Assessment of foliar-applied phosphorus fertiliser formulations to enhance phosphorus nutrition and grain production in wheat
Therese M. McBeath A B D , Evelina Facelli A , Courtney A. E. Peirce A C , Viran Kathri Arachchige A and Michael J. McLaughlin A
A B D , Evelina Facelli A , Courtney A. E. Peirce A C , Viran Kathri Arachchige A and Michael J. McLaughlin A
A Fertiliser Technology Research Centre, Soil Science, School of Agriculture, Food and Wine, The University of Adelaide, PMB 1, Waite Campus, Glen Osmond, SA 5064, Australia.
B CSIRO Agriculture and Food, Waite Campus, Locked Bag 2, Glen Osmond, SA 5064, Australia.
C Current address: South Australian Research and Development Institute, GPO Box 397, Adelaide, SA 5001, Australia.
D Corresponding author. Email: therese.mcbeath@csiro.au
Crop and Pasture Science 71(9) 795-806 https://doi.org/10.1071/CP20241
Submitted: 9 July 2020 Accepted: 19 September 2020 Published: 6 October 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC
Abstract
The ability to utilise foliar-applied phosphorus (P) as a strategy to increase the P status and yield of grain crops grown in dryland regions with variable climates is attractive. Several P formulations with varying pH, accompanying cations and adjuvants were tested for their effectiveness as foliar fertilisers for wheat (Triticum aestivum L.) plants, first under controlled and then under field conditions. Experiments under controlled conditions suggested that several formulations with specific chemistries offered promise with respect to wheat fertiliser-P recovery and biomass responses. These formulations were then evaluated in two field experiments, and although wheat grown at the sites showed substantive responses to soil-applied P, there was no significant grain-yield response to foliar-applied P. Following the limited responses to foliar-applied fertiliser in the field, we used an isotopic dilution technique to test the hypothesis that the variation in responses of wheat to foliar addition of P could be explained by a mechanism of substitution, whereby root P uptake is downregulated when P is taken up through the leaves, but this was proven not to be the case. We conclude that foliar P application cannot be used as a tactical fertiliser application to boost grain yield of wheat in dryland regions.
Keywords: adjuvant, foliar uptake, isotopic tracer, phosphorus source.
Introduction
A large proportion of soil-applied fertiliser phosphorus (P) will undergo chemical reactions that remove it from the plant-available pool (Hedley and McLaughlin 2005). Owing to the low recovery of soil-based P fertiliser in the season of application, a high application rate is often required on soils where soil P reserves are low. For soils where P has been managed such that P levels are at a maintenance phase (Weaver and Wong 2011), the fertiliser requirement is often marginal and dependent on in-season rainfall (McBeath et al. 2012). In Mediterranean systems such as southern Australia, application of P to soils occurs at sowing. This does not allow the subsequent climatic conditions to be taken into consideration, unlike management of nitrogen (N). Furthermore, the immobility of P in dryland cropping soils means that soil topdressing with P fertiliser later in the season is not effective (McLaughlin et al. 2011). Hence, management of crop P supply in synchrony with variation of in-season demand for P in response to growth conditions is difficult. With increasing costs of fertiliser P, this fertiliser input represents a large capital investment that could be managed more effectively. It is attractive to consider whether it is possible to develop more tactical P-application strategies, using foliar fertilisation to top up nutrients in-season as the crop requirements and climatic conditions dictate (Noack et al. 2010). However, consistent responses of wheat to foliar P applications in controlled and field conditions have been elusive (Noack et al. 2010).
Although wheat requirements for P are higher early in the growing season when most of the determinants of yield are set (Römer and Schilling 1986; Elliott et al. 1997; Grant et al. 2001), top-ups with foliar P have been found effective from first node visible to after anthesis in controlled conditions and/or field experiments (Sherchand and Paulsen 1985; Benbella and Paulsen 1998; Mosali et al. 2006; McBeath et al. 2011; Peirce et al. 2019). In pot experiments in controlled conditions, applications of foliar P were found effective between wheat flag-leaf emergence and booting, with different degrees of responsiveness in P uptake, biomass or grain yield (McBeath et al. 2011; Peirce et al. 2019), but earlier applications (at tillering, Zadoks growth stage GS22; Zadoks et al. 1974) were not effective (Peirce et al. 2019). However, there is a lack of consistency in responses, likely due to the interaction of factors that include physiological age of the crop, degree of crop P deficiency and leaf area of the crop available for spray interception (Noack et al. 2010).
In a previous study, we measured a 25% grain-yield response of wheat to foliar-applied phosphoric acid under controlled conditions in one of the two soils evaluated (McBeath et al. 2011). Further work focused on the use of phosphoric acid as the P source owing to this initial yield response and the availability of the product to farmers. However, yield responses to phosphoric acid have been inconsistent. This is despite the foliar uptake of P from phosphoric acid being high (>90% of the applied P) (Peirce et al. 2016). There is scope to investigate the effectiveness of other P formulations as foliar fertilisers because the accompanying cation and formulation pH can affect foliar P uptake (Koontz and Biddulph 1957). Previous studies have investigated the influence of replacing hydrogen from phosphoric acid with ammonium, sodium or potassium (K) and altering the pH (Tukey et al. 1956), but more recent evaluations of formulations is considered a gap (Froese et al. 2020). It is generally accepted that a low formulation pH of 2–3 facilitates more rapid uptake of foliar P (Swanson and Whitney 1953; Tukey et al. 1961; Bouma 1969), although it is often associated with necrotic spots and high leaf burn. For this reason, it is difficult to distinguish between the effect of pH and the effect of the associated cation, with most studies looking at the combined effect (Koontz and Biddulph 1957). The results from that study suggest that it is not a simple process of a lower pH resulting in higher uptake and translocation, given that at a pH of 5, NaH2PO4 had the highest translocation of P away from the treated area whereas KH2PO4 had the lowest. Furthermore, most previous studies that investigated the effect of pH and accompanying cation on foliar P absorption were conducted at P concentrations well below those used in the field (Koontz and Biddulph 1957; Tukey et al. 1961; Bouma 1969).
In addition to the source of P used, foliar fertilisation often includes the use of an adjuvant. An adjuvant is defined as any chemical added to a foliar spray solution that modifies the spray characteristics of the solution or aids in increasing the penetration of the leaf by the active ingredient (Hazen 2000). The use of adjuvants is especially important for wheat crops, because the hydrophobic nature of the wheat leaves render them difficult to wet with hydrophilic foliar sprays (Holloway 1969; Netting and von Wettstein-Knowles 1973; Peirce et al. 2016). The use of adjuvants can initially influence the deposition of the foliar fertiliser (through altering the spray characteristics including the droplet size) on the plant foliage (Spanoghe et al. 2007; Zabkiewicz 2007). Adjuvants also play a large role in increasing the retention of the sprays (particularly in the case of surfactants, which lower the surface tension of the formulation) and aiding the uptake of the foliar-applied P by increasing the diffusion of the active ingredient or increasing the hydration of the cuticle and hence its water permeability (Hess and Foy 2000). The role of adjuvants in the translocation of foliar P once it enters the plant cells is still relatively unknown, although Stolzenberg et al. (1982) showed that there was little movement of a labelled surfactant and its metabolites away from the initial application site. Although the type of adjuvant was not important for either uptake or translocation of foliar-applied P when used in combination with phosphoric acid as the P source (Peirce et al. 2016; Peirce et al. 2019), it is not known what interactions may occur between adjuvants and other P sources.
In mycorrhizal plants, the direct P uptake via roots is reduced when plants take up P through the mycorrhizal pathway, resulting in no biomass gains despite increases in P uptake compared with artificially induced, non-mycorrhizal controls (Grace et al. 2009; Facelli et al. 2014). It is possible that wheat plants behave in a similar way when P uptake is increased through leaves (as foliar-applied P) by downregulating uptake from soil, hence showing lack of yield responses to foliar P. Furthermore, foliar P fertilisation (which potentially alters leaf P status) could influence the long-distance signalling (movement of molecules from shoot to roots or vice versa) that regulates the expression of transporters involved in nutrient uptake and mobilisation (Liu et al. 2009) as well as the partitioning of carbohydrates (Huang et al. 2011).
The first of this series of experiments screened combinations of source of P, adjuvant for P recovery and wheat-plant response in a controlled-environment experiment. The best treatments from the controlled environment were then tested in field conditions in combination with relevant application timing and P dose for wheat grain-yield response. Finally, a controlled-environment experiment was used to investigate the possible substitution of root P uptake by P uptake through the leaves in wheat plants as a reason for lack of in-field responsiveness to foliar formulations.
Methods
Formulation screening in a controlled environment
The experiment was set up to investigate the uptake and translocation of seven different foliar P sources in combination with three adjuvants belonging to different classes (Table 1). This gave a total of 21 formulations of P plus a non-foliar (soil) P-application control. The experimental design was completely randomised with four replicates of each foliar fertiliser treatment and 12 replicates of the nil foliar control, with a total of 96 pots.

|
Plants were grown in soil (1.5 kg) in pots of diameter 10 cm and depth 17 cm that were closed systems. Plants were grown in a P-responsive soil (diffusive gradient in thin film (DGT) P, 4 µg/L; Colwell P, 3 mg/kg; and total P, 48 mg/kg) from Black Point, South Australia (34°36.776′S, 137°48.599′E), with a surface soil pH of 8.5 and additional soil characteristics as described in Peirce et al. (2014). Before sowing, the soil was wetted to 5% (w/w) (field capacity (FC) was 22.2% (w/w) as measured according to the method of Klute 1986), with the following basal nutrients (per pot) mixed through the soil with a mixer: N as CO(NH2)2, 75 mg N; P as H3PO4, 4.8 mg P (equivalent to 6 kg P/ha based on pot surface area); K as K2SO4, 100 mg K; magnesium as MgSO4.7H2O, 25 mg Mg; zinc as ZnSO4.7H2O, 15 mg Zn; copper as CuSO4.5H2O, 12 mg Cu; manganese as MnCl2.4H2O, 2 mg Mn; and total sulfur (S) applied in these reagents, 81 mg S. Additional N and K were added to the basal nutrients (including control pots) to balance the N and K applied in the foliar fertilisers.
After a week of equilibration, the soil was added to the pots and wetted to 70% FC before sowing four pre-germinated seeds of wheat (Triticum aestivum var. Axe) per pot at a depth of 1 cm. Cv. Axe was selected because it has a short growth cycle and is well suited to radionuclide experiments where decay needs to be considered in terms of safety of total dose used and the maximum length of experiment that can be managed. Immediately after sowing, the surface of the pot was covered with 50 g polyethylene granules to minimise evaporation from the soil surface, and the soil was wetted to 80% FC. When the plants were at the two-leaf growth stage, they were thinned to the two most uniform seedlings per pot. Plants were watered by weight every 2 days to maintain 80% FC and grown in a controlled-environment room (12-h cycle of 20°C day, 15°C night for 41 days; 23°C day, 15°C night thereafter until harvest at 62–65 days after sowing (DAS)), with their positions randomised every few days. To ensure that N was not limiting, at 15 and 27 DAS, 25 mg N (as urea) was applied to the surface of each pot and watered in.
Foliar fertilisers were applied 34 or 35 DAS at GS37, flag leaf visible. Treatments were applied early in the morning within a 2-h window to avoid changing environmental conditions. Owing to the large number of treatments, application occurred over 2 days with two of four replicate blocks treated each day. Isotopically labelled P fertiliser was made by adding carrier-free 33P (in the orthophosphate form) to give an activity of 144 kBq/pot at application. Fertilisers were applied with a Multipette M4 (Eppendorf, Hamburg), which controlled droplet size, delivering forty 2-µL drops to give an application rate of 1.6 mg P/pot, equivalent to 2 kg P/ha in a total volume 100 L/ha (0.65 m P). Fertiliser application was spread between the five leaves on the main stem excluding the flag leaf, provided they were healthy. If a leaf was not healthy, had been broken or had already senesced, it was excluded from the foliar application.
Aboveground plant material was harvested at the end of anthesis and separated into heads, treated leaves, tillers, and the main stem with the remaining leaves attached. All plant parts, including those of controls, were washed according to the method outlined in Fernández et al. (2014) to remove fertiliser P not taken up by the leaves. Plant parts were dried in an oven at 60°C for 72 h before being weighed, ground and digested in boiling nitric acid, then analysed for total P by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) (Zarcinas et al. 1987). A 2-mL sample of the digest was added to a vial with 10 mL scintillation fluid (Ultima Gold AB; PerkinElmer, Waltham, MA, USA), and 33P activity was determined by using a Quanta Smart liquid scintillation analyser (Model Tri-Carb B3110TR; PerkinElmer). Washing solutions were also retained to analyse both total and radioactive P by both ICP-AES and liquid scintillation counting. All counts were corrected for decay.
Total P in the plant was calculated as the sum of P (mg/pot) in all harvested plant parts after washing. The total P was then divided as P derived from the soil and seed and P derived from the labelled foliar fertiliser. Phosphorus derived from the foliar fertiliser was calculated according to Eqn 1:

By using the washing solution, the amount of P that was not absorbed by the plant was estimated by dividing the activity of the washing solution by the specific activity of the foliar fertiliser. Absolute recovery of the foliar fertiliser was then calculated as the sum of that recovered in the plant parts and in the washing solution as a percntage of the applied fertiliser (Eqn 2):
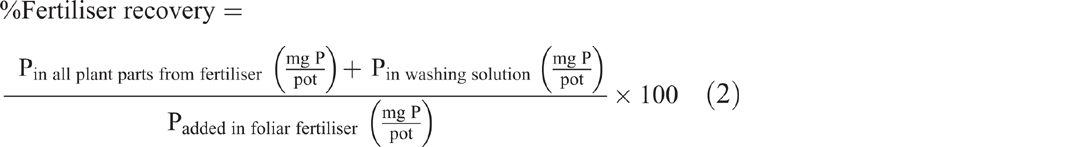
Both foliar P uptake and foliar P translocation were expressed as a percentage of the applied foliar fertiliser recovered in the plant parts after they were washed (Eqns 3 and 4). Translocated P (fertiliser P applied to leaves and translocated to other plant parts) was calculated as the percentage of foliar-applied P recovered in all plant parts at harvest excluding the treated leaves (Eqn 4):


Field experiments: testing formulations, timing and rates
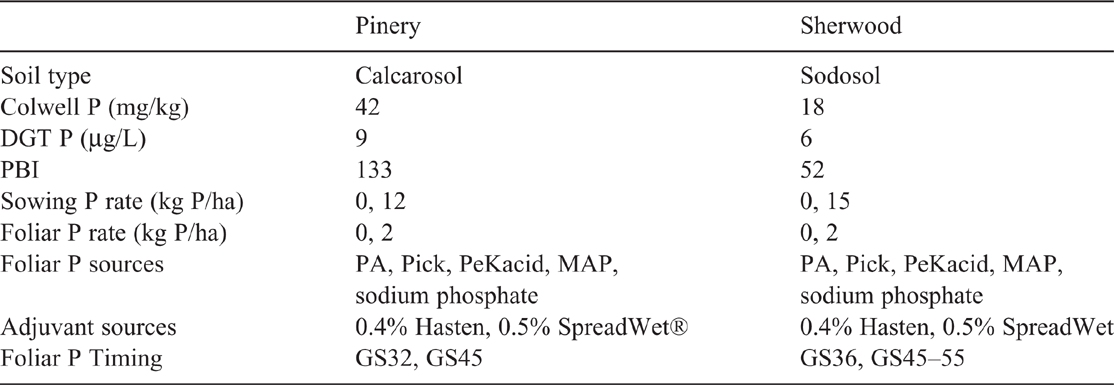
Replicated field experiments were established in 2015 at two locations in South Australian dryland cropping areas. Sites were selected at Sherwood in the Upper South East (36°04′S, 140°35′E) and Pinery in the Mid North (34°17′S, 138°28′E). Soil samples (0–10 cm) were taken from the sites before sowing to confirm soil P status. The soils were analysed, and the soil-test prediction was that the sites would produce wheat grain responsive to inputs of P fertiliser (Mason et al. 2010; Table 2). Rainfall was average for the growing season at Pinery, but below average for the months June and October. Rainfall throughout the growing season was well below average at Sherwood (Fig. 1).
|

|
Each experiment comprised two levels of soil starter-P and 10 foliar fertiliser formulations applied at two timings. The formulations resulted from the combination of five sources of P and two adjuvants (Table 2) and were compared with nil foliar-fertiliser controls. These formulations were chosen based on results from the first controlled-environment experiment. The rates for soil starter-P were selected to provide enough soil P to increase levels to a marginal P status. The rates were 12 kg P/ha for Pinery and 15 kg P/ha for Sherwood, added as mono-ammonium phosphate (MAP). Nitrogen was balanced across all plots by using urea. Balancing the small amounts of K and S added with the foliar fertiliser was considered unnecessary because soil tests at both sites showed test levels well in excess of sufficiency (data not shown). Following establishing rains in May, field plots of wheat (cv. Mace) were sown at 80 kg/ha to achieve establishment of at least 150 plants/m2. Cv. Mace was selected because it was the best adapted cultivar at the time and location of the experiments. These plots were sown with plot-scale equipment, and there were four replicate plots (2 m by 7 m; six rows with 0.25-m row spacing) per foliar fertiliser treatment at both levels of soil starter-P, arranged in a randomised complete block design.
At Pinery, the two timings for foliar P sprays were stem elongation, second node visible (GS32) and boots swollen (GS45). At Sherwood, timings of application were at sixth node visible (GS36) and boots swollen to half of inflorescence emerged (GS45–55). On the application date at Sherwood, plants growing in P0 plots were at GS45 whereas some of the plants in P15 plots were already at GS55. The application rate was equivalent to 2 kg P/ha in a total volume 100 L/ha. Sprays were applied with a 6-L handheld sprayer with two nozzles, each fitted with a constant pressure (100 kPa) valve at ~0.5 m above the crop. In-season plant nutrient concentrations were measured before the application of foliar treatments. Fifty plants were randomly sampled for youngest emerged blades from each control plot at GS36 and analysed for nutrient concentrations. Plant P concentration was measured according to the procedure outlined for the controlled-environment experiment. Plants from two 0.5-m sections of internal rows were randomly selected and cut at 2.5 cm above the ground, dried at 60°C and their dry weights were recorded.
At maturity, plots in all experiments were machine-harvested using a plot-scale harvester, and aboveground biomass was sampled by hand-cutting plants from four 0.5-m sections from each plot at ~2.5 cm above the ground.
Controlled environment experiment: substitution hypothesis
Wheat plants (cv. Axe) were grown in the same soil and conditions as the first controlled-environment experiment except that soil was treated to achieve two levels of basal soil P that mimicked deficient and marginal soil P levels for wheat growth. After a week of equilibration with basal nutrients, the soil was thoroughly mixed with labelled (33P, 3 MBq/kg) H3PO4 solutions to provide 3.2 or 8.5 mg P/kg (equal to 6 or 16 kg P/ha) and allowed to equilibrate for a further 10 days. Soil was then added to pots and wetted to 80% FC before sowing four pre-germinated seeds per pot. When the plants were at the two-leaf growth stage, they were thinned to the two most uniform seedlings per pot. Plants were watered by weight to 80% FC every 2 days and were grown in a controlled environment room (12-h cycle of 20°C day, 15°C night; average irradiance 230 μmol/m2.s) with their positions randomised at watering. To ensure N was not limiting, 25 mg N/pot as urea was applied to the surface at 15, 27 and 34 DAS and watered in.
The foliar fertiliser formulations Pick 15-42 and Maxi Phos in combination with SpreadWet 1000 were selected because they had contrasting effects on wheat dry mass and P uptake in the first controlled-environment experiment. Foliar fertilisers were applied as described for the first controlled-environment experiment at 35 DAS (GS 37, flag leaf visible). For each plant, fertiliser application was spread between three healthy leaves on the main stem and tillers (excluding flag leaf). The extra N and K applied in the foliar fertilisers was balanced in the corresponding treatments by addition of N (as urea) and K (as K2SO4) to the surface of the soil (watered in) the day before foliar fertilisation. Plants were harvested at early anthesis (GS63) and processed and analysed according to the procedures used in the first controlled-environment experiment.
Statistical analyses
For both controlled-environment experiments, analysis of variance (ANOVA) was used to test for treatment effects. For field experiments, ANOVA or t-tests were used to analyse the data. Genstat Release 16.1 (VSN International, Hemel Hempstead, UK) was used for analysis. Assumptions of distribution normality and constant variance error were tested for all data analysed. Least significant difference (l.s.d.) between treatments was calculated using Fisher’s protected l.s.d. at P = 0.05.
Results
Growth room formulation evaluation
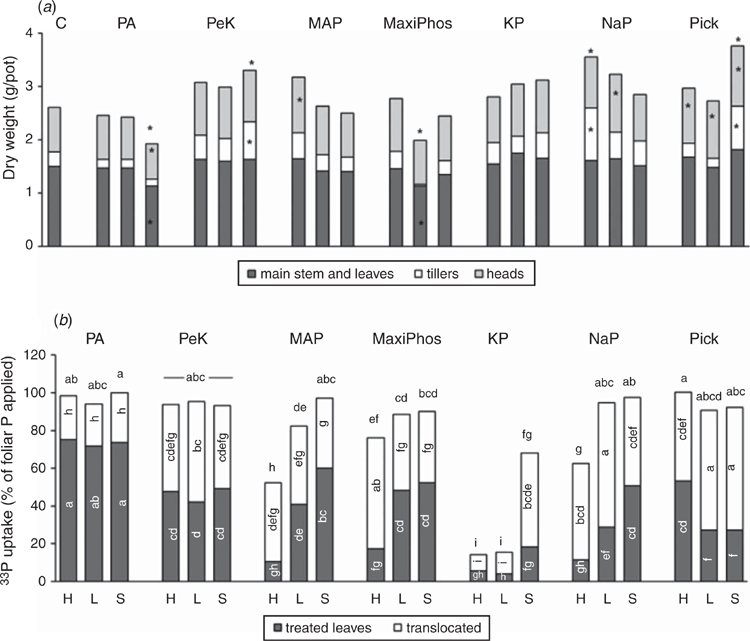
There were differences in aboveground dry weight between treated plants and the controls (Fig. 2a). Three treatments resulted in an increase in tiller biomass and total aboveground biomass compared with the control: PeKacid + SpreadWet, sodium phosphate + Hasten, and Pick + SpreadWet. Five treatments resulted in greater head biomass than the controls: MAP + Hasten, sodium phosphate + SpreadWet, and Pick with all three adjuvants.
The uptake of foliar P by plants was >80% of that applied for 15 of the 21 treatments (Fig. 2b). In most cases, nearly all of the recovered foliar fertiliser was located within the plant parts; only a small proportion (0–3%) of the fertiliser did not adhere to the leaves. Most of the products also had a large proportion of the foliar P translocated out of the treated leaves (Fig. 2b). The treatments that had lower foliar uptake (<80% of applied P) were MAP + Hasten, Maxi Phos + Hasten, potassium phosphate with all three adjuvants, and sodium phosphate + Hasten. These treatments represented the range from ammonium phosphate, potassium phosphate and sodium phosphate products. The pH of the first five of these formulations was ~4.3, whereas the pH of sodium phosphate + Hasten was 6.5.
Of the products that were commercially sourced (phosphoric acid, PeKacid, Maxi Phos, Pick), which varied in pH and associated cations, the uptake of foliar P was high (76–100% of applied amount) regardless of which adjuvant was utilised (Fig. 2b). The products that were analytical grade reagents (MAP, potassium phosphate, sodium phosphate) had variable uptake results. Phosphoric acid differed from all other products in the amount of foliar P that was translocated to other plant parts; for phosphoric acid, only 22–26% of the applied P was translocated, with the remainder (72–75%) located in the treated leaves at harvest (Fig. 2b). By comparison, all other products had a significantly higher proportion of their P translocated out of the treated leaves (38–82% of the foliar P recovered in the plant parts).
Field experiment: testing formulations, timing and rates
The results from the soil analyses were complemented by tissue analyses (Table 3) indicating that plant P concentrations at the first timing of foliar P application were close to the range given for diagnosis of P deficiency (0.21–0.25% total P for youngest emerged blades, GS31; Reuter and Robinson 1997). The concentrations of soil N, K and S (elements present in the foliar formulations tested at Pinery and Sherwood) were above adequate ranges (data not shown), suggesting that plants would not be responsive to further addition of these nutrients.
There was no effect of foliar P on grain yield (Fig. 3). However, there were increases in shoot P concentration and biomass due to applications of soil fertiliser P at both sites; the positive biomass response of the crops to soil starter-P was evident at anthesis, as it was in grain yield response at maturity at both sites (Table 3). From anthesis to maturity, the differences in biomass between nil P and added soil P decreased at both sites but there were still significant benefits of up to 38% higher grain yield with soil-applied P (Table 3). There was no effect of soil starter-P on grain protein, grain P concentration or harvest index at either site, although there was an increase in grain P uptake in line with the grain yield response at Sherwood (Table 3).
Controlled environment experiment: substitution hypothesis
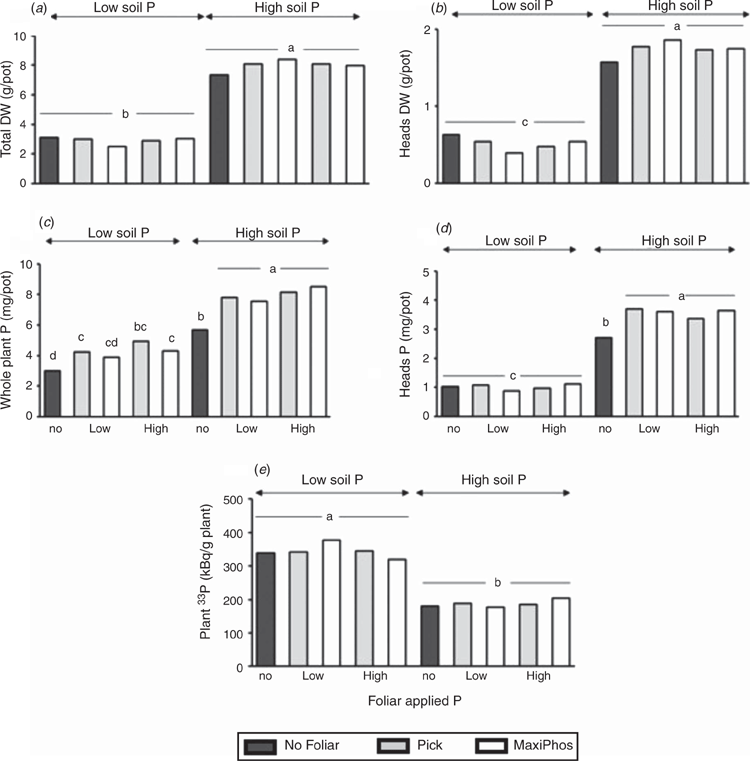
Aboveground and head biomass values were higher in pots with higher soil P, but there was no effect of foliar P application (Fig. 4a, b). However, the effects of foliar P application were significant for plant P uptake, and at each level of soil P there was an overall increase in plant P due to foliar P application (Fig. 4c). Similarly, there was an increase in head P due to foliar treatments in plants grown at high soil P, but in plants grown at low soil P, there was no difference in head P between foliar P treatments and the controls (Fig. 4d). The 33P activity per g plant was not different between foliar treatments and the controls within each level of soil fertility (Fig. 4e), indicating no effect of foliar P on uptake of P from soil (i.e. substitution).
Discussion
Growth room formulation evaluation
The responses measured in the controlled environment do not seem highly dependent on source of P (pH, accompanying cations) or adjuvant type. The products Pick and PeKacid in combination with SpreadWet produced the most consistent biomass increase over the control. An increase in biomass due to increased uptake of P from foliar application has previously been reported (Sherchand and Paulsen 1985; Mosali et al. 2006; McBeath et al. 2011). The high recovery (>60% in most cases) of the foliar pathway found in this study is consistent with findings from other studies that used foliar P at rates relevant for field applications (McBeath et al. 2011; Peirce et al. 2014, 2016). The efficiency of foliar P uptake was not affected by formulation, with only potassium phosphate having lower efficiency, owing to crystallisation of the fertiliser on the leaf surface, which was subsequently washed off the leaves. It is expected that higher P uptake from foliar fertiliser, and therefore more P resources for the plant to grow, would lead to aboveground biomass increases compared with the control, particularly if the tissue P concentration was low.
For a foliar application to be effective, once it is absorbed by the leaf, it must be able to move to the growing plant parts and be utilised for growth. Phosphorus is a nutrient that has been shown to be very effectively translocated from senescing plant parts to the grain when grown through to maturity (Batten et al. 1986). Phosphoric acid had high overall uptake across all formulations with the three different adjuvants. However, only 22–26% was translocated out of the treated leaves. It is likely that the small amount of foliar P that was translocated, regardless of the high uptake, is responsible for the lack of biomass response for this product. This low translocation is consistent with previous work at similar foliar P rates (Peirce et al. 2014, 2016).
There were three products that contained P in combination with potassium: PeKacid, potassium phosphate and Pick. The best performing of these three products was Pick, which consistently increased plant biomass across the three adjuvants. It also had high foliar P uptake (>90%) and translocation (47–65%). This product is a commercial potassium phosphate and potassium citrate solution that had an average pH of 8.7 when applied to the plants in combination with the adjuvants. By contrast, the analytical reagent potassium phosphate had very low uptake and translocation with Hasten and LI700, both of which formed a crystalline deposit on the leaf as the fertiliser dried. Reed and Tukey (1978) also observed decreased absorption for foliar fertilisers that dried as salt deposits on the leaf. They observed that for potassium phosphates, the highest absorption by chrysanthemum leaves was at a pH of 2, with absorption decreasing as the pH increased to 7, after which absorption increased again (Tukey et al. 1956). Those absorption trends with pH are consistent with our study. Interestingly, several field studies have documented wheat yield increases with application of potassium phosphate (Sherchand and Paulsen 1985; Benbella and Paulsen 1998; Mosali et al. 2006). However, those studies did not indicate the pH of the foliar fertilisers applied, but it would be expected to be similar to the potassium phosphate in our study if prepared with high-quality water. It is not known whether those yield responses were to the P or K in the fertilisers because the K applied in the foliar fertilisers was not balanced in the soil as was done in our study, and little information was provided on the K status of the plants. Yield responses in the study by Mosali et al. (2006) were documented in a field site with low soil K levels.
For the products that contained P in combination with N (MAP and Maxi Phos), only MAP + Hasten produced a positive head-biomass response, despite both products having the same pH. The only difference between the products was the N : P ratio. When applied at the same P rate of 1.6 mg/pot, MAP added 0.7 mg N/pot to the leaves and Maxi Phos added 1 mg N/pot to the leaves. It is therefore difficult to say why the products did not produce the same biomass response. Foliar uptake was variable for the products, ranging from 52% to 97%, but in all cases the translocation of foliar P was high (37–59%). In a study on the foliar uptake of P by bean leaves, comparing treatments across the pH range 2–7 and the accompanying cations of K, Na and ammonium, ammonium phosphate solutions at a pH of 2–3 performed best (Tukey et al. 1956). Likewise, Reed and Tukey (1978) found that ammonium phosphate absorption was highest at pH 2 and was much lower (only 5–8%) at all other pH values. Despite those results, we found high uptake of P from ammonium phosphates suggesting that at higher concentrations of P, total foliar absorption increases.
Sodium phosphate was the second-best performing product and produced some positive biomass responses when it was applied in combination with Hasten or LI700. Foliar uptake ranged from 62% to 98%, with high translocation particularly for sodium phosphate +LI700 (66%). Our results are comparable to those of Thorne (1957) who found 79–87% of foliar-applied P from sodium phosphate was recovered in the plant parts of swede and sugar beet plants. Tukey et al. (1956) showed that sodium phosphate absorption and translocation to the roots of bean plants was highest at a pH of 2–3 and second highest at a pH of 5 when the foliar fertilisers were applied at pH values ranging from 2 to 7, although the amount of P absorbed as a percentage of P applied was not discussed. Reed and Tukey (1978) also found that P absorption when using sodium phosphate reduced as the pH increased (from 22% at pH 2 to 5% at pH 6). However, our results did not indicate a relationship between P uptake from sodium phosphate and solution pH.
The inconsistent results regarding the use of adjuvants and their effect on foliar uptake and yield make generalisations difficult. Given that the three adjuvants were chosen across different classes, they might be expected to influence the uptake by different mechanisms. Hasten is an esterified oil with non-ionic surfactants, whereas LI700 is an emulsion of soyal phospholipids and propionic acid with some surfactants that the manufacturer claims to acidify the formulation. Given the low concentration of LI700 in the fertiliser formulation and the high pH-buffering capacity of phosphate in solution, we saw negligible pH differences among formulations as a result of the adjuvants (data not shown). SpreadWet is a pure non-ionic surfactant. The action of the adjuvant to increase the retention of the fertilisers on the leaves was performed equally well by all three adjuvants. In all cases, <3% of the foliar fertiliser volume for each treatment was not retained by the leaves. We have shown in previous work that uptake of foliar-applied P was high regardless of the adjuvant used, provided the adjuvant reduced the surface tension of the fertiliser to allow it to be retained on the leaf surface (Peirce et al. 2016, 2019).
Field experiments: testing formulations, timing and rates
Although foliar-applied P appears to be effectively taken up by wheat leaves and can increase biomass for some treatments in a controlled environment, a translation to increased grain yield was not measured in the field. Lack of grain-yield response to foliar-applied P in wheat in the field has been reported before (Sherchand and Paulsen 1985; Mosali et al. 2006; Froese et al. 2020) and is in line with the inconclusive results reviewed by Noack et al. (2010), and with more recent investigations where grain P concentrations (Ali et al. 2014) or P-use efficiency (Froese et al. 2020) were increased as a result of foliar applications but no benefits in yield were found. Overall, ~50% of the experiments reported in the literature (including both controlled environment and field experiments) have been non-responsive (Noack et al. 2010). The failure to achieve grain yield increases has been attributed mainly to the use of non-responsive soils. However, we selected soils that were deficient to marginal for P supply to wheat, and plants were evidently responsive to soil-applied P through to maturity.
In controlled conditions, the recovery of foliar fertiliser has been mostly >80% (McBeath et al. 2011; Peirce et al. 2016), but in field conditions where the fertiliser is sprayed instead of applied in small drops, a much lower recovery is expected. Our target rate of 2 kg P/ha has been effective in our experiments in controlled conditions (although responses were small) and it is within the recommended range for P applied as foliar sprays in the field (Noack et al. 2010). Increasing the rate in one application may lead to leaf damage via scorch, but not all damage precludes benefits of foliar P (Peirce et al. 2016). In the field experiments, only phosphoric acid produced scorch (but without biomass or grain yield penalty); thus, there is scope for rate increases. An increase in rate could also be achieved through multiple applications but they may not be economically feasible for dryland cropping.
The application times were within the time ‘window’ where wheat plants, after the early responses to soil-applied P (Rodríguez et al. 1999), may require a small amount of P to complete grain filling without P stress. By the end of stem elongation, wheat takes up most of the required P for maximum production of biomass, but it may need a small amount during grain filling to achieve maximum yield. However, the efficiency of utilisation of P in production of grain is directly proportional to water availability (Clarke et al. 1990), and overall growth conditions around flowering are associated with variation in wheat yield (French and Schultz 1984; Fischer 2015). Phosphorus added in foliar sprays at later stages could be used to boost plant growth and grain filling in cases where soil-available P is limiting or dry soils prevent soil P uptake, but this will be productive only if the stress induced by lack of water has not affected leaf P metabolism (Sutton et al. 1983; Grant et al. 2001). One of the promises for using foliar P as a ‘top-up’ is to be able to complement the soil starter-P in seasons with adequate yield potential. Overall, we were not able to detect significant yield responses to inputs of P applied in-season as foliar fertiliser.
The field experiments showed a wheat yield increase in response to soil starter-P but a response to foliar-applied P was lacking. At Sherwood the annual rainfall in the year of the experiments (2015) was markedly below the historical mean (Fig. 1), and at Pinery there were critical points in the growing season (e.g. June and October) with rainfall below the historical mean (Fig. 1). Although there was an increase of ~45% in grain yield in response to starter P, the maximum yield achieved in the experiment was still half of the 5-year regional yield average (1 t/ha vs 2 t/ha, PIRSA 2016). Importantly, rainfall was particularly low (Fig. 1) and temperatures unseasonably high around anthesis (October) at Sherwood and Pinery. The October mean temperatures were 20% and 30% above the long-term average at Pinery and Sherwood, respectively, with both sites having ³3 days with temperatures >35°C. Thus, the utilisation of P in the foliar ‘top-up’ could have been thwarted by unfavourable climatic conditions at the time when grain-yield components such as spikelet and floret numbers, which depend on growth conditions just before anthesis, were determined (French and Schultz 1984; Calderini et al. 2001; Fischer 2015).
Controlled environment experiment- Substitution hypothesis
The lack of any foliar P effect on anthesis biomass despite the increase in P uptake is in line with results reviewed in the literature (Noack et al. 2010) and reported from more recent greenhouse and field experiments (Ali et al. 2014; Peirce et al. 2016). It is surprising, though, that Pick did not increase anthesis biomass and Maxi Phos increased P uptake, because these results contrast with findings in the first controlled-environment experiment. These results highlight the complexity of predicting the efficacy of foliar-applied P. These plants may have had their physiology compromised despite the soil-P levels being only marginally limiting for wheat plant growth, because leaf P status and function are determinants of the capacity of plants to be able to uptake and translocate P (Fernández et al. 2014; Peirce et al. 2016). The lack of treatment effect on the plant 33P activity indicates that there was not a reduction in the uptake of P by roots in foliar-fertilised plants compared with plants that were not foliar-fertilised. Given there was no effect of foliar P addition on root P uptake from soil, it is not possible to use the concept of substitution to explain why foliar P uptake had an effect on plant P uptake but not on biomass.
Conclusions
Under controlled environment conditions, plant uptake of P from foliar-applied solutions was high. Except for potassium phosphate, plant uptake of P was 80–95% of the amount applied to the leaves for most formulations. The foliar route is therefore an effective pathway for P acquisition by crops. Biomass responses, and the efficiency of foliar P uptake and translocation were not consistently related to fertiliser pH, adjuvant type or accompanying cation. However, P applied as phosphoric acid appears to be poorly translocated from treated leaves, perhaps because of serious scorch damage to leaves. Responses to foliar P application are therefore possible, but dependent on product formulation.
Our results show that despite foliar P increasing P uptake by wheat in soils with concentrations of available P considered marginal for wheat production under controlled conditions, there were no significant effects observed under field conditions. Furthermore, wheat plants did not downregulate uptake of P by roots in response to foliar P fertilisation, suggesting that the plant was not substituting foliar P uptake for root P uptake. Why the increase in P uptake from foliar fertilisation is not translated into increases in biomass or grain yield remains unclear. Other mechanisms need to be investigated to explain the variation in responses of wheat to foliar addition of P. There remains insufficient evidence to recommend foliar-applied P as a management option for dryland wheat P nutrition.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
Thanks to the GRDC for Project UA00139, the Grains Industry Research Scholarship and the Fluid Fertilizer Foundation (USA) for financial support. Thanks to Eduardo Lopes Cancellier, Catherine Fiebiger, Hasbullah, Tanja Lenz, Trung Ta Nguyen and Sean Mason for technical assistance. We are grateful to the host landholders for their collaboration in field experiments and to Glenn McDonald for his help on site selection and field experiment management.
References
Ali MS, Sutradhar A, Edano ML, Edwards JT, Girma K (2014) Response of winter wheat grain yield and phosphorus uptake to foliar phosphite fertilisation. International Journal of Agronomy 2014, 801626| Response of winter wheat grain yield and phosphorus uptake to foliar phosphite fertilisation.Crossref | GoogleScholarGoogle Scholar |
Batten GD, Wardlaw IF, Aston MJ (1986) Growth and distribution of phosphorus in wheat developed under various phosphorus and temperature regimes. Australian Journal of Agricultural Research 37, 459–469.
| Growth and distribution of phosphorus in wheat developed under various phosphorus and temperature regimes.Crossref | GoogleScholarGoogle Scholar |
Benbella M, Paulsen GM (1998) Efficacy of treatments for delaying senescence of wheat leaves: II. Senescence and grain yield under field conditions. Agronomy Journal 90, 332–338.
| Efficacy of treatments for delaying senescence of wheat leaves: II. Senescence and grain yield under field conditions.Crossref | GoogleScholarGoogle Scholar |
Bouma D (1969) The response of subterranean clover (Trifolium subterraneum L.) to foliar applications of phosphorus. Australian Journal of Agricultural Research 20, 435–445.
| The response of subterranean clover (Trifolium subterraneum L.) to foliar applications of phosphorus.Crossref | GoogleScholarGoogle Scholar |
Burkitt LL, Moody PW, Gourley CJP, Hannah MC (2002) A simple phosphorus buffering index for Australian soils. Australian Journal of Soil Research 40, 497–513.
| A simple phosphorus buffering index for Australian soils.Crossref | GoogleScholarGoogle Scholar |
Calderini DF, Savin R, Abeledo LG, Reynolds MP, Slafer GA (2001) The importance of the period immediately preceding anthesis for grain weight determination in wheat. In ‘Wheat in a global environment’. (Eds Z Bedö, L Láng) pp. 503–509. (Springer: Dordrecht, The Netherlands)
Clarke JM, Campbell CA, Cutforth HW, DePauw RM, Winkleman GE (1990) Nitrogen and phosphorus uptake, translocation, and utilization efficiency of wheat in relation to environment and cultivar yield and protein levels. Canadian Journal of Plant Science 70, 965–977.
| Nitrogen and phosphorus uptake, translocation, and utilization efficiency of wheat in relation to environment and cultivar yield and protein levels.Crossref | GoogleScholarGoogle Scholar |
Elliott DE, Reuter DJ, Reddy GD, Abbott RJ (1997) Phosphorus nutrition of spring wheat (Triticum aestivum L.). 1. Effects of phosphorus supply on plant symptoms, yield, components of yield, and plant phosphorus uptake. Australian Journal of Agricultural Research 48, 855–867.
| Phosphorus nutrition of spring wheat (Triticum aestivum L.). 1. Effects of phosphorus supply on plant symptoms, yield, components of yield, and plant phosphorus uptake.Crossref | GoogleScholarGoogle Scholar |
Facelli E, Duan T, Smith SE, Christophersen HM, Facelli JM, Smith FA (2014) Opening the black box: outcomes of interactions between arbuscular mycorrhizal (AM) and non-host genotypes of Medicago depend on fungal identity, interplay between P uptake pathways and external P supply. Plant, Cell & Environment 37, 1382–1392.
| Opening the black box: outcomes of interactions between arbuscular mycorrhizal (AM) and non-host genotypes of Medicago depend on fungal identity, interplay between P uptake pathways and external P supply.Crossref | GoogleScholarGoogle Scholar |
Fernández V, Guzman P, Peirce CAE, McBeath TM, Khayet M, McLaughlin MJ (2014) Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant and Soil 384, 7–20.
| Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (2015) Definitions and determination of crop yield, yield gaps, and of rates of change. Field Crops Research 182, 9–18.
| Definitions and determination of crop yield, yield gaps, and of rates of change.Crossref | GoogleScholarGoogle Scholar |
French RJ, Schultz JE (1984) Water use efficiency of wheat in a Mediterranean-type environment. I. The relation between yield, water use and climate. Australian Journal of Agricultural Research 35, 743–764.
| Water use efficiency of wheat in a Mediterranean-type environment. I. The relation between yield, water use and climate.Crossref | GoogleScholarGoogle Scholar |
Froese S, Wiens JT, Warkentin T, Schoenau JJ (2020) Response of canola, wheat and pea to foliar phosphorus fertilization at a phosphorus deficient site in eastern Saskatchewan. Canadian Journal of Plant Science
| Response of canola, wheat and pea to foliar phosphorus fertilization at a phosphorus deficient site in eastern Saskatchewan.Crossref | GoogleScholarGoogle Scholar |
Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE (2009) Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytologist 181, 938–949.
| Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes.Crossref | GoogleScholarGoogle Scholar | 19140934PubMed |
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Canadian Journal of Plant Science 81, 211–224.
| The importance of early season phosphorus nutrition.Crossref | GoogleScholarGoogle Scholar |
Hazen JL (2000) Adjuvants: terminology, classification, and chemistry. Weed Technology 14, 773–784.
| Adjuvants: terminology, classification, and chemistry.Crossref | GoogleScholarGoogle Scholar |
Hedley MJ, McLaughlin MJ (2005) Reactions of phosphate fertilisers and by-products in soils. In ‘Phosphorus: agriculture and the environment’. (Ed. AN Sharpley) pp. 181–252. (American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA)
Hess FD, Foy CL (2000) Interaction of surfactants with plant cuticles. Weed Technology 14, 807–813.
| Interaction of surfactants with plant cuticles.Crossref | GoogleScholarGoogle Scholar |
Holloway PJ (1969) The effects of superficial wax on leaf wettability. Annals of Applied Biology 63, 145–153.
| The effects of superficial wax on leaf wettability.Crossref | GoogleScholarGoogle Scholar |
Huang CY, Shirley N, Genc Y, Shi B, Langridge P (2011) Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiology 156, 1217–1229.
| Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley.Crossref | GoogleScholarGoogle Scholar | 21606317PubMed |
Klute A (Ed.) (1986) ‘Water retention: laboratory methods.’ (American Society of Agronomy, Soil Science Society of America: Madison, WI, USA)
Koontz H, Biddulph O (1957) Factors affecting absoprtion and translocation of foliar applied phosphorus. Plant Physiology 32, 463–470.
| Factors affecting absoprtion and translocation of foliar applied phosphorus.Crossref | GoogleScholarGoogle Scholar | 16655030PubMed |
Liu TY, Chang CY, Chiou TJ (2009) The long-distance signaling of mineral macronutrients. Current Opinion in Plant Biology 12, 312–319.
| The long-distance signaling of mineral macronutrients.Crossref | GoogleScholarGoogle Scholar | 19481493PubMed |
Mason SD, McNeill AM, McLaughlin MJ, Zhang H (2010) Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant and Soil 337, 243–258.
| Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods.Crossref | GoogleScholarGoogle Scholar |
McBeath TM, McLaughlin MJ, Noack SR (2011) Wheat grain yield response to and translocation of foliar applied phosphorus Crop & Pasture Science 62, 58–65.
| Wheat grain yield response to and translocation of foliar applied phosphorusCrossref | GoogleScholarGoogle Scholar |
McBeath TM, McLaughlin MJ, Kirby J, Armstrong RD (2012) The Effect of soil water status on fertiliser, topsoil and subsoil phosphorus utilisation by wheat. Plant and Soil 358, 337–348.
| The Effect of soil water status on fertiliser, topsoil and subsoil phosphorus utilisation by wheat.Crossref | GoogleScholarGoogle Scholar |
McLaughlin MJ, McBeath TM, Smernik RJ, Stacey SP, Ajiboye B, Guppy C (2011) The chemical nature of P accumulation in agricultural soils-implications for fertiliser management and design: an Australian perspective. Plant and Soil 349, 69–87.
| The chemical nature of P accumulation in agricultural soils-implications for fertiliser management and design: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Mosali J, Desta K, Teal RK, Freeman KW, Martin KL, Lawles JW, Raun WR (2006) Effect of foliar application of phosphorus on winter wheat grain yield, phosphorus uptake, and use efficiency. Journal of Plant Nutrition 29, 2147–2163.
| Effect of foliar application of phosphorus on winter wheat grain yield, phosphorus uptake, and use efficiency.Crossref | GoogleScholarGoogle Scholar |
Netting AG, von Wettstein-Knowles P (1973) The physico-chemical basis of leaf wettability in wheat. Planta 114, 289–309.
| The physico-chemical basis of leaf wettability in wheat.Crossref | GoogleScholarGoogle Scholar | 24458788PubMed |
Noack SR, McBeath TM, McLaughlin MJ (2010) Potential for foliar phosphorus fertilisation of dryland cereal crops: a review. Crop and Pasture Science 61, 659–669.
| Potential for foliar phosphorus fertilisation of dryland cereal crops: a review.Crossref | GoogleScholarGoogle Scholar |
Peirce CAE, McBeath TM, Fernandez V, McLaughlin MJ (2014) Wheat leaf properties affecting the absorption and subsequent translocation of foliar-applied phosphoric acid fertiliser. Plant and Soil 384, 37–51.
| Wheat leaf properties affecting the absorption and subsequent translocation of foliar-applied phosphoric acid fertiliser.Crossref | GoogleScholarGoogle Scholar |
Peirce CAE, Priest C, McBeath TM, McLaughlin MJ (2016) Uptake of phosphorus from surfactant solutions by wheat leaves: spreading kinetics, wetted area, and drying time. Soft Matter 12, 209–218.
| Uptake of phosphorus from surfactant solutions by wheat leaves: spreading kinetics, wetted area, and drying time.Crossref | GoogleScholarGoogle Scholar |
Peirce CAE, McBeath TM, Priest C, McLaughlin MJ (2019) The timing of application and inclusion of a surfactant are important for absorption and translocation of foliar phosphoric acid by wheat leaves. Frontiers in Plant Science 10, 1532
| The timing of application and inclusion of a surfactant are important for absorption and translocation of foliar phosphoric acid by wheat leaves.Crossref | GoogleScholarGoogle Scholar | 31824546PubMed |
PIRSA (2016) Crop and Pasture Report South Australia. 2015–16 Harvest. Primary Industries and Regions SA, Adelaide. Available at: https://pir.sa.gov.au/__data/assets/pdf_file/0003/270057/PIRSA_Crop_and_Pasture_Report_Jan_16.pdf (accessed 20 August 2020).
Reed DW, Tukey HB (1978) Effect of pH on foliar absorption of phosphorus compounds by Chrysanthemum. Journal of the American Society for Horticultural Science 103, 337–340.
Reuter DJ, Robinson JB (Eds) (1997) ‘Plant analysis: an interpretation manual.’ (CSIRO Publishing: Melbourne, Vic.)
Rodríguez D, Andrade FH, Goudriaan J (1999) Effects of phosphorus nutrition on tiller emergence in wheat. Plant and Soil 209, 283–295.
| Effects of phosphorus nutrition on tiller emergence in wheat.Crossref | GoogleScholarGoogle Scholar |
Römer R, Schilling G (1986) Phosphorus requirements of the wheat plant in various stages of its life cycle. Plant and Soil 91, 221–229.
| Phosphorus requirements of the wheat plant in various stages of its life cycle.Crossref | GoogleScholarGoogle Scholar |
Sherchand K, Paulsen GM (1985) Response of wheat to foliar phosphorus treatments under field and high temperature regimes. Journal of Plant Nutrition 8, 1171–1181.
| Response of wheat to foliar phosphorus treatments under field and high temperature regimes.Crossref | GoogleScholarGoogle Scholar |
Spanoghe P, De Schampheleire M, Van der Meeren P, Steurbaut W (2007) Influence of agricultural adjuvants on droplet spectra. Pest Management Science 63, 4–16.
| Influence of agricultural adjuvants on droplet spectra.Crossref | GoogleScholarGoogle Scholar | 17173344PubMed |
Stolzenberg GE, Olson PA, Zaylskie RG, Mansager ER (1982) Behavior and fate of ethoxylated alkylphenol nonionic surfactant in barley plants. Journal of Agricultural and Food Chemistry 30, 637–644.
| Behavior and fate of ethoxylated alkylphenol nonionic surfactant in barley plants.Crossref | GoogleScholarGoogle Scholar |
Sutton PJ, Peterseon GA, Sander DH (1983) Dry matter production in tops and roots of winter wheat as affected by phosphorus availability during various growth stages. Agronomy Journal 75, 657–663.
| Dry matter production in tops and roots of winter wheat as affected by phosphorus availability during various growth stages.Crossref | GoogleScholarGoogle Scholar |
Swanson CA, Whitney JB (1953) Studies on the translocation of foliar-applied P32 and other radioisotopes in bean plants. American Journal of Botany 40, 816–823.
| Studies on the translocation of foliar-applied P32 and other radioisotopes in bean plants.Crossref | GoogleScholarGoogle Scholar |
Thorne GN (1957) The effect of applying a nutrient in leaf sprays on the absorption of the same nutrient by roots. Journal of Experimental Botany 8, 401–412.
| The effect of applying a nutrient in leaf sprays on the absorption of the same nutrient by roots.Crossref | GoogleScholarGoogle Scholar |
Tukey HB, Wittwer SH, Teubner FG (1956) Utilization of radioactive isotopes in resolving the effectiveness of foliar absorption of plant nutrients. In ‘Proceedings International Conference on the Peaceful Uses of Atomic Energy’. Vol. 12, pp. 138–148. (United Nations: New York)
Tukey HB, Wittwer SH, Bukovac MJ (1961) Absorption of radionuclides by aboveground plant parts and movement within the plant. Agricultural and Food Chemistry 9, 106–113.
| Absorption of radionuclides by aboveground plant parts and movement within the plant.Crossref | GoogleScholarGoogle Scholar |
Weaver DM, Wong MTF (2011) Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices. Plant and Soil 349, 37–54.
| Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices.Crossref | GoogleScholarGoogle Scholar |
Zabkiewicz JA (2007) Spray formulation efficacy: holistic and futuristic perspectives. Crop Protection 26, 312–319.
| Spray formulation efficacy: holistic and futuristic perspectives.Crossref | GoogleScholarGoogle Scholar |
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| A decimal code for the growth stages of cereals.Crossref | GoogleScholarGoogle Scholar |
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric acid digestion and multi-element analysis of plant material by inductively-coupled plasma spectrometry. Communications in Soil Science and Plant Analysis 18, 131–146.
| Nitric acid digestion and multi-element analysis of plant material by inductively-coupled plasma spectrometry.Crossref | GoogleScholarGoogle Scholar |