Environmental chemistry: a discipline standing on two shoulders
Montserrat FilellaA Institute F.-A. Forel, University of Geneva, Route de Suisse 10, CH-1290 Versoix, Switzerland. Email: montserrat.filella@unige.ch
B SCHEMA, Rue Principale 92, L-6990 Rameldange, Luxembourg.
Environmental Chemistry 11(1) 37-40 https://doi.org/10.1071/EN13180
Submitted: 6 October 2013 Accepted: 16 December 2013 Published: 25 February 2014
Environmental chemistry, a science at a crossroads
Many chemists working in the field of environmental chemistry will have experienced the feeling of being looked down on by other chemists doing ‘real science’. The roots of this attitude may lie in a profound misunderstanding of what environmental chemistry is actually about, a misunderstanding fostered by the unusual position of environmental chemistry among scientific disciplines. Some causes and effects are examined below.
According to Oreskes’ perceptive analysis,[1] scientific disciplines can be grouped in two families:
-
Earth sciences, which seek to understand nature ‘as it is’ rather than to reduce it to its constituent parts. Traditionally, Earth Sciences study the context itself and work by inductive generalisation from observation. This methodological approach favours explanation.
-
Physical sciences (including chemistry), which isolate phenomena from their context in nature in order to understand their invariant characteristics. This reductionist approach, whose ultimate objective is establishing the ‘laws of nature’, emphasises prediction. It has become mainstream nowadays that prediction is part and parcel of the scientific method.
Clearly, as a discipline, environmental chemistry straddles the inductive explanatory approach linked to any study of Earth systems and the reductionist predictive approach characteristic of chemistry. The tension that this situation generates is often not fully understood, even by environmental chemists. Thus, titles such as ‘From environmental analytical chemistry to ecotoxicology – a plea for more concepts and less monitoring and testing’,[2] written 30 years ago when the discipline was at an early stage of its development, already reflects a certain lack of understanding of its roots in implying that ‘mature science’ is not compatible with extensive field measuring and data collection (i.e. ‘monitoring’). Indeed the authors added ‘Monitoring data can rarely be generalised unless one knows the significant interrelationships and interactions between the parts of an ecological system’. The authors take-home message relies on the conception that collecting data – and any associated inductive approach – is merely the first step in the development of a field as it matures into a ‘real science’ where deduction and ‘laws’ reign. This is nothing more than a reflection of Popper’s caricature of inductive science as a ‘bucket method’ of acquiring knowledge. Founding books of environmental chemistry (e.g. Stumm and Morgan,[3] Buffle,[4] Morel and Hering[5] and Schwarzenbach et al.[6] to cite just a few in my area of expertise) have continued in this direction, emphasising the study of processes and largely neglecting the possible value of inductive approaches. It is undeniable that many of these texts have played a key role in the development of the discipline, but they may perhaps have also smothered some of its potential strengths.
Furthermore, the common description of natural systems as ‘dirty’ is emblematic of (some) environmental chemist’s unease when faced with natural systems, exemplified in statements such as ‘…environmental systems … are much ‘dirtier’ (i.e. more complex)’.[7] Such examples abound in the literature and may reflect the difficulty of applying strict deductive ‘recipes’, and ways of thinking, to the study of natural systems. Probably, one of the cases where the ‘dirtiness’ and, consequently, the limits of applying the deductive approach to an environmental issue, are most evident is with humic substances whose complexity and elusive nature have largely eluded traditional chemical approaches. Chemists’ amazement when faced with these substances is perfectly expressed in Schnitzer’s statement: ‘The molecular heterogeneity of humic materials is the main obstacle to arriving at a valid concept of the chemical structure of these materials. As structural organic chemistry is based primarily on the availability of molecularly homogeneous materials, the structure of humic substances will be a major preoccupation of SOM [soil organic matter] chemists for some time to come’.[8]
Curiously, the general acceptance of deduction and prediction as a sine qua non for ‘practising real science’ has recently been counterbalanced by the current vogue for studying ‘complexity’ and ‘emergence’. Sentences such ‘The failure of reductionism’[9] or ‘when faced with multidimensional problems take a crude look at the whole’ (Revkin[10] citing Gell-Mann) have become widespread in the scientific literature, even if sometimes tempered by authors such as Anderson in his classic article ‘More is different’,[11] ’the reductionist hypothesis does not by any means imply a constructionist one’. Defining ‘complexity’ and related terms is not straightforward. As pointed out by Johnson in a clarifying text,[12] complex system research suffers from a lack of precision when people refer to ‘emergent properties’ and a wide range of views are covered by a common umbrella. Nevertheless, these concepts have expanded and proved extremely useful in many fields such as the study of neural networks, ecosystems, etc., but they are rarely found in environmental chemistry – a close and potentially receptive discipline – where the notion of complexity is still largely equated with complication. For instance, a review of Environmental Chemistry searching for ‘emergence’ retrieved zero articles, whereas ‘complexity’ retrieved seven – but in all cases the word was used as a synonym of ‘complicated’ (e.g. ‘Neither of these models considers the complexity of internal subcellular fractionation’[13] or ‘…because of its complexity, it remains unproven today’[14]). A search in Environmental Science and Technology produced five articles that included the word ‘complexity’ in their titles, but again always as a synonym of ‘complicated’ (e.g. ‘Complexity behind biotech corn not addressed’[15] or ‘Model complexity needed for quantitative analysis of high resolution isotope and concentration data from a toluene-pulse experiment’[16]). Four titles included ‘emergence’ but also with a difference in meaning (e.g. ‘Emergence flux declines disproportionately to larval density along a stream metals gradient’[17] or ‘The emergence of treatment wetlands’[18]). A search for ‘emergent properties’ in Environmental Science and Technology (anywhere in the text) gave 25 articles. After browsing them, only four papers were deemed possibly relevant, and a detailed reading of these showed that none really dealt with or used such properties: either the words appeared in isolated sentences pointing to tentative hypotheses (‘A key question is whether the different relaxation time compartments identified in the spectra can be assigned to separate some soil components or whether they are an emergent property produced by interactions leading to the structure of the soil mixture.’[19]), only in the abstract (‘The use of coupled process models in the system model of CO2-PENS provides insights into the emergent behaviour of aggregate processes that could not be obtained by using individual process models.’[20]) or expressed wishes rather than facts (‘Once the properties and scales of emergence have been delineated, new conceptual models can be created that will encompass physical/chemical heterogeneities as well as scale related chemical behaviors.’[21]). It seems clear that the notions of ‘complexity’ and ‘emergence’ are perceived as positive but that the concepts have not yet permeated the discipline.
Environmental chemistry conceptual toolkit
The future of environmental chemistry as a scientific discipline is dependent on its ability to reconcile inductive and reductionist approaches in a purposeful way. This means simultaneously embracing both approaches without granting either one intellectual superiority over the other. In practice, we need to adopt the following.
An inductive attitude
We should put all data on a given subject together and look for patterns and correlations. This is different from collecting data just for narrative reviews or even data digestion. Paraphrasing Oreskes when commenting on Whewell, ‘induction is an act of creative insight that gives meaning to large bodies of observational evidence’[1,22] and environmental chemistry as a discipline needs to exploit this fully. These last 30 years an incredible amount of data has been collected, both from natural compartments (air, soil, water) and from environmentally relevant processes in the laboratory. We need to exploit the potential hidden in existing data by applying appropriate data mining and treatment procedures. I personally explored, in a modest and tentative way, this path in a somewhat controversial study[23] and I remain convinced of the power of the approach even if I also experienced the difficulty of convincing the directly involved scientific community of the value of the approach.
A reductionist attitude
Without any doubt, detailed knowledge of molecular and microscopic scale processes is needed to understand what is observed at the macro scale. Although examples are probably not necessary here, let us consider, for instance, the elegant example provided in Harada and Takahashi,[24] where the different behaviour of Se and Te in seawater is explained by the different inner v. outer sphere complexation behaviour towards ferrihydrite of these otherwise similar elements (Fig. 1). However, even if the importance of molecular and microscale understanding is generally accepted, some points need to be addressed. First, this type of causative link will always remain tentative to some degree as it is not generally possible to modify environmental systems in the same way that conditions in a chemical experiment in the laboratory can be (with the exception of a few interesting but inherently restricted cases such as the Experimental Lakes Area in western Ontario, Canada[25]). Second, the fact that causation is inherently tentative does not mean scientific articles need to include a long discussion section full of more or less justified causative links and be plagued with sentences starting by ‘possibly’, ‘probably’, ‘may be’, etc. Third, science being considered equivalent to prediction has led to taking mechanistic prediction across temporal and spatial scales in environmental systems for granted. A detailed discussion of the subject of prediction is outside the scope of this paper; the reader is referred to Oreskes’ insightful texts[1,26] on the subject. However, because it is often ignored that ‘testing the models with laboratory or field data to assess their ‘correctness’ or ‘validity’ really tests their consistency with data, not necessarily their proximity to truth’[27] and that this consistency is often reached through extensive (hidden) parameter fitting, it is clear that there is an urgent need to acknowledge the (hidden) degree of empiricism associated to the current use of apparently mechanistic-based modelling in many fields such as, for instance, ecotoxicicity assessment.[28] This, of course, opens the question of the extent to which considering environmental chemistry a strictly deductive science might be an illusion.

|
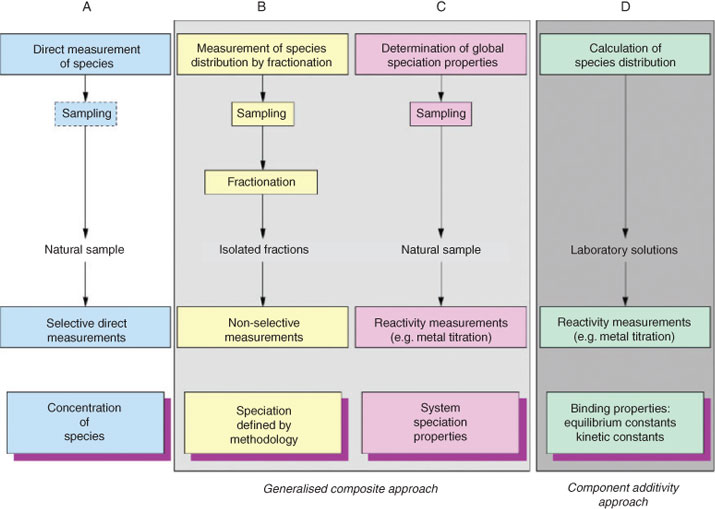
Without neglecting the potential interest of effectively introducing ‘complexity’ concepts into current environmental chemistry practice, we should already make better use of global parameters when integrating inductive and deductive strategies: environmental chemistry is characterised by the extensive use of operationally defined parameters. They are often considered as unfortunately unavoidable and, somehow, the lesser of two evils (the other option being having nothing whatsoever!) because they do not measure the ‘real’ pieces of the environmental jigsaw and have poor ‘predictive value’. However, some of these parameters provide information that is interesting per se because it integrates the results of different ‘real’ processes. If properly used and understood, such global parameters may provide a better insight on natural systems than the hypothetical possibility of being able to measure all the ‘real’ pieces of the jigsaw while, in the end, ignoring how they combine in nature (i.e. using global parameters as a kind of ‘low-cost’ complexity descriptors). A nice example of an existing use of global parameters is offered by trace element speciation studies. Together with the measurement of some pieces of the jigsaw (column A in Fig. 2) and strictly reductionist-based procedures (i.e. traditional thermodynamic speciation modelling, column D in Fig. 2), speciation techniques also include Generalised Composite methods (columns B and C in Fig. 2) with the measurement of global parameters, such as water complexation capacities and binding constants (column C).[29] Coexistence of two different strategies is also found in other fields, such as in the study of environmental colloids[30] where an approach that tends to emphasise complexity and heterogeneity and attempts to describe colloid behaviour by using global descriptors (power-laws) coexists with a second, reductionist approach that targets selected fractions for detailed analysis of specific features that might be extrapolated back to the bulk pool. Many years ago, Tipping already recognised that drawing the different approaches together was a major, ‘perhaps insurmountable’, challenge in the field.[31] Theoretical thinking about these issues is rare in environmental chemistry.
|
Corollary
Understanding that two scientific traditions support environmental chemistry as a discipline will foster its development by, on the one hand, exploiting the value hidden in the huge amounts of existing data and, on the other, correctly applying deductive-based prediction tools. As Murray Gell-Mann once said ‘We have to get rid of the idea that careful study of a problem in some narrow range of issues is the only kind of work to be taken seriously, while integrative thinking is relegated to cocktail party conversation’.[32]
Acknowledgements
I thank the three referees that read the manuscript. They provided me with a perfect laboratory to test the effects of my text in ‘real samples’ and helped to improve some aspects of it.
References
[1] N. Oreskes, Why predict? Historical perspectives on prediction in Earth Science, in Prediction. Science, Decision Making, and the Future of Nature (Eds D. Sarewitz, R. A. Pielke Jr, R. Byerly Jr.) 2000, pp. 23–40 (Island Press: Washington, DC).[2] W. Stumm, R. Schwarzenbach, L. Sigg, From environmental analytical chemistry to ecotoxicology – a plea for more concepts and less monitoring and testing. Angew. Chem. Int. Ed. Engl. 1983, 22, 380.
| From environmental analytical chemistry to ecotoxicology – a plea for more concepts and less monitoring and testing.Crossref | GoogleScholarGoogle Scholar |
[3] W. Stumm, J. J. Morgan, Aquatic Chemistry. An Introduction Emphasizing Chemical Equilibria in Natural Waters 1970 (Wiley: New York).
[4] J. Buffle, Complexation Reactions in Aquatic Systems. An Analytical Approach 1988 (Ellis Horwood: Chichester, UK).
[5] F. M. M. Morel, J. G. Hering, Principles and Applications of Aquatic Chemistry 1993 (Wiley: New York).
[6] R. P. Schwarzenbach, P. M. Gschwend, D. M. Imboden, Environmental Organic Chemistry 1993 (Wiley: New York).
[7] W. H. Glaze, Environmental chemistry comes of age. Environ. Sci. Technol. 1994, 28, 169A.
| Environmental chemistry comes of age.Crossref | GoogleScholarGoogle Scholar |
[8] M. Schnitzer, Soil organic matter – the next 75 years. Soil Sci. 1991, 151, 41.
| Soil organic matter – the next 75 years.Crossref | GoogleScholarGoogle Scholar |
[9] R. Sapolsky, Anecdotalism, in This Will Make You Smarter (Ed J. Brockman) 2012, pp. 278–280 (Harper Perennial: New York).
[10] A. Revkin, Anthropophilia, in This Will Make You Smarter (Ed J. Brockman) 2012, pp. 386–388 (Harper Perennial: New York).
[11] P. W. Anderson, More is different. Science 1972, 177, 393.
| More is different.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38XltVGlu7s%3D&md5=aec1d27601f9949402d65e657b77fe90CAS | 17796623PubMed |
[12] C. W. Johnson, What are emergent properties and how do they affect the engineering of complex systems? Reliab. Eng. Syst. Saf. 2006, 91, 1475.
| What are emergent properties and how do they affect the engineering of complex systems?Crossref | GoogleScholarGoogle Scholar |
[13] W.-X. Wang, P. S. Rainbow, Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms. Environ. Chem. 2006, 3, 395.
| Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlWnur7N&md5=0664fcade12cb95b1bd70655a9391037CAS |
[14] M. Harvey, The iron CLAW. Environ. Chem. 2007, 4, 396.
| The iron CLAW.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVanurvJ&md5=14989d378e2bf4cc7b5c8d8445db93c4CAS |
[15] J. Pelley, Complexity behind biotech corn not addressed. Environ. Sci. Technol. 2002, 36, 227A.
| Complexity behind biotech corn not addressed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xkt1ehsb4%3D&md5=3cf9ec1504edab08b3e7c780ba34d635CAS |
[16] D. Eckert, S. Qiu, M. Elsner, O. A. Cirpka, Model complexity needed for quantitative analysis of high resolution isotope and concentration data from a toluene-pulse experiment. Environ. Sci. Technol. 2013, 47, 6900.
| 1:CAS:528:DC%2BC3sXnsVeqsLk%3D&md5=02c2af6e69144c060824ff883b5cfc98CAS | 23668814PubMed |
[17] T. S. Schmidt, J. M. Kraus, D. M. Walters, R. B. Wanty, Emergence flux declines disproportionately to larval density along a stream metals gradient. Environ. Sci. Technol. 2013, 47, 8784.
| 1:CAS:528:DC%2BC3sXps1yhs70%3D&md5=3c8bcb5e9cfd6d98059db891141f83c9CAS | 23781899PubMed |
[18] S. Cole, The emergence of treatment wetlands. Environ. Sci. Technol. 1998, 32, 218A.
| The emergence of treatment wetlands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXivVyitr0%3D&md5=11d1239ce0cd44e9c3b6e9e97628e07bCAS | 21662202PubMed |
[19] T. Todoruk, C. H. Langford, A. Kantzas, Pore-scale redistribution of water during wetting of air-dried soils as studied by low-field NMR relaxometry. Environ. Sci. Technol. 2003, 37, 2707.
| Pore-scale redistribution of water during wetting of air-dried soils as studied by low-field NMR relaxometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjsVCitbg%3D&md5=d3b31b5ba90c7838a77cd297ca0e7cecCAS | 12854709PubMed |
[20] H. S. Viswanathan, R. J. Pawar, P. H. Stauffer, J. P. Kaszuba, J. W. Carey, S. C. Olsen, G. N. Keating, D. Kavestki, G. D. Guthrie, Development of a hybrid process and system model for the assessment of wellbore leakage at a geologic CO2 sequestration site. Environ. Sci. Technol. 2008, 42, 7280.
| Development of a hybrid process and system model for the assessment of wellbore leakage at a geologic CO2 sequestration site.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVWhs77O&md5=85655bfb0d74def54e121935a5357b75CAS | 18939559PubMed |
[21] A. W. Miller, D. R. Rodriguez, B. D. Honeyman, Upscaling sorption/desorption processes in reactive transport models to describe metal/radionuclide transport: a critical review. Environ. Sci. Technol. 2010, 44, 7996.
| Upscaling sorption/desorption processes in reactive transport models to describe metal/radionuclide transport: a critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1OqurzN&md5=ebde2a0991b7bec16e47a1e840af32a1CAS | 20942399PubMed |
[22] W. Whewell, The philosophy of the inductive sciences, founded upon their history, 2nd edn 1847 Available at http://openlibrary.org/books/OL7229132M/The_philosophy_of_the_inductive_sciences [Verified 28 December 2013].
[23] R. Town, M. Filella, Dispelling the myths: Is the existence of L1 and L2 ligands necessary to explain metal ion speciation in natural waters? Limnol. Oceanogr. 2000, 45, 1341.
| Dispelling the myths: Is the existence of L1 and L2 ligands necessary to explain metal ion speciation in natural waters?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXntFCitbo%3D&md5=8ef04715f580f513fcd9e935ac10553aCAS |
[24] T. Harada, Y. Takahashi, Origin of the difference in the distribution behavior of tellurium and selenium in a soil–water system. Geochim. Cosmochim. Acta 2008, 72, 1281.
| Origin of the difference in the distribution behavior of tellurium and selenium in a soil–water system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXis1WgurY%3D&md5=229d6a649bc9d0fe0414517c67053d10CAS |
[25] E. Stokstad, Canada’s experimental lakes. Science 2008, 322, 1316.
| Canada’s experimental lakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsV2jtrbJ&md5=90b3912baf8ec0a37bfe8b40bfdaf285CAS | 19039113PubMed |
[26] N. Oreskes, K. Shrader-Frechett, K. Belitz, Verification, validation, and confirmation of numerical models in the earth sciences. Science 1994, 263, 641.
| Verification, validation, and confirmation of numerical models in the earth sciences.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvit1OrsQ%3D%3D&md5=d535904851de4653844eeacb2ffb992dCAS | 17747657PubMed |
[27] D. K. Nordstrom, On evaluating and applying aqueous geochemical models. Eos Trans. AGU 1993, 74, 326.
[28] M. Filella, Quantifying ‘humics’ in freshwaters: purpose and methods. Chem. Ecol. 2010, 26, 177.
| Quantifying ‘humics’ in freshwaters: purpose and methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVGgsL7J&md5=28c6bf407c66b744f4e1bce57dbf081cCAS |
[29] M. Filella, Food for thought: a critical overview of current practical and conceptual challenges in trace element analysis in natural waters. Water 2013, 5, 1152.
| Food for thought: a critical overview of current practical and conceptual challenges in trace element analysis in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1GksbbM&md5=270aec124b04f4846f2c29744b9600e1CAS |
[30] M. Filella, Colloidal properties of submicron particles in natural waters, in Environmental Colloids and Particles: Behaviour, Separation and Characterisation (Eds K. W. Wilkinson, J. Lead) 2007, pp. 17–93 (Wiley: New York).
[31] E. Tipping, Colloids in the aquatic environment. Chem. Ind. 1988, 485.
| 1:CAS:528:DyaL1cXkvFygsrs%3D&md5=fc1ffbb2b72f22ac5d965ff72de905a7CAS |
[32] M. Gell-Mann, Transformations of the twenty-first century: transitions to greater sustainability, in Global Sustainability – A Nobel Cause (Eds H. J. Schellnhuber, M. Molina, N. Stern, V. Huber, S. Kadner) 2010, pp. 1–7 (Cambridge University Press: Cambridge, UK).
[33] Y. Takahashi, Origin of difference in solubility between tellurium and selenium into water at Earth’s surface, in Environmental Science, pp. 134–135. Available at http://www.spring8.or.jp/pdf/en/res_fro/08/134-135.pdf [Verified 28 December 2013].
[34] S. Goldberg, L. J. Criscenti, D. R. Turner, J. A. Davis, K. J. Cantrell, Adsorption–desorption processes in subsurface reactive transport modeling. Vadose Zone J. 2007, 6, 407.
| Adsorption–desorption processes in subsurface reactive transport modeling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpslegurc%3D&md5=9a0990dece6d7da6bea5db6496c69114CAS |


