Seed priming and soil application of zinc decrease grain cadmium accumulation in standard and zinc-biofortified wheat cultivars
Ayta Umar A and Shahid Hussain A *
A *
A Department of Soil Science, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan 60800, Pakistan.
Crop & Pasture Science 74(4) 284-293 https://doi.org/10.1071/CP22255
Submitted: 22 July 2022 Accepted: 24 October 2022 Published: 15 November 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Cadmium (Cd) is a toxic metal for both plants and humans. Wheat grown on Cd-contaminated soils may accumulate toxic levels of Cd in grains.
Aim: This study aimed to compare soil zinc (Zn) application and seed Zn-priming for decreasing grain Cd concentration in standard and Zn-biofortified wheat cultivars grown on Cd-spiked soil.
Methods: Standard (Jauhar-2016) and Zn-biofortified (Zincol-2016) wheat cultivars were grown in pots filled with Cd-spiked soil (8 mg Cd kg−1). The tested Zn treatments were un-primed, hydro-primed, and Zn-primed seeds with and without soil Zn application at 8 mg kg−1.
Key results: Zinc treatments significantly mitigated the toxic effects of Cd on the growth and physiological parameters of both cultivars. As compared to control, all Zn treatments significantly increased Zn and decreased Cd concentration in grains of the cultivars. On average, the maximum increase in grain Zn concentration over control was approximately 36% with Zn-priming + soil Zn. The same treatment, as compared to control, decreased grain Cd concentration by 42% in Zincol-2016 and 35% in Jauhar-2016. Grain Cd concentration was within the permissible level (≤0.2 mg kg−1) in Jauhar-2016 at all Zn treatments and in Zincol-2016 at Zn-priming + soil Zn.
Conclusion: Soil Zn application, seed Zn-priming, and their combination were effective in decreasing grain Cd accumulation in wheat grown on Cd-contaminated soil.
Implication: Zinc treatments, especially the combination of soil Zn application and seed Zn-priming, should be recommended for wheat grown on Cd-contaminated soil.
Keywords: accumulation, biofortification, cadmium, calcareous soil, seed priming, soil application, wheat, zinc, zincol-2016.
Introduction
At above-permissible levels, cadmium (Cd) is toxic for both plants and humans. In plants, Cd disturbs several key physiological processes like ion metabolism, photosynthesis, and mineral uptake (Khan et al. 2017). It affects photosynthesis by disturbing photosystems, photosynthetic pigments, carbon dioxide (CO2) reduction pathways, and electron transport systems (Parmar et al. 2013). Moreover, it inhibits plant growth by disrupting enzyme activities, protein synthesis, and hormone balance (Alharby et al. 2021). In humans, it causes oxidative stress that directly weakens the antioxidant defence system. It also damages DNA (Singh et al. 2021) and causes carcinogenesis and serious diseases like cardiovascular disease, and osteoporosis (Bimonte et al. 2021). Therefore, it is important to restrict Cd uptake from soil and its accumulation into plant edible parts.
Metal mining, wastewater irrigation, automobile exhaust, and phosphate fertilisers are the major anthropogenic sources of soil Cd contamination (Cai and Li 2022). From soils, Cd is accumulated in edible plant parts and ultimately enters the human food chain (Suhani et al. 2021). One clear strategy to save plant-based food from Cd exposure is to remediate contaminated soils through physical, chemical, and biological approaches (Liu et al. 2018). However, soil remediation techniques are expensive and mostly impractical for large-scale soil remediation projects. Another strategy, especially for marginally Cd-contaminated soils, is to increase the supply of zinc (Zn) to crop plants (Rizwan et al. 2017; Umar and Hussain 2020). Zinc is essential for plant metabolism and acts as a co-factor for some important enzymes. According to Hart et al. (2002), the root uptake, shoot transport, and grain loading of both Zn and Cd are facilitated by common transporters in plants. Therefore, these metals compete for uptake from the root and translocation to grains. Apart from this direct competition, Zn can also decrease Cd stress on plants in many other ways. These may include increasing the photosynthetic rate (Hussain et al. 2018), decreasing oxidative stress (Hassan et al. 2005), and balancing the nutrients (Rizwan et al. 2017).
There are significant genotypic variations in the extent of Zn effects on Cd accumulation in wheat (Zhou et al. 2020; Ali et al. 2022). In previous studies, it was reported that Zn-biofortified wheat can take up and accumulate more Cd in grains as compared to standard wheat (Hussain et al. 2019; Zia et al. 2020). Moreover, it is well known that both soil and foliar Zn applications increase Zn and decrease Cd accumulation in wheat grains (Murtaza et al. 2017; Forster et al. 2018; Zhou et al. 2020). Another Zn treatment, seed Zn-priming, is a more cost-effective technique of Zn application to cereals grown in calcareous soils (Harris et al. 2008). However, the effectiveness of seed Zn-priming relative to soil Zn application is unknown for decreasing grain Cd accumulation in Zn-biofortified and standard wheat cultivars. In the present study, therefore, our key objective was to compare soil Zn application and seed Zn-priming for decreasing grain Cd concentration in standard (Jauhar-2016) and Zn-biofortified (Zincol-2016) wheat cultivars grown in Cd-spiked soil.
Materials and methods
The surface layer (0–15 cm) of uncontaminated soil was collected from the Agricultural Farm (30.273°N, 71.514°E) of Bahauddin Zakariya University, Multan, Pakistan. The collected soil was air-dried, ground, sieved (through a 2 mm sieve), and thoroughly mixed. A representative sub-sample of the soil was analysed for physical and chemical properties by following the standard procedures. Soil texture was loamy with 44% sand, 26% silt, and 30% clay, as determined by Hydrometer Method (Gee and Bauder 1979). The soil was non-saline (with an electrical conductivity [EC] of 2.69 dS m−1 determined in saturated soil paste extract) but alkaline in reaction (with pH 7.8 determined in the saturated soil paste). Determined by following the Walkley and Black method (Nelson and Sommers 1996), the soil had 0.53% organic matter. Calcium carbonate in the soil was 49.8 g kg−1 as determined by the acid dissolution method (Loeppert and Suarez 1996). Plant available Zn and Cd were, respectively, 0.64 mg kg−1 and 0.02 mg kg−1 determined on an atomic absorption spectrophotometer (240FS AA, Agilent, Santa Clara, USA) after extraction by the diethylenetriaminepentaacetic acid (DTPA) method (Soltanpour and Workman 1979).
A known amount of soil was spiked at 8 mg Cd kg−1 by spraying a solution of CdCl2 followed by thorough mixing. The Cd-spiked soil was then covered with a polyethylene sheet and was allowed to equilibrate for 4 weeks (Lair et al. 2008). After that, 36 polyethylene-lined pots were each filled with 8 kg of Cd-spiked soil.
Before seed sowing, 60 mg N kg−1 as urea ((NH2)2CO), and 20 mg P kg−1 and 25 mg K kg−1 as potassium di-hydrogen phosphate (KH2PO4) were applied to pots in solution form. As per the treatment plan detailed in the following paragraph, two soil Zn rates (0 and 8 mg Zn kg−1) were applied as a solution of Zn sulfate heptahydrate (ZnSO4·7H2O). After nutrient application, the soil in each pot was thoroughly mixed.
There were 12 treatments comprising all combinations of two wheat cultivars (standard Jauhar-2016 (pedigree: KAUZ/PASTOR//V.3009) and Zn-biofortified Zincol-2016 (pedigree: OASIS/SKAUZ//4 × BCN/3/2 × PASTOR/4/T) and six Zn treatments [(1) un-primed seeds/control, (2) hydro-primed seeds, (3) Zn-primed seeds, (4) un-primed seeds + soil Zn, (5) hydro-primed seeds + soil Zn, and (6) Zn-primed seeds + soil Zn)]. For hydro-priming, the seeds were soaked in aerated distilled water at 1:3 ratio for 12 h. In the same conditions, the seeds for Zn-priming were soaked in 4 mM ZnSO4·7H2O. The treatments in the glasshouse were laid out in 2-factorial completely randomised design with three replications.
Seven healthy seeds were sown in each pot. After germination, plants were thinned to four per pot at the four-leaf stage. Plants were irrigated with distilled water to maintain moisture contents at 90% of the field capacity. At emergence, weeds were manually removed from the pots. To ensure randomisation, the positions of pots in the glasshouse were randomly changed each week. Additional splits of N (each at 60 mg kg−1) were applied as urea at stem elongation and booting stages.
At the anthesis stage (Feekes 10.5), a fully expanded uppermost leaf was selected from each plant for measuring chlorophyll contents (SPAD [Soil Plant Analysis Development] values) and photosynthetic parameters. Chlorophyll contents (SPAD values) were measured by using the SPAD-502 (Minolta, Osaka, Japan) meter. Photosynthetic rate, stomatal conductance, and transpiration rate were measured with CIRAS-3, a portable photosynthesis system (PP Systems, Amesbury, USA).
Two wheat plants from each pot were harvested at booting (Feekes 10) and the remaining two were harvested at full maturity (Feekes 11). After harvesting at maturity, plants were separated into stems, leaves, chaff, and grains. All plant parts were washed with distilled water to remove dust particles and then blotted dry with tissue papers. The plant samples were then oven-dried at 70°C for 48 h. Grains from the spikes were threshed by hand. Thousand-grain weights were calculated from the grain yield and the number of grains per pot. After the final harvest, post-harvest soil samples were collected from the pots for the determination of plant-available Zn and Cd by the DTPA method (Soltanpour and Workman 1979).
All plant samples were ground in a metal-free grinder followed by acid digestion in a di-acid mixture of nitric acid (HNO3) and perchloric acid (HClO4) (Jones and Case 1990). Zinc and Cd concentrations were analysed in the digests by using an atomic absorption spectrophotometer (240FS AA, Agilent, Santa Clara, USA). The total plant contents of Zn and Cd at two crop stages were calculated by multiplying dry weights with the concentrations of the respective element in each plant part.
The collected data were statistically analysed by using Statistix 8.1® for windows (Analytical Software, Tallahassee, USA). For the assessment of treatment effects, a two-way analysis of variance (ANOVA) test was run followed by Tukey’s honestly significant difference (HSD) test at P ≤ 0.05 (Quinn and Keough 2002). The interactive effect of wheat cultivars and Zn treatments was significant only for grain Cd concentration. Therefore, only the significant main effects were presented for the other parameters, and the interactive effect was presented for grain Cd concentration.
Results
Post-harvest soil zinc and cadmium
The main effect of Zn treatments influenced (P < 0.001) the post-harvest availability of Zn in the soil (Table 1). Averaged across cultivars, DTPA-extractable Zn was increased in all the treatments with soil Zn application as compared to control (Supplementary Table S1). Soil DTPA-extractable Zn ranged from 0.61 to 0.63 mg kg−1 in treatments without soil Zn and from 0.83 to 0.85 mg kg−1 in treatments with soil Zn. On the other hand, the imposed treatments did not affect DTPA-extractable Cd (Table 1). On average, the DTPA-extractable Cd in the soil after the harvest was 0.13 mg kg−1 (Table S1).

|
Photosynthetic parameters
The photosynthetic parameters, measured in the flag leaves at anthesis, were influenced by the main effects of wheat cultivars and Zn treatments (Table 1). Averaged across Zn treatments, stomatal conductance, photosynthesis rate and transpiration rate were significantly higher in Jauhar-2016 than in Zincol-2016 (Table 2). Averaged across cultivars and as compared to control, soil Zn combined with Zn-priming and hydro-priming increased transpiration rate, SPAD values, and stomatal conductance. However, the increase was non-significant in stomatal conductance with soil Zn + hydro-priming.
Plant growth and grain yield
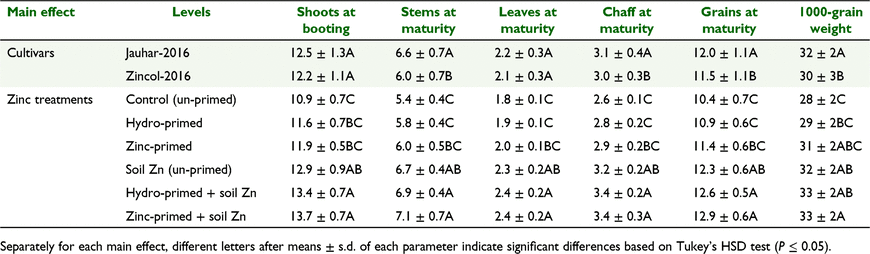
The main effects of cultivars and Zn treatments influenced shoot dry matter at booting, and stem, leaf, chaff and grain dry matter at maturity (Table 1). Averaged across Zn treatments, dry matters of stems, chaff and grains at maturity were significantly higher in Jauhar-2016 than in Zincol-2016 (Table 3). Averaged across cultivars, shoot dry matter at booting was increased by 18–25% with the treatments having soil Zn over control. Dry matters of stems, leaves, chaff, and grains at maturity were significantly increased by, respectively, 23–30%, 27–35%, 23–31%, and 18–25% with the treatments having soil Zn relative to control.
|
Thousand-grain weight was also affected by the main effects of cultivars (P = 0.005) and Zn treatments (P < 0.001) (Table 1). On average, the weight of 1000 grains was 6% higher in Jauhar-2016 than in Zincol-2016 (Table 3). Averaged across cultivars and as compared to control, 1000-grain weight was significantly increased (by 14–20%) with the treatments having soil Zn application.
Zinc concentration and contents
The main effects of cultivars and Zn treatments influenced Zn concentration and contents in shoots at booting, and in stems, leaves, chaff and grains at maturity (Table 1). On average, Zincol-2016 had significantly higher shoot Zn concentration (~18% more) and contents (~16% more) than Jauhar-2016 at booting (Tables 4, 5). At maturity, Zn concentration in stems, leaves, chaff and grains was, respectively, 14%, 14%, 18%, and 19% higher in Zincol-2016 than Jauhar-2016.

|

|
Averaged across cultivars, grain Zn concentration varied from 25 mg kg−1 in un-primed control to 34 mg kg−1 in Zn-priming + soil Zn (Table 4). Averaged across cultivars and as compared to control, Zn-priming + soil Zn increased shoot Zn concentration at booting by 51%. As compared to control, Zn-priming + soil Zn increased Zn concentration in stems, leaves, chaff and grains by 64%, 45%, 48% and 38%, respectively.
Averaged across cultivars, both at booting and maturity, shoot Zn contents were higher in Zn-treated pots than in control (Table 5). However, hydro-priming also significantly increased shoot Zn contents at maturity as compared to control. Averaged across Zn treatments, Zincol-2016 had higher (~16% more) shoot Zn content than Jauhar-2016 at maturity. In the cultivars, most of the total plant Zn was accumulated in grains (62–66%) followed by stems (18–21%), chaff (9–10%) and leaves (7–8%). However, Zn partitioning in stems was greater in Jauhar-2016 than Zincol-2016 whereas Zn partitioning in grains was greater in Zincol-2016 than Jauhar-2016.
Cadmium concentration and contents
On average, shoot Cd concentration at booting, and stem and chaff Cd concentrations at maturity were higher (P < 0.001) in Zincol-2016 than in Jauhar-2016 (Table 6). Averaged across cultivars and as compared to control, all Zn-containing treatments significantly decreased shoot Cd concentration at booting, and stem, leaf and chaff Cd concentration at maturity. The decrease with hydro-priming over control was non-significant in shoot Cd concentration at booting and in stem, leaf and chaff Cd concentration at maturity.

|
Grain Cd concentration ranged from 0.16 mg kg−1 in Jauhar-2016 (with Zn-priming + soil Zn) to 0.35 mg kg−1 in Zincol-2016 (in un-primed control) (Fig. 1). The interactive effect of cultivars × Zn treatments was significant (P < 0.001) for grain Cd concentration (Table 1). In un-primed control, grain Cd concentrations in Zincol-2016 and Jauhar-2016 were 0.35 mg kg−1 and 0.25 mg kg−1, respectively (Fig. 1). As compared to control, grain Cd concentration was significantly decreased with Zn treatments in both Jauhar-2016 and Zincol-2016. The maximum decrease over respective controls was 35% in Jauhar-2016 and 42% in Zincol-2016, achieved with Zn-priming + soil Zn. In Jauhar-2016, all Zn-containing treatments decreased grain Cd concentration below the permissible level of 0.20 mg kg−1. However, in Zincol-2016, only Zn-priming + soil Zn decreased grain Cd concentration to ~0.20 mg kg−1.
The main effects of treatments and cultivars influenced shoot Cd contents at booting and maturity (Table 1). On average, Cd contents in various plant samples of booting and maturity were higher (P < 0.001) in Zincol-2016 than in Jauhar-2016 (Table 7). Averaged across cultivars and as compared to control, shoot Cd contents at booting were significantly decreased only by Zn-priming + soil Zn. At maturity, Zn-priming and all treatments with soil Zn significantly decreased shoot Cd contents than control.

|
On average, most of the total above-ground plant Cd in both cultivars was accumulated in stems (41–44%), followed by leaves (25–28%), grains (15–20%), and chaff (13–14%) (Table 7). However, grain Cd partitioning was relatively higher in Zincol-2016 than in Jauhar-2016. Averaged across cultivars, priming treatments (hydro- and Zn-priming) had a non-significant effect on grain Cd partitioning, but the treatments with soil Zn significantly decreased grain Cd partitioning as compared to control.
Discussion
Photosynthetic parameters and crop yield
Cadmium is a toxic heavy metal that adversely affects the photosynthetic parameters and growth of plants (Hussain et al. 2018). Cadmium stress hampers the synthesis of chlorophyll and also affects the functional proteins associated with photosystem-II (Abbas et al. 2017). Therefore, it damages photosystem-II and adversely affects the photosynthetic parameters. Zinc has an ameliorative effect on plant Cd stress (Sperdouli et al. 2022). Zinc application restores the photochemical efficiency of photosystem-II and improves the photosynthetic parameters. It was previously reported that, under Cd stress, Zn application significantly increases stomatal conductance and net transpiration rate (Zhou et al. 2019). Similarly, in the present study, Zn-priming + soil Zn was effective in decreasing Cd stress by increasing chlorophyll contents (SPAD values), stomatal conductance, photosynthesis rate, and transpiration rate as compared to control (Table 2). However, the increase in photosynthesis rate was only marginal as compared to the control.
Photosynthetic parameters are closely associated with crop yield and its components (Yang et al. 2022). In this study, all the treatments with soil Zn increased the shoot dry matter at booting, and stem, chaff, and grain dry matter at maturity (Table 3). In addition, 1000-grain weight was also increased by soil Zn, which agrees with Hussain et al. (2019).
The difference in photosynthetic rate and yield between Jauhar-2016 and Zincol-2016 might be because of the genetic differences between these cultivars (Table 2). Jauhar-2016 was specifically developed for high grain yield (Ali et al. 2019) and it had, as compared to Zincol-2016, higher stomatal conductance, photosynthetic rate, transpiration rate, shoot dry matter and 1000-grain weight (Tables 2, 3). On the other hand, Zincol-2016 is Zn-biofortified wheat that was developed for high Zn accumulation in grains (Hussain et al. 2019; Lowe et al. 2022). In the present study, Zincol-2016 had a 19% higher grain Zn concentration than Jauhar-2016 (Table 4).
Zinc and cadmium accumulation
Zinc biofortification is an effective approach to fulfil the Zn requirements of the increasing population (Praharaj et al. 2021). Both genetic and agronomic Zn biofortification are useful approaches to combat Zn hunger (Ishfaq et al. 2021). For agronomic Zn biofortification, researchers have suggested soil Zn application (Hussain et al. 2012), foliar Zn spray (Ishfaq et al. 2022) and seed Zn-priming (Hassan et al. 2019). In our study, averaged across cultivars, Zn-priming and soil Zn increased grain Zn concentration at maturity as compared to control (Table 5). Rizwan et al. (2019) and Hussain et al. (2018) recommended the application of Zn oxide nanoparticles to increase shoot Zn concentration. Tavarez et al. (2015) reported that Zn addition increased stem, husk, and leaf Zn concentration under Cd stress. In our study, on average, Zn-priming and the treatments with soil Zn application were effective in increasing grain Zn concentration over control (Table 4). This might be because the Zn-priming provides Zn and improves crop growth only during the initial stages of a crop, whereas soil Zn application may provide a continuous supply of Zn throughout the crop lifecycle. In our study, post-harvest soil analysis clearly showed higher Zn availability in soil applied with soil Zn (Table S1).
Cadmium, a toxic metal, has a chemical resemblance with the essential micronutrient Zn. It is well known that Zn application decreases the harmful effects of Cd on plants grown on Cd-contaminated soil (Wang et al. 2018; Hussain et al. 2019; Khan et al. 2019; Ali et al. 2022). In plants, both Zn and Cd are transported through the same carriers (ZRT1, YSL, and ZI proteins) (Zheng et al. 2012, 2018), therefore, Zn application in Cd-contaminated soil decreased Cd uptake from soil to root (Hart et al. 2002). Wu et al. (2020) found Zn application decreased stem, leaf and chaff Cd concentration in wheat grown in Cd-stressed conditions. In our study, both Zn-priming and soil Zn significantly decreased grain Cd concentration, with more decrease with the combined Zn-priming + soil Zn (Table 6). Additionally, all the Zn treatments significantly decreased stem, leaf, and chaff Cd concentration as compared to control.
Various studies compared Zn application methods like soil Zn application (Abbas et al. 2017; Forster et al. 2018), foliar Zn application (Hussain et al. 2019; Wu et al. 2020), and seed Zn-priming (Rizwan et al. 2019; Ali et al. 2022) for increasing grain Zn concentration under Cd stress. Soil factors such as pH, organic matter, cation exchange capacity, clay contents and availability of other competitive elements affect the phytoavailable fractions of Zn and Cd (Antoniadis et al. 2017). These factors also affect the effectiveness of various Zn application methods. In alkaline soils, phytoavailable Zn and Cd are much lower than in low pH soils. In such conditions, soil Zn application significantly increases shoot Zn uptake and decreases Cd uptake. However, a major portion of the added Zn may be specifically adsorbed in alkaline soils (Riaz et al. 2020). Higher seed Zn contents in Zn-primed seeds may partly fulfil Zn requirements during seed germination. These Zn enriched seeds, therefore, accelerate the Zn transporter family (ZIP) that restricts the Cd uptake within the plants (Kiran et al. 2020). Soil Zn application may increase the plant-available Zn in soil that in combination with seed Zn-priming may decrease the Cd uptake in alkaline soils. Therefore, in our study seed Zn-priming + soil Zn resulted in the highest decrease in the grain Cd concentration in both cultivars.
In Jauhar-2016, grain Cd concentration was above the permissible limit (0.2 mg kg−1) in only control and hydro-priming (Fig. 1). On the other hand, in Zincol-2016, grain Cd concentration was above the permissible limit in all treatments except with Zn-priming + soil Zn. Zincol-2016 is Zn-biofortified wheat (PARC 2017) and it was specifically bred for high grain Zn concentration. Therefore, it has a higher ability to uptake Zn from the soil. High Cd concentration in Zincol-2016 might be because of the enhanced expression of specific genes controlling the similar transporters of Zn and Cd (ZIP, YSL, and ABC transporters) (Song et al. 2017; Umar and Hussain 2020). Therefore, Zincol-2016 and other high Zn cultivars may accumulate above-permissible levels of Cd in grains when grown on Cd-contaminated soils.
Qaswar et al. (2017) also found that Zn application significantly decreases grain Cd concentration in Zincol-2016. However, they reported a relatively higher decrease in grain Cd concentration in Zincol-2016 as compared to the tested standard wheat cultivar. This might be because of genetic differences in the standard wheat cultivars used in the previous and present studies. Moreover, various soil factors may also have contributed to this response, including the differences in the types and levels of soil contaminants. In this study, uncontaminated soil was artificially contaminated with a low level of Cd (8 mg Cd kg−1). In contrast, Qaswar et al. (2017) used naturally contaminated soil that had toxic levels of many heavy metals.
The highest contents of plant total Cd are partitioned in roots, followed in order by stems, leaves, grains and chaff (Cai et al. 2020). Similar observations were made in the present study (Table 7). However, the cultivars had different pattern of leaf and grain Cd partitioning. Grain Cd partitioning was relatively higher in Zincol-2016 than in Jauhar-2016. This is because relatively more Cd was partitioned in leaves of Jauhar-2016 than Zincol-2016. Averaged across cultivars and as compared to control, grain Cd partitioning was decreased in all the treatments with soil Zn. This can be linked to the effect of Zn on Cd transport through all plant barriers from roots to the developing grains. The effects of Zn on Cd transport in plants are discussed above. Moreover, the findings of this study suggest work at the subcellular level to explain the Zn-mediated changes in Cd translocation and compartmentalisation in cereal crops.
Conclusions
Soil Zn application and seed Zn-priming, especially if combined, proved effective strategies for increasing grain yield and grain Zn concentration, and for decreasing grain Cd concentration under Cd contamination. Averaged across treatments, Zincol-2016 had higher grain Cd and Zn concentrations than Jauhar-2016. In Zincol-2016, only Zn-priming + soil Zn was effective in decreasing grain Cd concentration within the permissible levels (~0.20 mg Cd kg−1). On the other hand, in Jauhar-2016, all Zn-containing treatments decreased grain Cd concentration below the permissible limit. Therefore, seed Zn-priming and soil Zn application are important Zn management strategies for standard and Zn-biofortified wheat cultivars grown on Cd-contaminated soils.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no known conflicts of interest.
Declaration of funding
This research was financially supported by the Higher Education Commission of Pakistan through Indigenous Ph.D. Fellowship awarded to AU.
References
Abbas MS, Akmal M, Ullah S, Hassan MU, Farooq S (2017) Effectiveness of zinc and gypsum application against cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Communications in Soil Science and Plant Analysis 48, 1659–1668.| Effectiveness of zinc and gypsum application against cadmium toxicity and accumulation in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Alharby HF, Al-Zahrani HS, Hakeem KR, Alsamadany H, Desoky E-SM, Rady MM (2021) Silymarin-enriched biostimulant foliar application minimizes the toxicity of cadmium in maize by suppressing oxidative stress and elevating antioxidant gene expression. Biomolecules 11, 465
| Silymarin-enriched biostimulant foliar application minimizes the toxicity of cadmium in maize by suppressing oxidative stress and elevating antioxidant gene expression.Crossref | GoogleScholarGoogle Scholar |
Ali I, Mahmood MT, Zafar A (2019) The new wheat variety Jauhar-16: an addition in local high yielding and rust resistant germplasm. International Journal of Biology and Biotechnology 16, 933–943.
Ali S, Bani Mfarrej MF, Hussain A, Akram NA, Rizwan M, Wang X, Maqbool A, Nafees M, Ali B (2022) Zinc fortification and alleviation of cadmium stress by application of lysine chelated zinc on different varieties of wheat and rice in cadmium stressed soil. Chemosphere 295, 133829
| Zinc fortification and alleviation of cadmium stress by application of lysine chelated zinc on different varieties of wheat and rice in cadmium stressed soil.Crossref | GoogleScholarGoogle Scholar |
Antoniadis V, Levizou E, Shaheen SM, Ok YS, Sebastian A, Baum C, Prasad MNV, Wenzel WW, Rinklebe J (2017) Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation—a review. Earth-Science Reviews 171, 621–645.
| Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation—a review.Crossref | GoogleScholarGoogle Scholar |
Bimonte VM, Besharat ZM, Antonioni A, Cella V, Lenzi A, Ferretti E, Migliaccio S (2021) The endocrine disruptor cadmium: a new player in the pathophysiology of metabolic diseases. Journal of Endocrinological Investigation 44, 1363–1377.
| The endocrine disruptor cadmium: a new player in the pathophysiology of metabolic diseases.Crossref | GoogleScholarGoogle Scholar |
Cai K, Li C (2022) Ecological risk, input flux, and source of heavy metals in the agricultural plain of Hebei Province, China. International Journal of Environmental Research and Public Health 19, 2288
| Ecological risk, input flux, and source of heavy metals in the agricultural plain of Hebei Province, China.Crossref | GoogleScholarGoogle Scholar |
Cai Y, Zhang S, Cai K, Huang F, Pan B, Wang W (2020) Cd accumulation, biomass and yield of rice are varied with silicon application at different growth phases under high concentration cadmium-contaminated soil. Chemosphere 242, 125128
| Cd accumulation, biomass and yield of rice are varied with silicon application at different growth phases under high concentration cadmium-contaminated soil.Crossref | GoogleScholarGoogle Scholar |
Forster SM, Rickertsen JR, Mehring GH, Ransom JK (2018) Type and placement of zinc fertilizer impacts cadmium content of harvested durum wheat grain. Journal of Plant Nutrition 41, 1471–1481.
| Type and placement of zinc fertilizer impacts cadmium content of harvested durum wheat grain.Crossref | GoogleScholarGoogle Scholar |
Gee GW, Bauder JW (1979) Particle size analysis by hydrometer: a simplified method for routine textural analysis and a sensitivity test of measurement parameters. Soil Science Society of America Journal 43, 1004–1007.
| Particle size analysis by hydrometer: a simplified method for routine textural analysis and a sensitivity test of measurement parameters.Crossref | GoogleScholarGoogle Scholar |
Harris D, Rashid A, Miraj G, Arif M, Yunas M (2008) ‘On-farm’ seed priming with zinc in chickpea and wheat in Pakistan. Plant and Soil 306, 3–10.
| ‘On-farm’ seed priming with zinc in chickpea and wheat in Pakistan.Crossref | GoogleScholarGoogle Scholar |
Hart JJ, Welch RM, Norvell WA, Kochian LV (2002) Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiologia Plantarum 116, 73–78.
| Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings.Crossref | GoogleScholarGoogle Scholar |
Hassan MJ, Zhang G, Wu F, Wei K, Chen Z (2005) Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. Journal of Plant Nutrition and Soil Science 168, 255–261.
| Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice.Crossref | GoogleScholarGoogle Scholar |
Hassan N, Irshad S, Saddiq MS, Bashir S, Khan S, Wahid MA, Khan RR, Yousra M (2019) Potential of zinc seed treatment in improving stand establishment, phenology, yield and grain biofortification of wheat. Journal of Plant Nutrition 42, 1676–1692.
| Potential of zinc seed treatment in improving stand establishment, phenology, yield and grain biofortification of wheat.Crossref | GoogleScholarGoogle Scholar |
Hussain S, Maqsood MA, Rengel Z, Aziz T (2012) Biofortification and estimated human bioavailability of zinc in wheat grains as influenced by methods of zinc application. Plant and Soil 361, 279–290.
| Biofortification and estimated human bioavailability of zinc in wheat grains as influenced by methods of zinc application.Crossref | GoogleScholarGoogle Scholar |
Hussain A, Ali S, Rizwan M, Zia ur Rehman M, Javed MR, Imran M, Chatha SAS, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environmental Pollution 242, 1518–1526.
| Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants.Crossref | GoogleScholarGoogle Scholar |
Hussain S, Khan AM, Rengel Z (2019) Zinc-biofortified wheat accumulates more cadmium in grains than standard wheat when grown on cadmium-contaminated soil regardless of soil and foliar zinc application. Science of the Total Environment 654, 402–408.
| Zinc-biofortified wheat accumulates more cadmium in grains than standard wheat when grown on cadmium-contaminated soil regardless of soil and foliar zinc application.Crossref | GoogleScholarGoogle Scholar |
Ishfaq M, Wakeel A, Shahzad MN, Kiran A, Li X (2021) Severity of zinc and iron malnutrition linked to low intake through a staple crop: a case study in east-central Pakistan. Environmental Geochemistry and Health 43, 4219–4233.
| Severity of zinc and iron malnutrition linked to low intake through a staple crop: a case study in east-central Pakistan.Crossref | GoogleScholarGoogle Scholar |
Ishfaq M, Kiran A, ur Rehman H, Farooq M, Ijaz NH, Nadeem F, Azeem I, Li X, Wakeel A (2022) Foliar nutrition: potential and challenges under multifaceted agriculture. Environmental and Experimental Botany 200, 104909
| Foliar nutrition: potential and challenges under multifaceted agriculture.Crossref | GoogleScholarGoogle Scholar |
Jones JB, Case VW (1990) Sampling, handling, and analyzing plant tissue samples. In ‘Soil testing and plant analysis’. (Ed. RL Westerman) pp. 389–427. (Soil Science Society of America: Madison, WI, USA) https://doi.org/10.2136/sssabookser3.3ed.c15
Khan MA, Khan S, Khan A, Alam M (2017) Soil contamination with cadmium, consequences and remediation using organic amendments. Science of the Total Environment 601–602, 1591–1605.
| Soil contamination with cadmium, consequences and remediation using organic amendments.Crossref | GoogleScholarGoogle Scholar |
Khan ZS, Rizwan M, Hafeez M, Ali S, Javed MR, Adrees M (2019) The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environmental Science and Pollution Research 26, 19859–19870.
| The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions.Crossref | GoogleScholarGoogle Scholar |
Kiran A, Wakeel A, Ishaq R, Mubaraka R, Ishfaq M, Mahmood A (2020) Zinc priming of maize seed enhances root to shoot Zn translocation but not of analogous heavy metals. The Journal of Animal and Plant Sciences 31, 1043–1051.
| Zinc priming of maize seed enhances root to shoot Zn translocation but not of analogous heavy metals.Crossref | GoogleScholarGoogle Scholar |
Lair GJ, Graf M, Zehetner F, Gerzabek MH (2008) Distribution of cadmium among geochemical fractions in floodplain soils of progressing development. Environmental Pollution 156, 207–214.
| Distribution of cadmium among geochemical fractions in floodplain soils of progressing development.Crossref | GoogleScholarGoogle Scholar |
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Science of the Total Environment 633, 206–219.
| Remediation techniques for heavy metal-contaminated soils: principles and applicability.Crossref | GoogleScholarGoogle Scholar |
Loeppert RH, Suarez DL (1996) Carbonate and gypsum. In ‘Methods of soil analysis. Part 3. Chemical methods’. (Eds DL Sparks, AL Page, PA Helmke, RH Loeppert, PN Soltanpour, MA Tabatabai, CT Johnston, ME Sumner) pp. 437–474. (Soil Science Society of America and American Society of Agronomy: Madison, WI, USA) https://doi.org/10.2136/sssabookser5.3.c15
Lowe NM, Zaman M, Khan MJ, Brazier AKM, Shahzad B, Ullah U, Khobana G, Ohly H, Broadley MR, Zia MH, McArdle HJ, Joy EJM, Bailey EH, Young SD, Suh J, King JC, Sinclair J, Tishkovskaya S (2022) Biofortified wheat increases dietary zinc intake: a randomised controlled efficacy study of zincol-2016 in rural Pakistan. Frontiers in Nutrition 8, 809783
| Biofortified wheat increases dietary zinc intake: a randomised controlled efficacy study of zincol-2016 in rural Pakistan.Crossref | GoogleScholarGoogle Scholar |
Murtaza G, Javed W, Hussain A, Qadir M, Aslam M (2017) Soil-applied zinc and copper suppress cadmium uptake and improve the performance of cereals and legumes. International Journal of Phytoremediation 19, 199–206.
| Soil-applied zinc and copper suppress cadmium uptake and improve the performance of cereals and legumes.Crossref | GoogleScholarGoogle Scholar |
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In ‘Methods soil analysis. Part 2. Chemical methods’. (Eds DL Sparks, AL Page, PA Helmke, RH Loeppert, PN Soltanpour, MA Tabatabai, CT Johnston, ME Sumner) pp. 539–579. (Soil Science Society of America and American Society of Agronomy: Madison, WI, USA) https://doi.org/10.2134/agronmonogr9.2.2ed.c29
PARC (2017) Improve nutrition and public health by developing biofortified food crops at NARC. Pakistan Agricultural Research Council 2–4. http://www.parc.gov.pk/index.php/en/about-narc/12-news-events/news/1151-improve-nutrition-and-public-health-by-developing-biofortified-food-crops-at-narc
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Botanical Studies 54, 45
| Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress.Crossref | GoogleScholarGoogle Scholar |
Praharaj S, Skalicky M, Maitra S, Bhadra P, Shankar T, Brestic M, Hejnak V, Vachova P, Hossain A (2021) Zinc biofortification in food crops could alleviate the zinc malnutrition in human health. Molecules 26, 3509
| Zinc biofortification in food crops could alleviate the zinc malnutrition in human health.Crossref | GoogleScholarGoogle Scholar |
Qaswar M, Hussain S, Rengel Z (2017) Zinc fertilisation increases grain zinc and reduces grain lead and cadmium concentrations more in zinc-biofortified than standard wheat cultivar. Science of the Total Environment 605–606, 454–460.
| Zinc fertilisation increases grain zinc and reduces grain lead and cadmium concentrations more in zinc-biofortified than standard wheat cultivar.Crossref | GoogleScholarGoogle Scholar |
Quinn GP, Keough MJ (2002) ‘Experimental design and data analysis for biologists.’ (Cambridge University Press: New York, NY, USA) https://doi.org/10.1017/CBO9780511806384
Riaz MU, Ayub MA, Khalid H, ul Haq MA, Rasul A, ur Rehman MZ, Ali S (2020) Fate of micronutrients in alkaline soils. In ‘Resources use efficiency in agriculture’. (Eds S Kumar, RS Meena, MK Jhariya) pp. 577–613. (Springer Singapore: Singapore) https://doi.org/10.1007/978-981-15-6953-1_16
Rizwan M, Ali S, Hussain A, Ali Q, Shakoor MB, Zia-ur-Rehman M, Farid M, Asma M (2017) Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 187, 35–42.
| Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment.Crossref | GoogleScholarGoogle Scholar |
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Zia ur Rehman M, Waris AA (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214, 269–277.
| Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat.Crossref | GoogleScholarGoogle Scholar |
Singh P, Mitra P, Goyal T, Sharma S, Sharma P (2021) Blood lead and cadmium levels in occupationally exposed workers and their effect on markers of DNA damage and repair. Environmental Geochemistry and Health 43, 185–193.
| Blood lead and cadmium levels in occupationally exposed workers and their effect on markers of DNA damage and repair.Crossref | GoogleScholarGoogle Scholar |
Soltanpour PN, Workman S (1979) Modification of the NH4HCO3-DTPA soil test to omit carbon black. Communications in Soil Science and Plant Analysis 10, 1411–1420.
| Modification of the NH4HCO3-DTPA soil test to omit carbon black.Crossref | GoogleScholarGoogle Scholar |
Song Y, Jin L, Wang X (2017) Cadmium absorption and transportation pathways in plants. International Journal of Phytoremediation 19, 133–141.
| Cadmium absorption and transportation pathways in plants.Crossref | GoogleScholarGoogle Scholar |
Sperdouli I, Adamakis I-D, Dobrikova A, Apostolova E, Hanć A, Moustakas M (2022) Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochemical efficiency in Salvia sclarea plants. Toxics 10, 36
| Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochemical efficiency in Salvia sclarea plants.Crossref | GoogleScholarGoogle Scholar |
Suhani I, Sahab S, Srivastava V, Singh RP (2021) Impact of cadmium pollution on food safety and human health. Current Opinion in Toxicology 27, 1–7.
| Impact of cadmium pollution on food safety and human health.Crossref | GoogleScholarGoogle Scholar |
Tavarez M, Macri A, Sankaran RP (2015) Cadmium and zinc partitioning and accumulation during grain filling in two near isogenic lines of durum wheat. Plant Physiology and Biochemistry 97, 461–469.
| Cadmium and zinc partitioning and accumulation during grain filling in two near isogenic lines of durum wheat.Crossref | GoogleScholarGoogle Scholar |
Umar A, Hussain S (2020) The role of zinc in grain cadmium accumulation in cereals. In ‘Plant micronutrients’. (Eds T Aftab, KR Hakeem) pp. 455–470. (Springer International Publishing: Cham, Switzerland) https://doi.org/10.1007/978-3-030-49856-6_20
Wang H, Xu C, Luo Z-c, Zhu H-h, Wang S, Zhu Q-h, Huang D-y, Zhang Y-z, Xiong J, He Y-b (2018) Foliar application of Zn can reduce Cd concentrations in rice (Oryza sativa L.) under field conditions. Environmental Science and Pollution Research 25, 29287–29294.
| Foliar application of Zn can reduce Cd concentrations in rice (Oryza sativa L.) under field conditions.Crossref | GoogleScholarGoogle Scholar |
Wu C, Dun Y, Zhang Z, Li M, Wu G (2020) Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicology and Environmental Safety 190, 110091
| Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil.Crossref | GoogleScholarGoogle Scholar |
Yang Y, Wang L, Che Z, Wang R, Cui R, Xu H, Chu S, Jiao Y, Zhang H, Yu D, Zhang D (2022) Novel target sites for soybean yield enhancement by photosynthesis. Journal of Plant Physiology 268, 153580
| Novel target sites for soybean yield enhancement by photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Zheng L, Yamaji N, Yokosho K, Ma JF (2012) YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. The Plant Cell 24, 3767–3782.
| YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice.Crossref | GoogleScholarGoogle Scholar |
Zheng X, Chen L, Li X (2018) Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to Cd stress. Botanical Studies 59, 22
| Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to Cd stress.Crossref | GoogleScholarGoogle Scholar |
Zhou Z, Zhang B, Liu H, Liang X, Ma W, Shi Z, Yang S (2019) Zinc effects on cadmium toxicity in two wheat varieties (Triticum aestivum L.) differing in grain cadmium accumulation. Ecotoxicology and Environmental Safety 183, 109562
| Zinc effects on cadmium toxicity in two wheat varieties (Triticum aestivum L.) differing in grain cadmium accumulation.Crossref | GoogleScholarGoogle Scholar |
Zhou J, Zhang C, Du B, Cui H, Fan X, Zhou D, Zhou J (2020) Effects of zinc application on cadmium (Cd) accumulation and plant growth through modulation of the antioxidant system and translocation of Cd in low- and high-Cd wheat cultivars. Environmental Pollution 265, 115045
| Effects of zinc application on cadmium (Cd) accumulation and plant growth through modulation of the antioxidant system and translocation of Cd in low- and high-Cd wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Zia MH, Ahmed I, Bailey EH, Lark RM, Young SD, Lowe NM, Joy EJM, Wilson L, Zaman M, Broadley MR (2020) Site-specific factors influence the field performance of a Zn-biofortified wheat variety. Frontiers in Sustainable Food Systems 4, 135
| Site-specific factors influence the field performance of a Zn-biofortified wheat variety.Crossref | GoogleScholarGoogle Scholar |



