and measuring dissolved metal concentrations periodically (Symes and Kester 1985; Savenko and Shatalov 1998; Hidmi and Edwards 1999). When the dissolved concentrations plateau, this is assumed to be the limit of solubility of the selected phase. An alternative, less-utilised approach involves addition of an excess of dissolved metal to the solution to trigger precipitation. The mixtures are then equilibrated and sampled at various time intervals until dissolved metal concentrations are stable (Angel et al. 2016a, 2016b). It is assumed that the solubility limit is independent of the mass of precipitate in solution. It should be noted that the precipitation approach makes no prior assumptions on the identity of the precipitating phase. If the system is allowed to attain true thermodynamic equilibrium, both methods should yield the same results.
Copper is regarded as one of the more toxic metals in marine ecosystems. Dissolved copper concentrations in marine waters are typically in the range <0.01–0.1 µg L−1 in the open ocean (Kremling and Pohl 1989; Donat and van den Berg 1992; Angel et al. 2010a) and often orders of magnitude higher in coastal waters (Apte and Day 1998; Angel et al. 2010a; Gaulier et al. 2019) owing to numerous anthropogenic inputs of copper.
Surprisingly few studies have investigated copper solubility in seawater and they have tended to focus on identifying the minerals that are formed. In his classic work on metal precipitation in natural seawater conducted over 60 years ago, Krauskopf (1956) determined the solubility of copper to be between 0.4 and 0.8 mg L−1. In a preliminary test of copper solubility before conducting toxicity testing, Furuta et al. (2008) measured concentrations of dissolved copper of 0.5–0.8 mg L−1 after 24 h in copper-spiked natural and artificial seawater. Savenko and Shatalov (1998) measured the solubility of atacamite (Cu2(OH)3Cl) in artificial seawater at 22 °C over a range of pH values and reported that it was fairly stable between pH 7.8 and 8.2 at 0.083 ± 0.025 mg L−1. Copper predominantly forms precipitates with carbonates, chlorides, hydroxides and oxides. The minerals most commonly formed under oxygenated conditions are malachite, and the polymorphs atacamite (Cu2(OH)3Cl), clinoatacamite and botallackite (Bianchi and Longhi 1973; Grice et al. 1996; Jambor et al. 1996; Krivovichev et al. 2017). The formation of malachite and atacamite polymorphs is most commonly mentioned in seawater. There is strong competition between these two minerals that is dependent on the concentration of bicarbonate in seawater, with atacamite polymorphs slightly favoured in most natural seawaters and malachite favoured as the bicarbonate concentration increases (Bianchi and Longhi 1973; North and McLeod 1987).
In the present study, we investigate the solubility of copper in seawater in experiments that simulate the scenario of preparation of metal-spiked solutions for use in experimental studies such as toxicity testing. The aims of this study were to gain insight into factors affecting the solubility of copper in seawater at its natural pH and over timescales relevant to laboratory and field studies. Copper solubilities in both natural and artificial seawater were determined in order to assess the effects of natural organic matter on metal solubility. A comparison of filtered copper in <0.45- and <0.025-µm size fractions was measured to determine if colloidal copper was a significant component. To further inform the study, the composition of precipitates formed in seawater was investigated.
Experimental
General analytical procedures
All plasticware was acid-washed before use by soaking in 10 % (v/v) nitric acid (Tracepur, Merck, Darmstadt, Germany) for 24 h, followed by at least five rinses with deionised water (18 MΩ cm, Milli-Q, Millipore). All chemicals used in the study were of analytical reagent grade or better. Measurements of pH were carried out using a Wissenschaftlich-Technische Werkstattan (WTW, Weilheim, Germany) meter equipped with a pH probe (Orion Sure-flow combination pH 9165BN). The pH probe was calibrated against commercially available pH 4.00, 7.00 and 10.00 buffers (Orion Pacific, Sydney, NSW, Australia). Seawater used for solubility tests and calibration standards was collected from Cronulla, NSW, Australia (35‰, pH 8.15, dissolved organic carbon 1 mg L−1), filtered through 0.45-µm membrane filters (Millipore HA mixed-cellulose esters) and stored refrigerated (2–4 °C) in the dark for a maximum of 3 months before use. Artificial seawater was prepared according to the recipe of Kester et al. (1967). Major cation concentrations were checked using inductively coupled plasma atomic emission spectrometry (ICPAES, Varian 730 ES) to confirm the solution composition.
Metal, chloride and sulfur concentrations were determined by ICPAES using matrix-matched standards prepared by the addition of MES-04-1 multielement standard (Accustandard, New Haven, CT, USA) to filtered seawater and preserved with 0.2 % nitric acid (Tracepur) as described by Angel et al. (2016a). Copper concentrations were determined using the mean of ICPAES wavelength lines 324.754 and 327.395 nm with a limit of detection (LOD) of 0.4 µg L−1 (calculated as 3× the standard deviation of the mean seawater blank, n = 18). The quality control and quality assurance for each sample batch analysis included deionised water filtration blanks, spike recoveries on at least 10 % of samples as a check of matrix effects, and analysis of an independent copper standard (Accustandard) as a check of the calibration accuracy.
Copper solubility in seawater
A 1000 mg Cu L−1 stock solution was prepared in a polycarbonate container by dissolving 1.96 g of CuSO4.5H2O (Ajax Finechem, Univar®, analytical reagent grade) in 0.5 L of deionised water. The final pH of this solution was 4.57.
Copper solubility test treatments were conducted in 200 mL of natural and artificial seawater prepared in triplicate in polycarbonate containers. Three separate tests were performed for each water type. Each test contained a range of, but not always all, concentration treatments, so that the total number of replicates for a given treatment tested in all three tests was in the range 3 to 9. Nominal treatment concentrations of 0.5, 1, 2, 5, 10 and 20 mg Cu L−1 were prepared by pipetting appropriate aliquots (≤2 % of final volume to maintain salinity and prevent significant decreases in the solution carbonate concentration) of the stock solution into filtered natural and artificial seawater. The treatments were shaken vigorously for 1 min, the pH measured, and for most treatments because the pH varied by 0.1–0.2 pH units, an adjustment to pH 8.15 ± 0.05 was performed by the addition of a small volumes of 1 M NaOH (Angel et al. 2016b). The treatments were contained in capped vials that were mixed during aging by placing on a bottle agitator (60 rpm) at 22 °C in the dark in a temperature-controlled room, and the pH of the solutions was checked every few days to ensure they remained in the desired range (8.15 ± 0.05). The vials were uncapped for ~30 min during measurement of pH to allow gas exchange of the headspace. To determine if mixing had any effect on solubility, an additional 5 mg L−1 total copper treatment was allowed to stand without mixing and sampled at the same time as a mixed 5 mg L−1 treatment.
At selected times (0, 0.08, 0.33, 1, 2, 3, 4, 7, 14, 28 days), the solutions were shaken for 10 s and 15 mL subsamples were withdrawn using a syringe (Terumo) and filtered (deionised water-rinsed 0.45-µm cellulose nitrate filter, 25 mm, Sartorius, Minisart, Goettingen, Germany) into receiving vials for the analysis of dissolved (<0.45 µm) metals. For the 0.5, 1, 2 and 5 mg L−1 treatments, a subsample was withdrawn by pipette for total copper analysis and another subsample was filtered through a 0.025-µm membrane filter (47 mm, mixed-cellulose esters, type HA, Millipore) to check for the presence of colloidal copper in the 0.025–0.45-µm size range. The samples were preserved by acidification to 0.2 % (v/v) with concentrated nitric acid (Tracepure) immediately after filtration. The total copper concentrations measured represented 88–99 % of the nominal concentration (Fig. S1b, Supplementary material). Deviations between nominal and total copper increased slightly as the concentration increased, suggesting deposition of particulates between shaking and subsampling was responsible for the difference rather than copper adsorption to plasticware surfaces. The spike recoveries were in the range 91–107 % (n = 89) and 90–102 % (n = 116) for natural and artificial seawater copper analysis respectively by ICPAES.
Copper precipitate composition
The X-ray diffraction (XRD) spectra, and metal, sulfur, carbon and chloride contents of the precipitates were measured to provide information on the likely geochemical composition of the precipitates formed 1, 7 and 28 days after spiking with copper. For measurements of precipitate mass, copper, chloride and sulfur concentrations, 2-L treatment volumes of 20 mg L−1 total copper in natural and artificial seawater were prepared in polyethylene bottles (Nalgene) and equilibrated for 1, 7 and 28 days as described previously, before filtering onto preweighed membrane filters (47 mm, mixed cellulose esters, 0.45 µm membrane filters type HA) (Angel et al. 2016b). The filtration apparatus was then rapidly rinsed with ~20 mL of deionised water while under vacuum to remove residual seawater salts that would contribute to the mass. The precipitates were dried in an oven at 50 °C, cooled in a desiccator and reweighed to constant weight.
The analysis of precipitate copper, chloride and sulfur was performed by transferring the filters into acid-washed 250-mL polycarbonate vials followed by the addition of 18 mL of deionised water. The treatments were then mixed and stored for 2 days to allow hydration of the precipitates to prevent passivation by the subsequent addition of nitric acid, which was observed in preliminary tests by the presence of undigested precipitates. After 2 days of hydration, 2 mL of concentrated nitric acid (Tracepure) was pipetted into the treatments to achieve 10 % v/v. The treatments were mixed vigorously and allowed to stand for a further 48 h at room temperature before dilutions of these solutions were performed in 0.2 % v/v nitric acid (Tracepure). The analytes analysed by ICPAES included Ag, Al, As, B, Ba, Be, Ca, Cd, Ce, Cl, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Na, Ni, P, Pb, S, Se, Sr, Ti, V, Zn. Membrane filter blanks showed no significant metal contribution and spike recoveries of 93–104 % indicated negligible contamination or matrix artefacts during analysis.
For XRD analyses and measurement of the carbonate content of the precipitate, 2 L of a 20 mg L−1 total copper solution was prepared in Nalgene bottles as above for each of the 1-, 7- and 28-day durations in natural and artificial seawater. At each time point, the treatment solutions were decanted into centrifuge tubes and centrifuged at 2000 g and 22 °C for 5 min to form a pellet, followed by removal of the supernatant. The precipitate pellets were thoroughly rinsed with deionised water, followed by drying in an oven at 50 °C.
The method described by Ohlsson and Wallmark (1999) was used to determine the carbon content of the precipitates. In brief, the dried samples were weighed into tin capsules and introduced sequentially into an elemental analyser (Thermo Fisher Flash 2000 HT EA) where the carbon was combusted (1020 °C) into CO2 in a furnace. The gas was then passed through a water trap before the CO2 was passed through a gas chromatography column at 40 °C followed by transfer to an isotopic ratio mass spectrometer (Thermo Fisher Conflo IV) for measurement of total carbon.
For the XRD analysis, the dried precipitates were deposited onto silicon single-crystal low-background holders with light pressure applied to the front of the sample using a glass slide to provide a flat surface for analysis. XRD patterns were measured with a PANalytical X’Pert Pro Multipurpose diffractometer using Fe-filtered Co Kα radiation, auto divergence slit, 1 ° antiscatter slit and fast X’Celerator Si strip detector. The diffraction patterns were recorded in steps of 0.016 ° 2θ with a total counting time of 30 min.
Thermodynamic modelling
Predictions of copper solubility in seawater were performed using Visual Minteq version 3.1 (Gustafsson 2015). The calculations were run with the potential precipitating mineral phases atacamite (clinoatacamite constants were not available in the database), malachite, tenorite and copper hydroxide. The results obtained using atacamite and malachite are discussed because their polymorphs (clinoatacamite) or amorphous precursors (dicopper hydroxycarbonate) were the dominant solid phases detected in test solutions. The following input parameters were used: salinity 35‰, the major ion composition for surface seawater as summarised by Pilson (1998), temperature 22 °C, ionic strength 0.7 mol L−1. The calculations were run using the thermodynamic constants from the Minteq database without any further modifications. Separate modelling runs were carried out using activity corrections computed using the extended Debye–Hückel equation and the Davies equation, whose outputs differed by less than 6 %.
Results
Measured copper solubility in seawater
The solubility measurements conducted in this study were made over a duration of 28 days. Although not stated in the text below, it should be noted that the solubility limits reported are ‘apparent’ limits of solubility because they are conditional on several factors such as the timescale of observations, major ion composition, temperature, pH and total copper concentration.
Dissolved copper concentrations in natural and artificial seawater measured at time points over 28 days for the different added copper concentrations are shown in Fig. 1 and Table S1 in the Supplementary material. Similar trends were generally observed in both natural and artificial seawater for any given added copper concentration over time. For 0.5 mg Cu L−1, the dissolved copper concentrations remained steady over the 28-day duration of the test. An initial phase of precipitation occurred rapidly within the first hour, ranging from 20 % of added copper precipitating in the 1 mg Cu L−1 treatment to 96 % at 20 mg Cu L−1. For total concentrations of ≥1 mg Cu L−1, the concentration of dissolved copper was highest in the first hour after spiking treatments and generally decreased with time. The highest dissolved copper concentrations, recorded immediately after spiking of the 5 mg Cu L−1 treatments, were 1180 and 1250 µg L−1 in natural and artificial seawater respectively.

|
The dissolved copper in the 1 mg Cu L−1 treatments exhibited a decrease in concentration in the first few days post spiking, followed by remaining relatively steady, with mean (±s.d.) concentrations of 0.65 ± 0.06 and 0.62 ± 0.15 mg Cu L−1 in natural and artificial seawater (at pH 8.15 and 22 °C) respectively.
For total copper concentrations ≥2 mg Cu L−1, the dissolved copper concentrations were highest in the first few days to weeks after spiking (Fig. 1). The relationships between the concentration of dissolved copper and the duration in seawater for these treatments, other than 10 and 20 mg L−1 natural seawater treatments, were characterised by decreases for 1–7 days after spiking test solutions, followed by stabilisation for a few days to weeks, before often exhibiting a sudden drop in concentration. As the concentration of total copper in seawater increased, the duration over which the dissolved copper concentration remained stable was shorter before the rapid decrease occurred. For the 10 and 20 mg L−1 total copper in natural seawater treatments, the concentration of dissolved copper decreased over time without a plateau. For the treatments that exhibited a rapid decrease, the mean concentrations of dissolved copper often had large error bars around the time the decrease occurred.
The copper precipitates, determined as the difference between the total and dissolved concentrations, comprised 34.6–99.7 % of the total copper in the ≥1 mg L−1 natural and artificial seawater treatments after 28 days. The relationship between the 28-day copper solubility and the copper precipitate concentration of the ≥1 mg Cu L−1 treatments is shown in Fig. 2. Over the range 1 to 5 mg L−1 of total copper, solubility decreased as the concentration of precipitate increased. However, at total copper concentrations of ≥10 mg L−1, the copper solubility was independent (i.e. not significantly different, P > 0.05) of the precipitate concentration.

|
The effect of mixing (used for experimental treatments) compared with not mixing on copper solubility was also tested with a 5 mg L−1 treatment over 28 days. The dissolved copper concentration of the non-mixed treatment represented 94 to 103 % of the mixed treatment, indicating mixing had a negligible effect on solubility.
Similar concentrations of copper were measured in the <0.45- and <0.025-µm size fractions over time (Fig. S1a, Supplementary material), indicating that colloidal copper was absent in the 0.025–0.45-µm size range at all points in time during precipitation. The lack of colloidal copper in the presence of precipitates suggests that the growth of precipitates occurs rapidly. There was also negligible filterable (<0.45 µm) aluminium, iron and manganese in all treatments (concentrations less than 1, 0.3 and 0.2 µg L−1 respectively), suggesting inorganic colloid concentrations were negligible in the test solutions.
When comparing the natural and artificial seawater treatments, the main difference in the behaviour of copper occurred in the ≥2 mg Cu L−1 treatments, where the concentration of dissolved copper in the natural seawater generally decreased more than in the artificial seawater in the 1- to 7-day period post spiking (Fig. 1). The concentration of dissolved copper after 28 days in the 10 and 20 mg L−1 total copper treatments was also significantly (P < 0.05) lower in natural seawater than in artificial seawater (Fig. 2). A major difference between the two 0.45-µm-filtered seawater types that may explain the lower solubility of copper in natural seawater is that it is likely to contain a combination of high-molecular-weight organic molecules, virus particles and small colloids, which may enhance copper precipitation through heterogeneous nucleation. Another explanation is that the dissolved organic carbon (DOC) present in natural seawater is incorporated into the copper precipitate and provides additional adsorption sites for dissolved copper binding (Lu and Allen 2006). Unfortunately, owing to its low concentration in seawater, potential decreases in DOC in the presence of copper precipitates could not be detected using total organic carbon (TOC) or spectrofluorimetric analyses.
Precipitate composition
Precipitates collected after 1, 7 and 28 days from natural and artificial seawater were analysed for their elemental content (ICPAES) and mineral composition (XRD). The copper underwent rapid precipitation after spiking of the natural and artificial seawater with 20 mg Cu L−1, with precipitate masses of 36.4 and 36.5 mg L−1 measured after 24 h respectively. The concentration of precipitate measured in the solutions decreased over time in both water types, probably owing to an inability to harvest all of it as the duration increased (Table S2, Supplementary material). During filtrations, the test bottles were observed to have a stronger bluish tinge as the time in solution increased, which could not be removed by vigorous mixing and rinsing, suggesting that some of the copper precipitate irreversibly stuck to the bottles over time. This meant that changes in precipitate masses could not be used as a line of evidence of precipitate mineral composition. All elemental analyses were performed on weighed portions of dry precipitate so that calculated percentages of elements in the precipitate were not affected by the loss of precipitate mass before filtrations.
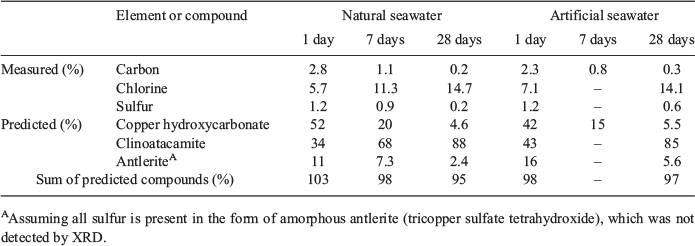
The results of the elemental analyses along with the elemental compositions of common copper minerals are presented in Table 1. The analyses of major cations (Ca, K, Mg and Na) and sulfur in natural and artificial seawater samples spiked with copper were similar to control waters, i.e. no significant decreases due to incorporation into precipitates. The precipitates harvested from the different treatments were blue with a green tinge. In preliminary tests that used oven temperatures of >50 °C to dry the precipitates, some changed to a dark grey colour, indicating oxidation to tenorite (Pollard et al. 1989; Hidmi and Edwards 1999). When nitric acid was added to the precipitates, there was noticeable effervescence generated by the 1- and 7-day precipitates (1 > 7 days) indicating the presence of carbonate, but none in the 28-day precipitates.

|
The carbon content of the precipitates decreased with time, with 2.3–2.8, 0.8–1.1 and 0.2–0.3 % measured after 1, 7 and 28 days respectively. This equates to a carbonate content between 1.2 and 14 %. The copper content in the precipitate increased slightly as the duration increased. In natural seawater, the mean ± s.d. percentage copper after 1, 7 and 28 days was 52.6 ± 0.9, 56.2 ± 0.7 and 57.3 ± 1.6 % respectively. In artificial seawater, the mean ± s.d. percentage copper after 1, 7 and 28 days was 53.1 ± 0.8, 55.8 ± 0.7 and 56.8 ± 1.6 % respectively.
Analysis of precipitate digests by ICPAES indicated that the concentrations of calcium, magnesium and sodium comprised <4 % of the precipitate mass as a result of carry-over from seawater; the precipitate mass was corrected for these salts by subtracting the respective masses of calcium carbonate, magnesium carbonate and sodium chloride, and the chlorine associated with sodium chloride was subtracted from the total chloride concentration that was measured in precipitate digests. The concentrations of potassium, phosphorus and trace metals were negligible (<0.2 %). The precipitates contained a relatively low but measurable amount of sulfur (0.2–1.2 %), with the percentage decreasing as duration in seawater increased. This probably indicates that a small amount of a sulfate-bearing copper mineral was present in the fresh precipitates and decreased over time.
The chloride content measured in the precipitates increased as the duration in seawater increased. In natural seawater, the mean ± s.d. percentage chloride after 1, 7 and 28 days was 5.7 ± 1.1, 11.3 ± 1.9 and 14.7 ± 1.2 % respectively. In artificial seawater, the mean ± s.d. percentage chloride after 1 and 28 days was 7.1 ± 1.2 and 14.1 ± 1.5 % respectively.
The XRD data are summarised in Table 2 and shown in Fig. 3 (natural seawater) and Fig. 4 (artificial seawater). The 1-day natural seawater precipitate showed only very broad bands of intensity indicative of non-crystalline material. The 1-day artificial seawater precipitate showed broad partially defined peaks. The 7-day natural and artificial seawater precipitates showed a series of broad but well-defined peaks. Some of the peaks at ~19 °, 38 ° and 46.5 ° 2θ were indicative of clinoatacamite while additional peaks at ~43 °, 54 ° and 70 ° 2θ were not identified. As shown in Figs S2 and S3 (Supplementary material), the polymorphs atacamite and botallackite did not account for the unidentified peaks at any of the durations in natural and artificial seawater. Fig. S4 (Supplementary material) shows a comparison of theoretical peaks of clinoatacamite and atacamite after 28 days in artificial seawater, indicating clinoatacamite dominates. The 28-day natural and artificial seawater precipitates showed broad, high-intensity, well-defined peaks of clinoatacamite with no unidentified peaks.

|
Discussion
Changes in precipitate composition and mineralisation during aging
The XRD analysis showed that the copper precipitates comprised an increasing proportion of clinoatacamite as they aged in natural and artificial seawater. The findings of higher initial concentrations of total carbon, effervescence and dominant amorphous phases detected by XRD in the 1 day-old precipitates indicated that the fresh precipitates contained significant amounts of copper hydroxycarbonate. Predictions of the copper compounds present in precipitates as they age, based on the elements they contain and the percentages of those elements in pure compounds, are shown in Table 3. All carbon was assumed to be present in copper hydroxycarbonate and all chloride was assumed to be in a dicopper trihydroxide chloride form (i.e. an amorphous or mineralised polymorph). Using the percentage of total carbon in the precipitates (2.3–2.8 %) compared with that in pure copper hydroxycarbonate (5.4 %) (Table 1), the 1-day-old precipitate is calculated to contain 42–52 % of this compound. Likewise, the presence of 5.7–7.1 % chlorine in the 1 day-old precipitate probably indicated that the fresh precipitates contained 34–43 % dicopper trihydroxide chloride, the amorphous precursor to clinoatacamite before crystal lattice formation. There are several copper hydroxysulfate compounds that include similar percentages of sulfur that may have been present as amorphous forms in the freshly formed precipitates (e.g. antlerite, langite, brochantite; Table 1). For the purpose of mass balance calculations, the small amount of sulfur measured in the precipitate was assumed to be amorphous antlerite (Cu3(SO4)(OH)4); the 1-day-old precipitate is calculated to contain 13–17 % of this compound. Copper hydroxycarbonate and copper hydroxysulfate decreased and dicopper trihydroxide chloride amounts increased and transformed into clinoatacamite as the precipitates aged so that after 28 days, they contained 4.6–5.5, 2.4–6.5 and 85–88 % of these compounds respectively. The sum of the percentages for these three compounds for the different-aged compounds was in the range 95–103 % (Table 3), supporting the copper predominantly being in the clinoatacamite and copper hydroxycarbonate forms. When the copper content measured in the precipitates was compared with the copper content predicted using the percentages of the three compounds in Table 3, the predictions were shown to be in the range 97–100 %, further supporting the predicted composition.
|
Previous studies that have made theoretical predictions of copper precipitation in seawater at ~pH 8 have shown there is competition between the formation of malachite and atacamite (clinoatacamite polymorph) that is dependent on the concentration of carbonate (Bianchi and Longhi 1973; North and McLeod 1987). The seawater used in the current study was collected from the Tasman Sea near the extreme south-western Pacific Ocean and contains ~2.22 × 10−3 mol kg−1 of bicarbonate. Bianchi and Longhi (1973) predicted that atacamite formation is favoured at bicarbonate concentrations <2.31 × 10−3 mol kg−1, supporting the results of the current study where the atacamite polymorph clinoatacamite was detected by XRD rather than malachite.
The mineral clinoatacamite is a polymorph of botallackite (monoclinic) and atacamite (orthorhombic) and was only discovered in the mid-1990s (Grice et al. 1996; Jambor et al. 1996; Krivovichev et al. 2017). Optical and X-ray methods are required to distinguish between the different polymorphs (Jambor et al. 1996). Studies before this time have mentioned the occurrence of the other polymorphs in samples such as oxidised ore bodies (Pollard et al. 1989), ocean geothermal vents (Hannington 1993) and corrosion products (North and McLeod 1987). Recent studies by Li et al. (2013) and Krivovichev et al. (2017) have found that clinoatacamite is the most stable polymorph so it is probable that it was present in at least some of the samples in these older studies but not detected by the measurement techniques available at that time.
The lattice energy of mineral polymorphs often only differs by a few kilojoules per mole (Pollard et al. 1989). Krivovichev et al. (2017) reported that the order of mineral crystallisation in the Cu2(OH)3Cl system follows the Ostwald step rule where the least stable and least complex polymorph forms initially, followed by the breaking and formation of chemical bonds to form more stable polymorphs. Botallackite is the least stable and therefore the rarest of the naturally occurring polymorphs, but it is a key intermediate of the other polymorphs and crystallises first under most conditions (Pollard et al. 1989). Transformations following the Ostwald rule then occur in the sequence of phases botallackite–atacamite–clinoatacamite where clinoatacamite may form directly from botallackite or from atacamite (i.e. clinoatacamite is the most stable at room temperature) (Krivovichev et al. 2017). The likely presence of metastable Cu2(OH)3Cl polymorphs in addition to amorphous copper hydroxycarbonate in the fresh precipitate is likely to have resulted in a higher solubility initially and even after 28 days than would be measured over longer time scales.
Solubility changes with time
In order to interpret the solubility results, it is important to take into account some of the basic principles of precipitation and crystal formation. When considering precipitation from a solution, it is widely recognised there are three zones of importance (Füredi-Milhofer 1980; Stumm and Morgan 1996; Pritula and Sangwal 2015): (i) the undersaturated zone, which lies below the solubility limit and consequently no precipitation is expected; (ii) the supersaturated zone, where the concentration of solute is so great that precipitation occurs spontaneously as soon the metal solute is added to the solution or there are other changes in condition such as changes in temperature that trigger precipitation; and (iii) the metastable zone, which is intermediate between the other two zones, where precipitation occurs slowly, which can often lead to the development of metastable equilibria. Such equilibria may persist for long durations before there is a decline in solute concentration and true thermodynamic equilibrium is established (Füredi-Milhofer 1980; Stumm and Morgan 1996). Precipitation is triggered by the presence of nucleation sites, which can be heterogenous or homogeneous. In the case of artificial seawater, it is likely that homogeneous nucleation predominates. However, in natural seawater, the presence of high-molecular-weight organic molecules, virus particles and small colloids is likely to additionally enhance precipitation through heterogeneous nucleation.
An additional complicating factor is the transformation of solid phases over the time course of the experiment. Consistent with the Ostwald step rule (Krivovichev et al. 2017), amorphous copper phases are predicted to transform to more crystalline thermodynamically stable phases with lower solubility. The solubility of freshly precipitated phases may be orders of magnitude higher than aged precipitate phases (Savenko and Shatalov 1998). Such transitions in precipitate composition are expected to drive the dissolved copper concentrations downwards.
A conceptual model of copper precipitation in seawater is shown in Fig. 5. The minimum solubility of 0.053 mg L−1 measured in the present study after 28 days is assumed to approximate the solubility limit for clinoatacamite, although it may decrease further over longer time scales. It was therefore assumed that the higher dissolved copper concentrations that persisted in solution for lengthy durations are in the zone of metastability, with the duration of metastability decreasing as the concentration of precipitates in solution increased. The higher dissolved copper concentration observed over the first 7 days is also likely to be influenced by the higher solubility of the amorphous phases that initially precipitate. At 0.5 mg L−1 total copper, dissolved copper concentrations remained stable over 28 days and no precipitates were detected, i.e. a metastable state persisted for the 28 days. The duration over which the 0.5 mg L−1 copper treatments will remain metastable is unknown, but at some stage heterogeneous nucleation (with solution colloids, etc.) will result in precipitation. However, at higher total copper concentrations (2–20 mg L−1), initial rapid precipitation occurred during the first hour, which was followed by the establishment of metastable equilibria that persisted for periods of several days. The concentration of dissolved copper reached as high as 1250 µg L−1 in the first few hours after spiking, before decreasing owing to exceedance of the metastable equilibrium concentration range. The metastable equilibrium solubility concentrations and durations were influenced by the total copper concentration and were typically in the range 0.6 to 0.9 mg L−1. After 5 to 15 days, a step change decrease in dissolved copper concentration was observed in the 2–20 mg L−1 total copper treatments, followed by a slow decline in concentration. The final dissolved copper concentrations observed after 28 days were related to the precipitate concentrations.

|
The rapid decreases in the concentration of dissolved copper that were often measured for the ≥2 mg Cu L−1 treatments generally occurred after 14–21 days and coincided with an increase in clinoatacamite (Figs 3 and 4). The mechanism leading to the rapid loss of dissolved copper is attributed to the lower solubility of clinoatacamite. The finding of decreasing dissolved copper concentrations in the solubility tests as the precipitates aged and/or changed composition is similar to previous findings reported for aluminium and lead solubility studies (Angel et al. 2016a, 2016b). The change in aluminium precipitate composition also coincided with decreased solubility, supporting the decreases in the current study being driven by changes in the composition of the precipitate.
Modelling of copper solubility in seawater
The solubility limits for copper in seawater (pH 8.15, 22 °C) were calculated using VMinteq, assuming malachite (mineralised copper hydroxycarbonate) and atacamite (clinoatacamite polymorph) as the precipitating phases. Malachite was not actually detected by XRD in the precipitates, but rather its amorphous precursor, copper hydroxycarbonate, is likely to have been present (Table 3), which is likely to have a higher solubility. Atacamite was selected because stability constants for clinoatacamite were not available in VMinteq. The predicted solubility limits were 0.018 and 0.022 mg L−1 for malachite and atacamite respectively, which were lower than the solubilities measured in our experiments where the lowest concentration recorded after 28 days was 0.053 mg L−1. These calculations illustrate the limited value of thermodynamic modelling calculations of solubilities for informing short-term experiments, where amorphous and metastable compounds control solubility rather than highly crystalline forms represented in thermodynamic modelling databases. Similar discrepancies have been reported between predicted and measured solubilities in other studies. Savenko and Shatalov (1998) reported that the solubility of atacamite in seawater between pH 7.8 and 8.2 was 0.083 ± 0.025 mg L−1.
Relevance to toxicity testing
The current study demonstrates that the formation of copper precipitates occurs quickly in marine toxicity test waters when total copper concentrations exceed 1 mg L−1. This has relevance for toxicity tests involving species of marine organisms that require higher concentrations of dissolved copper to obtain a full dose–response curve. Most species are quite sensitive to copper and effects concentrations will be lower than the solubility limits that we measured after 28 days in natural and artificial seawater. However, some species, particularly certain fish, require higher concentrations to elicit toxic effects (Chen and Lin 2001; Furuta et al. 2008; Adeyemi and Klerks 2012; Wu et al. 2019). Toxicity tests with fish are also generally longer than the test durations used in tests with lower trophic species, allowing greater precipitate formation and changes in composition to occur. A survey of the literature from the past 25 years indicated several studies where the highest exposure concentrations in toxicity tests have exceeded 5 mg Cu L−1 (e.g. Valdovinos and Zuniga 2002; Ma et al. 2020; Wang et al. 2020). Toxicity endpoints have also been reported at concentrations higher than the solubility limits of copper in seawater determined in the current study. For instance, the 7-day concentration that caused 50 % lethality (LC50) for marine medaka (Oryzias melastigma) larval survival was 10.2 mg L−1 (Wong et al. 1999) and the 96-h LC50 for the juvenile shrimp Penaeus japonicus Bate was 2.1 mg L−1 (Bambang et al. 1995). It is surprising that the authors did not report evidence of precipitation. Concentrations of total copper were also higher than the solubility limit in 24–96-h pulsed exposure tests with the amphipod Melita plumulosa (Angel et al. 2010b) (concentrations up to 1.2 mg L−1) and in 72-h toxicity tests with the green alga Dunaliella tertiolecta (Levy et al. 2008) (concentrations up to 0.9 mg L−1). Decreases in measured dissolved copper concentrations in these studies were attributed to adsorptive losses to the surface of organisms and test vessels but precipitation is also likely to have contributed. Total copper concentrations were not measured in these studies for comparison with dissolved concentrations to confirm this.
An understanding of the solubility limit and the factors that affect solubility and precipitate composition is important for interpreting organism responses in toxicity and bioaccumulation tests where metal precipitates may influence organism responses in addition to dissolved metal species (Golding et al. 2015). In cases where metal precipitates exert toxicity, changes in the composition or mineralisation of the precipitate are likely to alter the toxicity of the precipitate. When toxicity tests require total copper concentrations above the solubility limit in this study, it is recommended that flow-through systems or static renewal of test solutions be employed (Chen and Lin 2001; Furuta et al. 2008). These measures will minimise precipitation but may not prevent it entirely for high total copper concentrations that undergo rapid precipitation (Fig. 1). Measuring the dissolved concentrations of copper at the start and end of tests is recommended as a minimum requirement for understanding exposure. Additional measurements during the test and the measurement of total concentrations when using high concentrations are beneficial to better understand exposure.
Conclusions
This study illustrates the complexity of copper precipitation in seawater (pH 8.15, 22 °C) over a time scale of 0 to 28 days. Depending on the total copper concentration, metastable equilibria may persist for several days to weeks, resulting in dissolved copper concentrations in the range of 0.6 to 0.9 mg L−1 that are far higher than the estimated equilibrium solubility of 0.05 mg L−1 for the most stable mineral phase, clinoatacamite.
The precipitates that formed after 1 day comprised similar proportions of amorphous copper hydroxycarbonate and hydroxychloride species that transformed into mineralised clinoatacamite as they aged. A rapid decrease in the concentration of dissolved copper appeared to coincide with the formation of the less soluble clinoatacamite.
These findings have particular relevance for laboratory toxicity tests where there is a need to prepare test solutions of copper concentrations that span several orders of magnitude. Clearly, at total copper concentrations that exceed ~1 mg L−1, organisms will be exposed to a mixture of dissolved and particulate forms whose concentrations change depending on the precipitate concentration. Additionally, the chemical composition of the precipitate is dynamic and will change with time. This reinforces the need to monitor concentrations of dissolved and particulate copper in the toxicity test exposure system over time, in order to relate metal form to observed toxicity. Overall, the study highlights the need for experimental determination of solubility and characterisation of the mineral phases that precipitate from solution.
Supplementary material
The supplementary material includes means and standard deviations of dissolved copper concentrations measured in solubility test solutions, measured precipitate concentrations in test solutions, comparisons of <0.025 µm, <0.45 µm and total copper concentrations in test solutions and XRD diffractograms with the expected intensities of the clinoatacamite polymorphs atacamite and botallackite to demonstrate these polymorphs were not present.
Conflicts of interest
Graeme Batley is an Associate Editor. Despite this relationship, he did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor of this journal. The authors have no further conflicts of interest to declare.
Acknowledgements
Darren Koppel and Joshua King are thanked for internal review of the manuscript before submission. The anonymous journal reviewers are thanked for their review of the manuscript. No funding other than internal CSIRO funds were utilised for this study.
References
Adeyemi JA, Klerks PL (2012). Salinity acclimation modulates copper toxicity in the sheepshead minnow, Cyprinodon variegatus. Environmental Toxicology and Chemistry 31, 1573–1578.| Salinity acclimation modulates copper toxicity in the sheepshead minnow, Cyprinodon variegatusCrossref | GoogleScholarGoogle Scholar | 22511216PubMed |
Angel BM, Hales LT, Simpson SL, Apte SC, Chariton AA, Shearer DA, Jolley DF (2010a). Spatial variability of cadmium, copper, manganese, nickel and zinc in the Port Curtis Estuary, Queensland, Australia. Marine and Freshwater Research 61, 170–183.
| Spatial variability of cadmium, copper, manganese, nickel and zinc in the Port Curtis Estuary, Queensland, AustraliaCrossref | GoogleScholarGoogle Scholar |
Angel BM, Simpson SL, Jolley DF (2010b). Toxicity to Melita plumulosa from intermittent and continuous exposures to dissolved copper. Environmental Toxicology and Chemistry 29, 2823–2830.
| Toxicity to Melita plumulosa from intermittent and continuous exposures to dissolved copperCrossref | GoogleScholarGoogle Scholar | 20836070PubMed |
Angel BM, Apte SC, Batley GE, Golding LA (2016a). Geochemical controls on aluminium concentrations in coastal waters. Environmental Chemistry 13, 111–118.
| Geochemical controls on aluminium concentrations in coastal watersCrossref | GoogleScholarGoogle Scholar |
Angel BM, Apte SC, Batley GE, Raven MD (2016b). Lead solubility in seawater: an experimental study. Environmental Chemistry 13, 489–495.
| Lead solubility in seawater: an experimental studyCrossref | GoogleScholarGoogle Scholar |
Apte SC, Day GM (1998). Dissolved metal concentrations in the Torres Strait and Gulf of Papua. Marine Pollution Bulletin 36, 298–304.
| Dissolved metal concentrations in the Torres Strait and Gulf of PapuaCrossref | GoogleScholarGoogle Scholar |
Bambang Y, Thuet P, Charmantier-Daures M, Trilles J-P, Charmantier G (1995). Effect of copper on survival and osmoregulation of various developmental stages of the shrimp Penaeus japonicus bate (Crustacea, Decapoda). Aquatic Toxicology 33, 125–139.
| Effect of copper on survival and osmoregulation of various developmental stages of the shrimp Penaeus japonicus bate (Crustacea, Decapoda)Crossref | GoogleScholarGoogle Scholar |
Bianchi G, Longhi P (1973). Copper in seawater, potential–pH diagrams. Corrosion Science 13, 853–864.
| Copper in seawater, potential–pH diagramsCrossref | GoogleScholarGoogle Scholar |
Burton DT, Fisher DJ (1990). Acute toxicity of cadmium, copper, zinc, ammonia, 3,3′-dichlorobenzidine, 2,6-dichloro-4-nitroaniline, methylene chloride, and 2,4,6-trichlorophenol to juvenile grass shrimp and killifish. Bulletin of Environmental Contamination and Toxicology 44, 776–783.
| Acute toxicity of cadmium, copper, zinc, ammonia, 3,3′-dichlorobenzidine, 2,6-dichloro-4-nitroaniline, methylene chloride, and 2,4,6-trichlorophenol to juvenile grass shrimp and killifishCrossref | GoogleScholarGoogle Scholar | 2344484PubMed |
Chen J-C, Lin C-H (2001). Toxicity of copper sulfate for survival, growth, molting and feeding of juveniles of the tiger shrimp, Penaeus monodon. Aquaculture 192, 55–65.
| Toxicity of copper sulfate for survival, growth, molting and feeding of juveniles of the tiger shrimp, Penaeus monodonCrossref | GoogleScholarGoogle Scholar |
Donat JR, van den Berg CMG (1992). A new cathodic stripping voltammetric method for determining organic copper complexation in seawater. Marine Chemistry 38, 69–90.
| A new cathodic stripping voltammetric method for determining organic copper complexation in seawaterCrossref | GoogleScholarGoogle Scholar |
Frimmel FH, Huber L (1996). Influence of humic substances on the aquatic adsorption of heavy metals on defined mineral phases. Environment International 22, 507–517.
| Influence of humic substances on the aquatic adsorption of heavy metals on defined mineral phasesCrossref | GoogleScholarGoogle Scholar |
Füredi-Milhofer H (1980). Investigations of complex precipitation systems. Croatica Chemica Acta 53, 243–254.
Furuta T, Iwata N, Kikuchi K (2008). Effects of fish size and water temperature on the acute toxicity of copper for Japanese flounder, Paralichthys olivaceus, and Red Sea bream, Pagrus major. Journal of the World Aquaculture Society 39, 766–773.
| Effects of fish size and water temperature on the acute toxicity of copper for Japanese flounder, Paralichthys olivaceus, and Red Sea bream, Pagrus majorCrossref | GoogleScholarGoogle Scholar |
Gaulier C, Zhou C, Guo W, Bratkic A, Superville P-J, Billon G, Baeyens W, Gao Y (2019). Trace metal speciation in North Sea coastal waters. The Science of the Total Environment 692, 701–712.
| Trace metal speciation in North Sea coastal watersCrossref | GoogleScholarGoogle Scholar | 31539978PubMed |
Golding LA, Angel BM, Batley GE, Apte SC, Krassoi R, Doyle CJ (2015). Derivation of a water quality guideline for aluminium in marine waters. Environmental Toxicology and Chemistry 34, 141–151.
| Derivation of a water quality guideline for aluminium in marine watersCrossref | GoogleScholarGoogle Scholar | 25318392PubMed |
Grice JD, Szymanski JT, Jambor JL (1996). Crystal structure of clinoatacamite, a new polymorph of Cu2(OH)3Cl. Canadian Mineralogist 34, 73–78.
Gustafsson JP (2015). Visual Minteq ver. 3.1. Available at http://vminteq.lwr.kth.se [Verified 14 May 2015]
Hannington MD (1993). The formation of atacamite during weathering of sulfides on the modern sea-floor. Canadian Mineralogist 31, 945–956.
Hidmi L, Edwards M (1999). Role of temperature and pH in Cu(OH)2 solubility. Environmental Science & Technology 33, 2607–2610.
| Role of temperature and pH in Cu(OH)2 solubilityCrossref | GoogleScholarGoogle Scholar |
Hua X, Dong D, Liu L, Gao M, Liang D (2012). Comparison of trace metal adsorption onto different solid materials and their chemical components in a natural aquatic environment. Applied Geochemistry 27, 1005–1012.
| Comparison of trace metal adsorption onto different solid materials and their chemical components in a natural aquatic environmentCrossref | GoogleScholarGoogle Scholar |
Jambor JL, Dutrizac JE, Roberts AC, Grice JD, Szymanski JT (1996). Clinoatacamite, a new polymorph of Cu2(OH)3Cl, and its relationship to paratacamite and ‘anarakite’. Canadian Mineralogist 34, 61–72.
Kester DR, Duedall IW, Connors DN, Pytkowicz RM (1967). Preparation of artificial seawater. Limnology and Oceanography 12, 176–179.
| Preparation of artificial seawaterCrossref | GoogleScholarGoogle Scholar |
Krauskopf KB (1956). Factors controlling the concentration of thirteen trace metals in seawater. Geochimica et Cosmochimica Acta 9, 1–32.
| Factors controlling the concentration of thirteen trace metals in seawaterCrossref | GoogleScholarGoogle Scholar |
Kremling K, Pohl C (1989). Studies on the spatial and seasonal variability of dissolved cadmium, copper and nickel in north-east Atlantic surface waters. Marine Chemistry 27, 43–60.
| Studies on the spatial and seasonal variability of dissolved cadmium, copper and nickel in north-east Atlantic surface watersCrossref | GoogleScholarGoogle Scholar |
Krivovichev SV, Hawthorne FC, Williams PA (2017). Structural complexity and crystallization: the Ostwald sequence of phases in the Cu2(OH)3Cl system (botallackite–atacamite–clinoatacamite). Structural Chemistry 28, 153–159.
| Structural complexity and crystallization: the Ostwald sequence of phases in the Cu2(OH)3Cl system (botallackite–atacamite–clinoatacamite)Crossref | GoogleScholarGoogle Scholar |
Levy JL, Angel BM, Stauber JL, Poon WL, Simpson SL, Cheng SH, Jolley DF (2008). Uptake and internalisation of copper by three marine microalgae: comparison of copper-sensitive and copper-tolerant species. Aquatic Toxicology 89, 82–93.
| Uptake and internalisation of copper by three marine microalgae: comparison of copper-sensitive and copper-tolerant speciesCrossref | GoogleScholarGoogle Scholar | 18639348PubMed |
Li M, Wang LQ, Xia Y, Yang QY (2013). Research on the spectral analysis and stability of copper green. Guangpuxue Yu Guangpu Fenxi 33, 3293–3297.
Lu YF, Allen HE (2006). A predictive model for copper partitioning to suspended particulate matter in river waters. Environmental Pollution 143, 60–72.
| A predictive model for copper partitioning to suspended particulate matter in river watersCrossref | GoogleScholarGoogle Scholar |
Luoma SN, Rainbow PS (2008). Toxicity testing. In ‘Metal contamination in aquatic environments: science and lateral management’. pp. 232–259. (Cambridge University Press: Cambridge)
Ma Y, Rivera-Ingraham G, Nommick A, Bickmeyer U, Roeder T (2020). Copper and cadmium administration induce toxicity and oxidative stress in the marine flatworm Macrostomum lignano. Aquatic Toxicology 221, 105428
| Copper and cadmium administration induce toxicity and oxidative stress in the marine flatworm Macrostomum lignanoCrossref | GoogleScholarGoogle Scholar | 32035411PubMed |
North NA, McLeod ID (1987). Corrosion of metals. In ‘Conservation of marine archaeological objects’. (Ed. C Pearson) Ch. 4, pp. 68–98 (Butterworths: London)
Ohlsson K, Wallmark P (1999). Novel calibration with correction for drift and non-linear response for continuous-flow isotope ratio mass spectrometry applied to the determination of δ15N, total nitrogen, δ13C and total carbon in biological material. Analyst 124, 571–577.
| Novel calibration with correction for drift and non-linear response for continuous-flow isotope ratio mass spectrometry applied to the determination of δ15N, total nitrogen, δ13C and total carbon in biological materialCrossref | GoogleScholarGoogle Scholar |
Pilson MEQ (1998). ‘An introduction to the chemistry of the sea, 2nd edn.’ (Cambridge University Press: London)
Pollard AM, Thomas RG, Williams PA (1989). Synthesis and stabilities of the basic copper(II) chlorides atacamite, paratacamite and botallackite. Mineralogical Magazine 53, 557–563.
| Synthesis and stabilities of the basic copper(II) chlorides atacamite, paratacamite and botallackiteCrossref | GoogleScholarGoogle Scholar |
Pritula I, Sangwal K (2015). Fundamentals of crystal growth from solutions. In ‘Handbook of crystal growth, 2nd edn’. (Ed. P Rudolph) pp. 1185–1227. (Elsevier, BV: Amsterdam)
Savenko VS, Shatalov IA (1998). Atacamite solubilities and the physicochemical state of solute copper in seawater. Geokhimiya 8, 842–847.
Stumm W, Morgan JJ (1996). Precipitation and dissolution. In ‘Aquatic chemistry, chemical equilibria and rates in natural waters, 3rd edn’. pp. 356–357 (John Wiley & Sons, Inc.: New York, NY)
Symes JL, Kester DR (1985). Copper(II) interaction with carbonate species based on malachite solubility in perchorate medium at the ionic strength of seawater. Marine Chemistry 16, 189–211.
| Copper(II) interaction with carbonate species based on malachite solubility in perchorate medium at the ionic strength of seawaterCrossref | GoogleScholarGoogle Scholar |
Valdovinos C, Zuniga M (2002). Copper acute toxicity tests with the sand crab Emerita analoga (Decapoda: Hippidae): a biomonitor of heavy metal pollution in Chilean coastal seawater?. Bulletin of Environmental Contamination and Toxicology 69, 393–400.
| Copper acute toxicity tests with the sand crab Emerita analoga (Decapoda: Hippidae): a biomonitor of heavy metal pollution in Chilean coastal seawater?Crossref | GoogleScholarGoogle Scholar | 12177761PubMed |
Wang F, Chen J, Forsling W (1997). Modeling sorption of trace metals on natural sediments by surface complexation model. Environmental Science & Technology 31, 448–453.
| Modeling sorption of trace metals on natural sediments by surface complexation modelCrossref | GoogleScholarGoogle Scholar |
Wang R-F, Zhu L-M, Zhang J, An X-P, Yang Y-P, Song M, Zhang L (2020). Developmental toxicity of copper in marine medaka (Oryzias melastigma) embryos and larvae. Chemosphere 247, 125923
| Developmental toxicity of copper in marine medaka (Oryzias melastigma) embryos and larvaeCrossref | GoogleScholarGoogle Scholar | 31972495PubMed |
Wong PPK, Chu LM, Wang CK (1999). Study of toxicity and bioaccumulation of copper in the silver sea bream Sparus sarba. Environment International 25, 417–422.
| Study of toxicity and bioaccumulation of copper in the silver sea bream Sparus sarbaCrossref | GoogleScholarGoogle Scholar |
Wu J, Li L, Zhu Z, Zhong J, Su G, Zhou C, Lan K, Fang M (2019). Therapeutic effect of copper sulfate on cryptocaryoniosis in twospot puffer Takifugu bimaculatus. Fisheries Science 38, 305–312.




