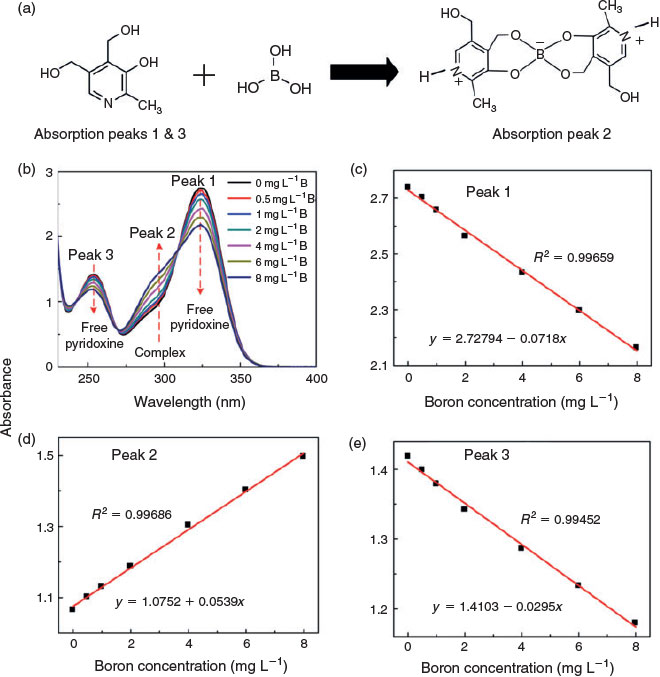
Boron detection and quantification based on the absorption spectra of pyridoxine and its boron complex
Fuming Chen A , Ye Ai A and Hui Ying Yang A BA Pillar of Engineering Product Development, Singapore University of Technology and Design, Singapore 487372, Singapore.
B Corresponding author. Email: yanghuiying@sutd.edu.sg
Environmental Chemistry 14(3) 135-140 https://doi.org/10.1071/EN16196
Submitted: 8 December 2016 Accepted: 16 February 2017 Published: 2 March 2017
Environmental context. Boron, an essential element for human health and the growth of animals and plants, can also be harmful when intake is excessive. Herein, a simple and efficient method for determining boron species in aqueous samples has been developed based on the optical absorption of the pyridoxine or boron–pyridoxine complex. This rapid method is suitable for online analysis, with great significance to drinking water and industrial water treatment.
Abstract. A simple and efficient method for the determination of boron species in aqueous samples is presented based on the optical behaviour of the pyridoxine or boron–pyridoxine complex. The boron concentration in the sample is proportional to the absorption intensity of the boron–pyridoxine complex, and is inversely proportional to that of free pyridoxine. The calibration plot is linear in the range of 0–8 mg L–1 boron element within a pH 5.72–9.30 range. The method was developed for freshwaters, but is also applicable to seawater without significant interference from other commonly occurring ions in water such as Na+, K+, Cl–, Zn2+, Mn2+, Co2+, Ni2+, NO3–, and SO42–. This simple and rapid method is suitable for incorporation into an online analyser, which will be of great significance to the water treatment industry.
Additional keywords: boron determination, boron sensor, pyridoxine, vitamin B6.
Boron is an essential element for human health and for the growth of animals and plants, but excessive intake becomes harmful. Boron compounds are widely used in food and industrial applications such as semiconductors, pharmacy, agricultural fertilisers and insecticides, and optical materials.
The guidelines for acceptable boron concentration may vary substantially among different boron-related industries. For example, ultrapure water containing less than 60 µg L–1 of boron is required for the high-technology semiconductor industry because a higher boron content can cause serious p-type doping of solid-state devices.[1] Normally, irrigation water for crops should contain less than 1 mg L–1 of boron; excess boron may result in dwarfing or death of plants to some extent, whereas lack of boron may affect the growth and yield of plants.[2,3] For drinking water, the recommended maximum permissible level of boron varies between countries and regions, although the World Health Organisation in 2011 recommended a value of 2.4 mg L–1 boron as the drinking water quality standard.[4] Therefore, it is of great importance to accurately determine the boron content in water, food, soil and industrial effluents in order to meet regulatory requirements and to provide a safeguard for human health and the environment.
A quick, accurate and inexpensive online boron analyser is required to provide continuous analysis, especially in the field of semiconductor manufacturing. Inductively coupled plasma mass spectrometry (ICP-MS) or inductively coupled plasma optical emission spectrometry (ICP-OES) are sensitive methods[5,6] commonly used for boron in research laboratories. However, the methods are not suitable for real-time analysis because the operations are time-consuming and complex. Electrical conductivity is utilised for boron measurements based on ionised complex formation with a reagent (e.g. mannitol),[7–10] whereupon the boron concentration in the sample is correlated mathematically to the conductivity differential caused by the ionised complex formation. Although this method is applicable for the development of online boron analysers, it can only measure samples with low salt content owing to interference from the conductivity of salt. Spectrophotometric techniques are also used for the determination of boron with a wide range of applications.[11–14] Some reagents such as azomethine-H can react with boron, causing an intensity change in the absorption spectra, which can be related to boron concentrations in aqueous samples. This technique provides low-cost operation, readily adapted for online or portable use, but in most cases, poisonous or hazardous reagents and complicated procedures are involved.
In view of the limitations of currently available methods for boron measurements, we aimed to develop a simple method applicable to the analysis of environmentally relevant samples. Herein, we report a novel, rapid and non-toxic spectrophotometric method for the determination of boron in aqueous samples based on the optical absorption behaviour of pyridoxine and the boron–pyridoxine complex.
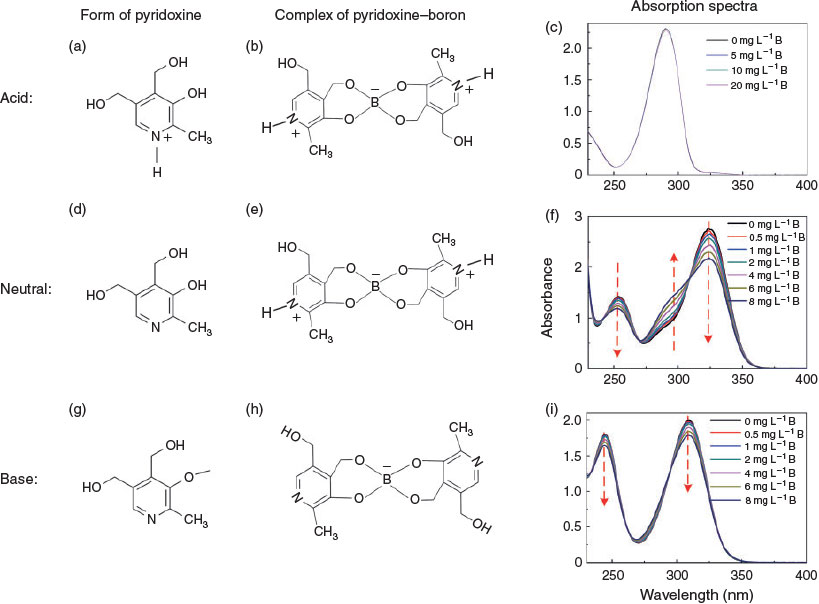
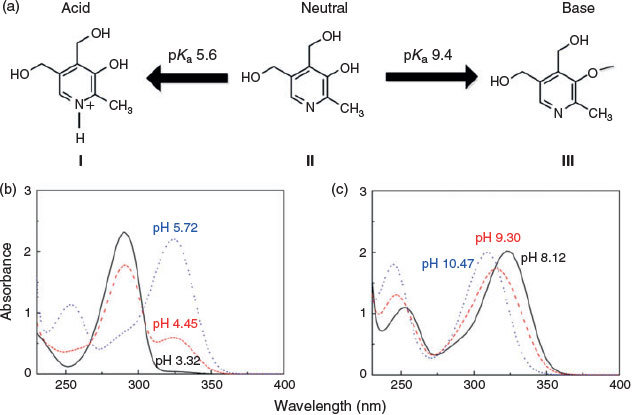
Pyridoxine is a bipolar molecule with acid dissociation constant (pKa) values of 5.6 and 9.4,[15,16] which indicates that it is amphoteric. It can exhibit three different forms under acidic, neutral and basic conditions,[17] as shown in Fig. 1a. Under neutral conditions (pH 5.72–8.12), pyridoxine is non-ionised or weakly ionised, and displays two main peaks in its absorption spectra at 254 and 325 nm (Fig. 1b; see Supplementary material for experimental details). As the pH value decreases, pyridoxine will be protonated gradually and exhibit a net positive charge. For example, at pH 4.55, two major peaks can be observed at 299 and 325 nm, corresponding to the absorption of forms I and II of pyridoxine that coexist in the solution. At the even lower pH of 3.32, all the pyridoxine is protonated, and there is only form I of pyridoxine in solution, with an absorption peak at 299 nm. When the pH increases from neutral to basic, the negatively charged pyridoxine dominates in the solution, resulting in a blue-shift from 325 nm (Fig. 1c), which indicates that the negative charge of the pyridoxine form III gradually becomes stronger with increasing pH.
|
Pyridoxine can react with boric acid to form a pyridoxine–boron complex by combining two molecules of pyridoxine and one molecule of boric acid,[18] as shown in Fig. 2a. At neutral pH, the non-ionised pyridoxine exhibits absorption peaks at 325 and 254 nm. Once it combines with boric acid in the sample, however, the complex exhibits amphoteric properties (Fig. 2a). The pyridinic-N of pyridoxine will be locally protonated owing to the negatively charged nature of boron in the complex. Two isosbestic points were observed at 269 and 309 nm, indicating equilibria of two forms of the ions in the system, whereas the complex has peak absorption at 299 nm (peak 2, Fig. 2b). When boron is not present in the sample, only two intrinsic absorption maxima, peaks 1 and 3, can be observed from the free pyridoxine in the sample. As the boron concentration increases, the intensity of peaks 1 and 3 decreases, indicating that free pyridoxine is consumed by the reaction with the boron species. At the same time, the intensity of peak 2 increases owing to the complex formation. Kinetics measurements of pyridoxine and the boron complex demonstrated that the intensity of the absorption spectra did not change when the time span was increased to 10 min (Fig. S1).
The boron concentration is linearly correlated with the intensity of the absorption spectra, with correlation coefficients >0.99 for all three peaks (Fig. 2c–e; Table S1). The limits of detection for the method (detected limit (DL) = 3.3 × s.d.blank/slope,[19] based on 20 blank measurements; see Supporting online material Fig. S2 and Tables S2 and S3) were 0.76 mg L–1 (peak 1), 0.18 mg L–1 (peak 2) and 0.15 mg L–1 (peak 3). These excellent analytical characteristics demonstrate that boron concentration can be accurately determined by the optical signal method with the addition of non-toxic pyridoxine.
Fig. 3 shows the forms of pyridoxine and its boron complex at acidic, neutral and basic pH and the change in absorption spectra with changing boron content. At acidic pH, pyridoxine took the same form whether free or complexed with boron, independently of the boron concentration, resulting in identical absorption spectra (Fig. 3a–c). With alkaline conditions, the peak position of absorption spectra was unvaried. However, the intensity decreased with increasing boron concentration (Fig. 3g–i) because the molar absorptivity of the complex is lower than that of pyridoxine under alkaline conditions.[20] Additionally, the absorption peak experienced a blue shift at the higher pH. In the neutral range, the form of free pyridoxine is different from that in the complex (Fig. 3e) – the pyridinic-N of the complexed pyridoxine is protonated owing to an enhanced electrostatic effect from the negatively charged boron once combined with boric acid. Two contrasting behaviours of peaks can be observed in the spectra (Fig. 3f); the intensity of peaks 1 and 3 decreases with increasing boron concentration owing to the consumption of free pyridoxine, whereas the peak 2 intensity increases as more complex is formed. Therefore, the method can serve as a boron sensor in the neutral range.
The pH of the sample solution is a key factor influencing the form of pyridoxine and the pyridoxine–boron complex. To further explore the applicable pH range of the sensor, we examined the effect of pH on the quantification of boron. We observed a shift in the position of peaks 1–3 with pH, with the maximum wavelength occurring at pH 7 (Fig. 4a). There was generally a blue shift of peaks 1 and 3 (pure pyridoxine) when the pH value is higher (basic conditions) or lower (acidic conditions) than pH 7. The blue shift observed under acidic pH conditions is less pronounced. Additionally, the absorption intensities for peaks 1–3 varied with pH, and differences were also observed in the calibration curves for boron measured at various pHs (Fig. 4b–d). These effects could influence the accuracy of boron determination. The most linear response was found at pH 7. Thus, to obtain the most accurate measurements during actual application, it is necessary to adjust the solution pH to a value of 7.
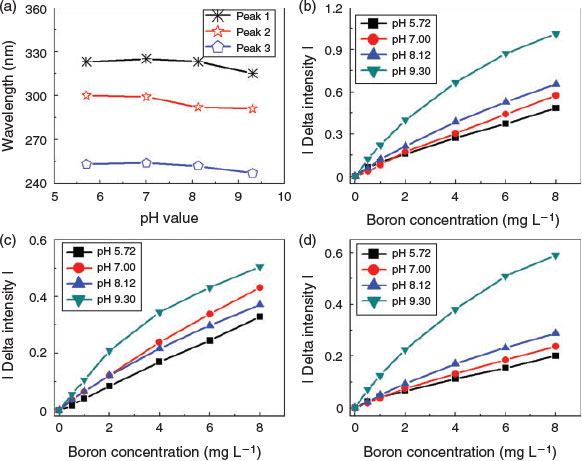
|
In practical applications, the sample solution could contain other species that might interfere with the analyte response, resulting in measurement error. Accordingly, the potential interfering effect of 11 ions on the signal obtained for a 0.5-mg L–1 boron solution at neutral pH was investigated (Table 1). A tolerance in absorbance of ±5 % caused by interfering ions was considered acceptable. There is no significant interference on the boron signal from ions commonly occurring in water such as Na+, K+, Cl–, Zn2+, Mn2+, Co2+, Ni2+, NO3– and SO42–. The response for boron, however, was very sensitive to Fe3+, with a tolerance limit of 0.5 mg L–1 Fe3+. At this concentration of Fe3+, a prominent increase in the intensity of absorbance for boron was recorded. This interference restricts the application of the current boron measurement method. The method is also affected by the presence of Al3+, with a tolerance limit of 0.4 mg L–1 Al3+. The presence of Al3+ ions can cause a blue shift in the boron peak due to the pH change, which is consistent with the results shown in Figs 3 and 4. There is no obvious interference from anions.
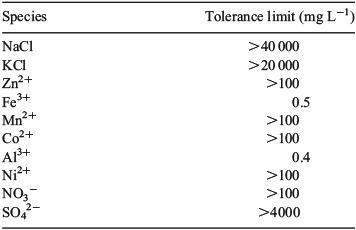
|
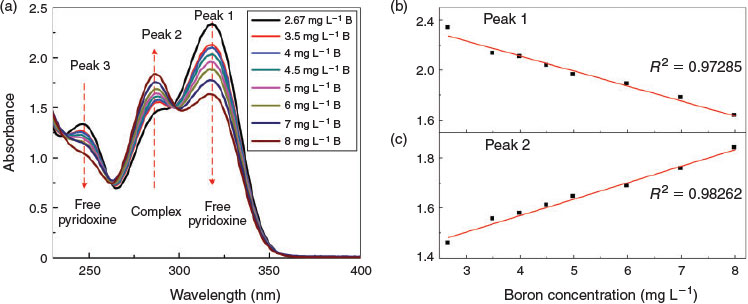
In order to explore the influence of other ions, seawater was used as a test sample and the method of standard additions was applied. Seawater, collected off the east coast of Singapore, and filtered to remove particles, had a pH of 8.15 and contained 2.67 mg L–1 of boron as measured by plasma atomic-emission spectrometry (Shimadzu ICPE-9820, Kyoto, Japan). Pyridoxine was added to the seawater sample followed by known amounts of boron as shown in Fig. 5a. The correlation coefficient (R2) of peaks 1 and 2 was 0.972 and 0.982 respectively, which indicates that the calibration plot is still accurate even in real seawater. For comparison, the calibration curves in freshwater and seawater are shown in Fig. S3. Based on the calibration curve, the calculated boron content in seawater was 2.15 mg L–1 for peak 1, and 2.31 mg L–1 for peak 2, which matches well the value obtained by atomic-emission spectrometry and demonstrates the applicability of the new method for measuring boron in a complex aqueous matrix such as seawater.
In summary, we demonstrate the use of pyridoxine as a novel reagent for a quick, accurate, portable and sensitive boron detector based on absorption spectra techniques. This simple and effective technology for measuring boron concentration in aqueous solutions is suitable for online analysers, and thus will have direct applications in environmental analysis.
Supplementary material
Materials used in the present study and experimental details are available from the Journal’s website.
Acknowledgement
This project was supported by the National Research Foundation, Prime Minister’s Office, Singapore, under its Environment & Water Research Program (project ref. no. 1301-IRIS-17). This program is administered by the Public Utilities Board (PUB), Singapore’s national water agency.
References
[1] R. Wickham, R. Godec, Controlling boron levels in semiconductor UPW using an experimental on-line boron analyzer, in Semiconductor Pure Water and Chemicals Conference Proceedings, Monterey, CA, 27 February–1 March 2001.[2] W.-W. Choi, K. Y. Chen, Evaluation of boron removal by adsorption on solids. Environ. Sci. Technol. 1979, 13, 189.
| Evaluation of boron removal by adsorption on solids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXitV2rurw%3D&md5=c4978637b99efd68d4ca56be7651f0afCAS |
[3] J. A. Rajaratnam, J. B. Lowry, P. N. Avadhani, R. H. V. Corley, Boron: possible role in plant metabolism. Science 1971, 172, 1142.
| Boron: possible role in plant metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3MXkslamurY%3D&md5=e8ecd627be569ede9b5db4f6ce1ce9abCAS |
[4] World Health Organization, Boron in Drinking Water – Background Document for Development of WHO Guidelines for Drinking-Water Quality WHO/HSE/WSH/09.01/2 2009 (World Health Organization: Geneva). Available at http://www.who.int/iris/handle/10665/70170 [verified 17 February 2017].
[5] D. Atanassova, V. Stefanova, E. Russeva, Co-precipitative pre-concentration with sodium diethyldithiocarbamate and ICP-AES determination of Se, Cu, Pb, Zn, Fe, Co, Ni, Mn, Cr and Cd in water. Talanta 1998, 47, 1237.
| Co-precipitative pre-concentration with sodium diethyldithiocarbamate and ICP-AES determination of Se, Cu, Pb, Zn, Fe, Co, Ni, Mn, Cr and Cd in water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXotFSltLY%3D&md5=86036030846d18be37d83ab16c8261c2CAS |
[6] Y. Muramatsu, S. Uchida, K. Tagami, S. Yoshida, T. Fujikawa, Determination of plutonium concentration and its isotopic ratio in environmental materials by ICP-MS after separation using and extraction chromatography. J. Anal. At. Spectrom. 1999, 14, 859.
| Determination of plutonium concentration and its isotopic ratio in environmental materials by ICP-MS after separation using and extraction chromatography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXislWkt78%3D&md5=7c9c5bca6701ce8652ed42148e73c13bCAS |
[7] S. D. Kumar, B. Maiti, P. K. Mathur, Determination of boron by flow injection analysis using a conductivity detector. Anal. Chem. 1999, 71, 2551.
| Determination of boron by flow injection analysis using a conductivity detector.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjtFOkt7c%3D&md5=c405138ca5e1c9bbc6aa99dd88dfa2d1CAS |
[8] C. Paul, P. Cohen, W. D. Fletcher, Method and Apparatus for Measuring the Concentration of Boron. US Patent 3468764 A 1969.
[9] E. M. Shvarts, R. T. Ignash, R. G. Belousova, Reactions of polyols with boric acid and sodium monoborate. Russ. J. Gen. Chem. 2005, 75, 1687.
| Reactions of polyols with boric acid and sodium monoborate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvF2ksA%3D%3D&md5=e26d5411a629fcc324ef5edca289609dCAS |
[10] P. P. Kosenka, K. J. O’Neill, R. D. Godec, Low-Level Boron Detection and Measurement. US Patent 6884356 B2 2005.
[11] D. L. Harp, Modifications to the azomethine-H method for determining boron in water. Anal. Chim. Acta 1997, 346, 373.
| Modifications to the azomethine-H method for determining boron in water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXktF2qurg%3D&md5=bc5eb428f93a7f12ac7778e3d7270aeaCAS |
[12] D. Sarkar, A. A. Sheikh, K. Batabyal, B. Mandal, Boron estimation in soil, plant, and water samples using spectrophotometric methods. Commun. Soil Sci. Plant Anal. 2014, 45, 1538.
| Boron estimation in soil, plant, and water samples using spectrophotometric methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXovVeit7Y%3D&md5=822a1660902ed87283c048f7a59d1a18CAS |
[13] Y.-M. Liu, K. Lee, Modifications of the curcumin method enabling precise and accurate measurement of seawater boron concentration. Mar. Chem. 2009, 115, 110 .
| Modifications of the curcumin method enabling precise and accurate measurement of seawater boron concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGku7fK&md5=49ab3c6a17ca3324b40d088ebcc53e2eCAS |
[14] D. M. C. Gomes, M. A. Segundo, J. L. F. C. Lima, A. O. S. S. Rangel, Spectrophotometric determination of iron and boron in soil extracts using a multi-syringe flow injection system. Talanta 2005, 66, 703.
| Spectrophotometric determination of iron and boron in soil extracts using a multi-syringe flow injection system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXks1Skur8%3D&md5=a5e0c2256f135d54926c6803f21d69ddCAS |
[15] M. Swain, chemicalize.org. J. Chem. Inf. Model. 2012, 52, 613.
| chemicalize.org.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVSqt70%3D&md5=21ac1ba48688c8c1520edcdaa6d876d3CAS |
[16] M. M. A. C. Ribeiro, J. M. Freitas, R. A. A. Munoz, C. L. do Lago, E. M. Richter, Fast determination of diphenhydramine, pyridoxine, and 8-chlorotheophylline by capillary electrophoresis with capacitively coupled contactless conductivity detection. Anal. Methods 2016, 8, 4432.
| Fast determination of diphenhydramine, pyridoxine, and 8-chlorotheophylline by capillary electrophoresis with capacitively coupled contactless conductivity detection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XntlSrt7g%3D&md5=a1a2724273ec39e2e0214223f284b38eCAS |
[17] A. K. Lunn, R. A. Morton, Ultra-violet absorption spectra of pyridoxine and related compounds. Analyst 1952, 77, 718.
| Ultra-violet absorption spectra of pyridoxine and related compounds.Crossref | GoogleScholarGoogle Scholar |
[18] D. A. Köse, B. Zumreoglu-Karan, O. Sahin, O. Büyükgüngör, Boric acid complexes with thiamine (vitamin B1) and pyridoxine (vitamin B6). Inorg. Chim. Acta 2014, 413, 77.
| Boric acid complexes with thiamine (vitamin B1) and pyridoxine (vitamin B6).Crossref | GoogleScholarGoogle Scholar |
[19] A. Shrivastava, V. B. Gupta, Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles of Young Scientists 2011, 2, 21.
| Methods for the determination of limit of detection and limit of quantitation of the analytical methods.Crossref | GoogleScholarGoogle Scholar |
[20] V. Ollilainen, HPLC Analysis of Vitamin B6 in Foods 2000 (Agricultural and Food Science: Finland). Available at http://journal.fi/afs/article/view/5632 [verified 17 February 2017].